Research Article Open Access
Microalgal-Bacterial Consortium in Polyaromatic Hydrocarbon Degradation of Petroleum Based Effluent
Egberomoh Godsgift Omojevwe* and Fagade Obasola Ezekiel
Department of Microbiology, Environmental Microbiology Unit, University of Ibadan, Ibadan, Oyo, Nigeria
- *Corresponding Author:
- Egberomoh Godsgift Omojevwe
Department of Microbiology, Environmental Microbiology
Unit, University of Ibadan, Ibadan, Oyo, Nigeria
Tel: +2348037905257
E-mail: egberomohgo@gmail.com
Received date: June 22, 2016; Accepted date: July 06, 2016; Published date: July 07, 2016
Citation: Gods'gift OE, Fagade OE (2016) Microalgal-Bacterial Consortium in Polyaromatic Hydrocarbon Degradation of Petroleum – Based Effluent. J Bioremed Biodeg 7:359. doi:10.4172/2155-6199.1000359
Copyright: © 2016 Gods'gift OE, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The recurrent and indiscriminate discharge of flow stations' crude oil effluent has led to an increased polyaromatic hydrocarbons (PAHs) level in the environment. PAHs are high-priority environmental pollutants of significant human health concerns, thus considerable effort has been made in this study to biodegrade these PAHs using microalgalbacterial consortia. Crude oil effluent samples collected from the saver pit at Egbaoma flow station in the Niger Delta region of Nigeria were analysed biochemically and microbiologically. Three species of bacteria and two species of microalgae isolated, characterized and screened emerged as best hydrocarbon utilizers. The consortia comprised of Chlorella minutissimma and Aphanocapsa sp. as microalgae inoculants, while Citrobacter sp. SB9, Pseudomonas aeruginosa SA3 and Bacillus subtilis SA7 as bacterial inoculants. Growth dynamics, pH and degradation of the effluents' PAH content were analyzed for a period of ten days. Consortium BCC (bacteria inoculants and Chlorella minutissimma), BCCA (all inoculants) and BCA (bacteria inoculants and Aphanocapsa sp.) had PAH degradation percentage of 92.09%, 67.76% and 47.19% and optical density (OD) of 0.1255°A, 0.0669°A and 0.0703°A from an initial OD of 0.0126°A respectively. Only BCA had an acidic pH after ten days. Effective synergism and excellent PAH degradation is achievable and highly recommendable with microalgal-bacterial consortium yet its success is highly dependent on the consortium assembling, therefore, proper selection of the microbes for biodegradation is paramount to the success and efficiency of the degradation process.
Keywords
Crude oil effluent; Polycyclic aromatic hydrocarbon; Biodegradation; Microalgae; Bacterial microalgae consortium
Introduction
Petroleum-based effluent is the largest waste stream generated in the petroleum industry. On shore, crude oil effluent generated in flow stations is collected in a series of pit where it is mechanically treated with API (American Petroleum Institute) oil-water separator before being discharged into surrounding water bodies [1]. The effluent contains significant amount of oil and other impurities [2]. It has been reported that toxic compounds such as aliphatic hydrocarbons, aromatic hydrocarbons, phenols, heavy metals/metal derivatives and other chemicals used during processes such as de-emulsification, are always present in crude oil effluent [3,4]. The accumulation of the toxic compounds from the discharged effluent in the receiving water bodies and its surrounding environment results in serious environmental and health consequences [5]. Polycyclic aromatic hydrocarbons (PAHs), a major constituent of petroleum-based effluent, are a group of hydrocarbons containing two or more fused aromatic rings in linear, angular or clustered arrangements. They are naturally present in crude-oil and generated during fuel combustion, waste incineration or as by-product of industrial processes such as petroleum exploration [6]. PAHs have been reported as high priority pollutants of the environment and are of significant health concerns because of their ability to solubilized in water, toxicity, recalcitrant nature and bioaccumulation potential [7-9] amongst other researcher, have reported that the toxicity and recalcitrant nature of PAHs are the major reasons for the relative inefficiency in PAHs degradation by bacteria and fungi both in pure form and in consortium, therefore researchers have reportedly explored the bioaccumulation and biodegradation potentials of microalgae [10-13]. Microalgae play an important role in the environmental cleaning of pollutants. These are a diverse group of prokaryotes with varying morphological and physiological properties, and have attracted increasing interests because of their immense potentials [7,14-18]. Their most common feature is their ability to carry out oxygenic photosynthesis which is similar to that in higher plants, and this makes a large contribution to the equilibrium of the earth’s atmosphere by producing oxygen and removing carbon (IV) oxide [19]. Vast interest in microalgae obtained from the environment has grown considerably because they represent an innovative approach to obtain new underexplored resources [20]. In nature and long term laboratory algal cultures, most microalgae are found in association with other aerobic or anaerobic microorganisms. The bacterial assemblages are known to influence the development or decline of algal blooms [21]. The molecular oxygen from algal photosynthesis is used as an electron acceptor by bacteria to degrade organic matter while the carbon dioxide (CO2) from the bacterial mineralization completes the photosynthetic cycle. The symbiotic interactions of microalgae and bacteria form the basis of the biological oxygen demand (BOD) removal in the wastewater treatment ponds, first reported by Ref. [22]. Furthermore, microalgae can help in co-metabolic degradation or produce biosurfactants and extracellular matters to enhance bacterial activity for increasing pollutant bioavailability [23]. Additionally, microalgae can accumulate hydrocarbons, and subsequently make those compounds available to the associated hydrocarbon-utilizing bacteria [24]. The cooperation of phototrophic microalgae and heterotrophic bacteria could potentially improve the degradation of environmental contaminants including oil pollutants [25,26]. Since microalgae and bacteria have been reported to biodegrade environmental pollutants with varying degrees of success, this study has been conducted to ascertain the potential of microalgal-bacterial consortia in degrading PAH fraction of petroleum-based effluent.
Methodology
All chemicals were of analytical grade; crude oil was obtained from Egbaoma flow station, Nigeria. Unless otherwise specified, all experiments were conducted in triplicate at 28 ± 2°C under sterile conditions. The microbial strains were isolated from the saver pit sample.
Sample site and collection
Sampling was conducted in June and September at the Egbaoma flow station’s saver pit in Umutu, Niger Delta region of Nigeria. Subsurface effluent samples were collected into 250 mL amber bottles for hydrocarbon analysis and 1 L plastic bottle for physicochemical and microbiological analysis, although, pH and temperature were recorded in situ. Samples were transported in ice packs containing dry ice at 4°C to the laboratory for analysis.
Sample analysis
Physicochemical assessment: The physicochemical analysis of the waste water samples were conducted using standard methods [27,28]. The pH and electrical conductivity were measured using pH meter 3015, Jenway, and conductivity meter as described by Ref. [29] while dissolved oxygen (DO) and total dissolved solids (TDS) were measured using [30] DO and TDS meter respectively. Turbidity was measured using ref [30] direct spectrophotometer (method 8237) and then estimated against deionized water as blank at 450 nm. Nitrate (NO3-), phosphate (PO43-), sulphate (SO42- ) and total petroleum hydrocarbon (TPH) were determined using [17] spectrophotometer while total hardness, alkalinity, and biological oxygen demand (BOD) were determined volumetrically [27,28]. Salinity was determined using Ref. [31] equipment while temperature was determined with a mercury thermometer.
PAH assessment: PAHs were analyzed using a gas chromatography fitted with flame ionization detector (GC-FID). The extractions were carried out using n-hexane and methanol followed by procedures [27,28].
Microbiological assessment: Total cultural heterotrophic bacteria and microalgae counts were done using the pour plate techniques on nutrient agar (Oxoid, UK) as described by Ref. [32,33] on Allen medium respectively. The total hydrocarbon utilizing bacteria and microalgae counts were determined using the vapour phase transfer method [8,34] Hydrocarbon utilizing microalgae and bacteria were screened for pollutant tolerance/utilization using Allen medium and minimal salt medium of Ref. [35] respectively supplemented with 1% (v/v), 1.5% (v/v) and 3% (v/v) crude oil. This was done by subculturing the isolates from 1% (v/v) to 1.5% (v/v) and finally to 3% (v/v) after an incubation period of 5 days each for bacteria and 7 days each for microalgae. Isolates with the best hydrocarbon utilizing ability were characterized and identified based on their morphological, physiological and biochemical characteristics presented in Bergey’s Manual of Determinative Bacteriology [36] and the API kit profiles [37] while that of microalgae was done by comparison with those documented in the Identification Guide to freshwater and terrestrial algae [38]. These isolates were used to make up three consortia in the following order,
BCC – Pseudomonas aeruginosa SA3, Bacillus subtilis. SA7, Citrobacter sp. SB7 and Chlorella minutissima SA1
BCA – Pseudomonas aeruginosa SA3, Bacillus subtilis. SA7, Citrobacter sp. SB7, and Aphanocapsa sp. SB5 BCCA – Pseudomonas aeruginosa SA3, Bacillus subtilis. SA7, Citrobacter sp. SB7, Chlorella minutissima SA1 and Aphanocapsa sp. SB5
The ability of isolates to degrade PAHs was determined using BG11 medium [39] as described by Ref. [12] with a slight modification. Thirty milliliters of BG11 medium and ten milliliters of filtered effluent sample was dispensed aseptically into one hundred milliliters Erlenmeyer flasks after which three milliliters suspension of isolates in a ratio of 2:1 for microalgae and bacteria was aseptically dispensed into the flask. Polyaromatic hydrocarbon degradation assessment was carried out for 240 hrs in 120 hrs intervals on a rotary shaker (G24 environmental incubator shaker, New Brunswick Scientific Co. Inc. Edison, New Jersey, USA.) at 160 rpm. The initial absorbance of the medium was measured at 600 nm on a UV-Visible Spectrophotometer (Camspec M105) and standardized at 0.8°A while pH was adjusted to 6.5.
Results
The physicochemical analysis of the crude oil effluent sample showed that total nitrogen (September samples only), sulphur, conductivity, salinity and total petroleum hydrocarbon, for both samples, were well above the [40] limit while other parameters were relatively within the allowable limits (Table 1). The mean heterotrophic bacteria and microalgae counts were 30.0 × 106 cfu/ml and 530 cells/ml respectively while the of hydrocarbon utilizing bacteria and microalgae counts were 6.01 × 103 cfu/ ml and 189 cells/ml respectively. The ability of isolates to tolerate and utilize hydrocarbon showed three bacteria isolates, Pseudomonas aeruginosa SA3, Bacillus subtilis SA7 and Citrobacter sp. SB9, grew very well on all concentrations of crude oil while seven microalgae isolates survived at 3%v/v crude oil with two isolates, Chlorella minutissima and Aphanocapsa sp, emerging as the most dominant having counts of 66 cells/ml and 52 cells/ml respectively.
| Properties | June sample | September sample | DPR limits |
|---|---|---|---|
| Total dissolved solids (mg/L) | 1140 | 1720 | 2000 |
| Total Nitrogen (mg/L) | 27 | 10.2 | 0-8.1 |
| pH | 6.85 | 7.88 | 6.5-8.5 |
| Temperature (°C) | 23 | 26 | 25-30 |
| Turbidity (NTU) | 9.97 | 10.1 | 10 |
| Available phosphorus (mg/L) | 2.72 | 3.99 | 3 |
| Sulphur (mg/L) | 280.18 | 334.16 | 200 |
| Conductivity (µs/cm) | 1607.4 | 1870.7 | 400-1250 |
| Total hardness (mg/L) | 14.13 | 15.41 | 10-144 |
| Total alkalinity (mg/L) | 87.55 | 79 | 300 |
| Salinity (mg/L) | 1867.9 | 2100 | 600 |
| Dissolved oxygen (mg/L) | 5.8 | 4.2 | 5 |
| Biological oxygen demand (mg/L) | 11.58 | 8.68 | 10 |
| Total petroleum hydrocarbon (%) | 29.6 | 36.1 | 10 |
Table 1: Physico-chemical properties of the effluent samples from the saver pit at Egbaoma flow station.
TRL – MSM+1% (v/v) crude oil effluent
BCC – Pseudomonas aeruginosa SA3, Bacillus subtilis. SA7, Citrobacter sp. SB7 and Chlorella minutissima SA1+MSM+1% (v/v) crude oil effluent
BCA – Pseudomonas aeruginosa SA3, Bacillus subtilis. SA7, Citrobacter sp. SB7, and Aphanocapsa sp. SB5+MSM+1% (v/v) crude oil effluent
BCCA – Pseudomonas aeruginosa SA3, Bacillus subtilis. SA7, Citrobacter sp. SB7, Chlorella minutissima SA1 and Aphanocapsa sp. SB5+MSM+1% (v/v) crude oil effluent
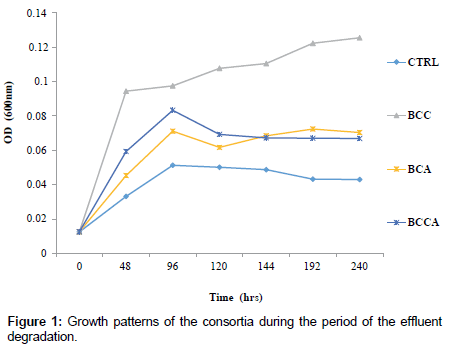
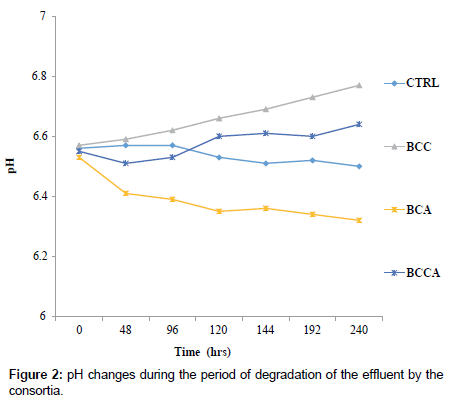
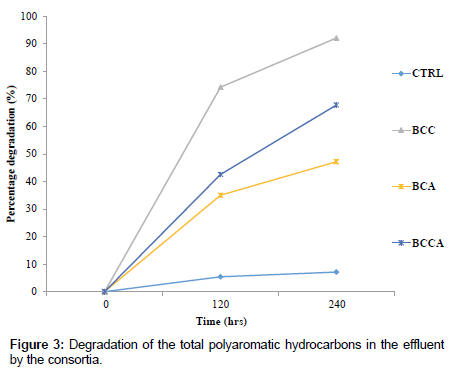
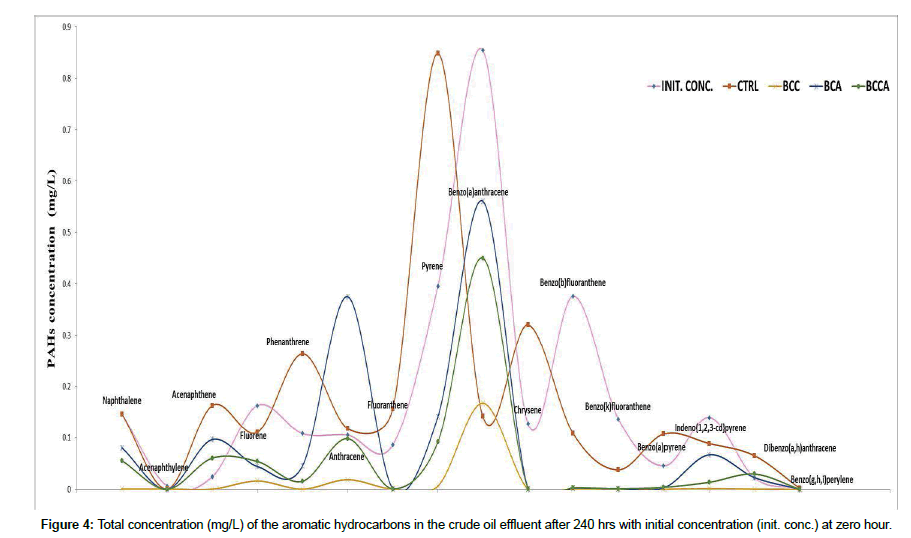
BCA, BCC and BCCA respectively (Figure 2) with consortium BCA falling below the initial pH level. The optical density (OD) of the degradation medium increased from 0.0126°A to 0.1255°A, 0.0703°A and 0.0669°A for consortium BCC, BCA and BCCA respectively (Figure 1) with consortium BCC having the highest optical density after 240 hrs while pH change observed ranged from 6.5 to 6.3, 6.8 and 6.6 for consortium. Figure 3 shows the percentage degradation of total PAH by the consortia. Consortium BCC had the highest percentage of 92.07%, preceded by BCCA with a percentage of 67.76% while the least was observed in BCA with percentage of 47.19%. Consortium BCC was able to remove over 90% of sixteen PAHs detected in the crude oil effluent after 240 hrs with an exception of acenaphthylene, anthracene and benzo (a) anthracene (Figure 4).
Discussion
According to Ref. [41,42], the percentage of hydrocarbon utilizing microbial counts as compared to the heterotrophic microbial counts obtained from the saver pit sample could be attributed to the percentage of total petroleum hydrocarbon in the sample. These researchers proved that there’s always a correlation between the level of hydrocarbon pollution and the ratio of heterotrophic microorganisms to hydrocarbon utilizing microorganisms present in the effluent. The emergence of Pseudomonas aeruginosa SA3, Bacillus subtilis. SA7, Citrobacter sp. SB7, Chlorella minutissima SA1 and Aphanocapsa sp. SB5 as best hydrocarbon utilizers indicates that these species are capable of utilizing and metabolizing the various hydrocarbon components contained in the crude oil [10,23,43]. The optical density, pH and PAH degradation percentage observed for the consortia in this study indicates successful synergistism of inoculant, utilization and degradation of the PAH fraction of the effluent. This is affirmative to the findings of Ref. [12,44,45] who achieved similar results with different species of bacteria and microalgae in consortium. The increased concentration of some PAHs such as anthracene and benzo(a)anthracene by the consortia (Figure 4) affirms the findings of Ref. [13,30] who proved that these PAHs are refractory organic compounds. The accumulation of some PAHs such as acenaphthene, dibenzo(a,h)anthracene, indicated by increased concentrations in Figure 4 is in accordance with the findings of Ref. [46,47] who proved that some hydrocarbons especially PAHs tend to increase during biodegradation in relative or absolute amounts suggesting that these compounds do not only tend to resist biodegradation but are formed de novo by condensation and/or displacement reactions of the biodegradation derivatives. The fluctuations observed for the control samples does not indicate degradation or accumulation, it simply indicates the condensation and/or displacement reactions of the biodegradation components that occurs in the natural environment.
Conclusion
This study has proven that microalgae-bacteria consortium isolated from a crude oil polluted site possess the ability to biodegrade crude oil effluent based-PAHs and preferentially attack high molecular PAHs that has been branded by the US EPA as high priority pollutants.
References
- Nnaji CC, Agunwamba JC (2014) Quality Assessment of Water Receiving Effluents from Crude oil. Water and Environmental Journal 28: 104-113
- Fakhru’l-Razi A, Alireza P, Luqman CA, Dayang RAB, Sayed SM, et al.(2009) Review of technologies for Oil and Gas Produced Water Treatment. Journal of Hazardous Materials 170: 530-551.
- Li Q, Kang C, Zhang C (2005) Waste Water Produced from an Oilfield and Continuous Treatment with an Oil-Degrading Bacterium. Process Biochemistry 40: 873-877.
- Uzoekwe SA, Oghosanine FA (2011) The Effect of Refinery and Petrochemical Effluent on water quality of Ubeji creek Warri, Southern Nigeria. Ethiopian Journal of Environmental Studies and Management 4: 107-116.
- Nweke CO, Okpokwasili GC (2004) Effects of Bioremediation Treatments on the Bacterial Populations of Soil at Different Depths. Nigerian Journal of Microbiology 18: 363-372.
- Igwo-Ezikpe MN, Gbenle OG, Ilori MO, Okpuzor J, Osuntoki AA (2010) High Molecular Weight Polycyclic Aromatic Hydrocarbons Biodegradation by Bacteria Isolated from Contaminated Soils in Nigeria. Research Journal of Environmental Sciences 4: 127-137.
- Apt KE, Behrens PW (1999) Commercial Development in Microalgae Biotechnology. Journal of Phycology 35: 215-226.
- Subashchandrabose SR,RamakrishnanB,MegharajM,VenkateswarluK, NaiduR(2011) Consortia of Cyanobacteria/microalgae and Bacteria: Biotechnological Potential. Biotechnology Advances 29: 896-907.
- Warshawsky D, LaDow K, Schneider J (2007) Enhanced Degradation of benzo[a]pyrene by Mycobacterium sp. in Conjunction with Green alga. Chemosphere 69: 500-506.
- Ifeanyi VO, Anyanwu BN, Ogbulie JN, Nwabueze RN, Chukwuma V, et al (2013) Ex-Situ Bioremediation Of Crude Oil Polluted Soil Using Algal Species (AphanocapsaElachista). Asian Journal of Science and Technology 5: 001-003.
- Cohen Y (2002) Bioremediation of oil by Marine Microbial Mat. International Microbiology 5:189-193
- Tang X, Dang Z, He LY, Lu GN, Tao XQ, et al. (2010) Biodegradation of Crude oil By Artificial Microalgal-Bacterial Consortium. Scientific Reports 1: 1-4
- Tang X, He LY, Tao XQ, Dang Z, Geo C (2010) Construction of an Artificial Microalgal-Bacterial Consortium that Efficiently Degrades Crude oil. Journal of Hazardous Material 181: 1158-1162.
- Chisti Y, Grima EM, Belarbia EH, Fernandez FGA, Medina AR (2003) Recovery of Microalgal Biomass and Metabolites: Process Options and Economics. Biotechnology Advances 20: 491-515.
- Cohen Z (1999) Chemicals from microalgae. Taylor and Francis Publishers, London, UK,p: 419
- Lee CG (1999) Calculation of light penetration depth in photobioreactors. Biotechnology and Bioprocess Engineering 4: 78-81.
- Olaizola M (2003) Commercial development of microalgae biotechnology from the test-tube to the market place. Biomolecular Engineering,pp: 1-8
- Tramper J, Battershill C, Brandenburg W, Burgess G, Hill R, et al. (2003) What to do in marine biotechnology. Biomolecular Engineering,pp: 1-5
- Tandeau de Marsac N, Houmard J (1993) Adaptation of cyanobacteria to environmental stimuli: new steps towards molecular mechanisms. FEMS Microbiology Reviews 104: 119-190.
- Romano I, Bellitti MR, Nicholaus B, Lama L, Manca CM, et al. (2000). Lipid profile a useful chemotaxonomic marker for classification of a new cyanobacterium in Spirulina genus. Phytochemical 54: 289-294
- Fukami, Nishijima T, Ishida Y (1997) Stimulative and Inhibitory Effects of Bacteria on the Growth of Microalgae. Hydrobiologia 358: 185-199.
- Oswald W, Gotaas H, Ludwig H, Lynch V (1953) Algae Symbiosis in Oxidation Ponds: III. Photosynthetic Oxygenation. Sewage Industrial Wastes 25: 692-705.
- Muñoz R, Guieysse B, Mattiasson B (2003) Phenanthrene Biodegradation by an Algal-Bacterial Consortium in Two-phase Partitioning Bioreactors. Applied Microbiology and Biotechnology 61: 261-267.
- Radwan SS, Al-Hasan RH, Ali N, Salamah S, Khanafer M (2005) Oil-consuming microbial consortia floating in the Arabian Gulf. International Biodeterioration and Biodegradation 56: 28-33.
- Chaillan F, Gugger M, Saliot A, Coute A, Oudot J (2006) Role of cyanobacteria in the biodegradation of crude oil by a tropical cyanobacterial mat. Chemosphere 62: 1574-1582
- Safonova ET, Dmitrieva IA, Kvitko (1999) The interaction of algae with alcanotrophic bacteria in black oil decomposition. Resources Conservation and Recycling 27: 193-201.
- American Public Health Association (1992) Standard Methods for the Examination of a Water and Wastewater. 18th edn,American Public Health Association, American Water Works Association and Water Environment Federation, Washington, USA.
- APHA (1985) Standard method for the examination of water and waste water. American Public Health Association, Washington DC, USA, p: 256.
- Richard LA (1954) Diagnoses and Improvement of Saline and Alkali Soils. Agriculture Hand Book,p: 60
- Jahasz AL, Britz ML, Stanley GA (1997) Degradation of Phenanthrene, Pyrene, Benzo(a)anthracene and dibenzo(a,h)anthracene by Burkholderiacepacia. Journal of Applied Microbiology 83: 189-196.
- HACH (2001) Hach Company. Direct Reading (DR)/3000 Spectrophotometer Manual. DR/2010 Spectrophotometer Handbook.
- Fagade EO (1990) Bacterial Degradation of Crude oil from the Niger Delta area of Nigeria. Unpublished PhD Thesis, Department of Botany and Microbiology, University of Ibadan, Nigeria,pp: 77-79.
- Stein JR (1973) Culturing Methods and Growth Measurements. Hand Book of Phycological Methods, 3rd edn. Cambridge University Press. pp: 234-252
- Raymond RL, Audson JO, Jamison VW (1976) Oil Degradation in soil. Applied Environmenta Microbiology 31: 522-535.
- Mills AL, Breuil C, Colwell (1978) Enumeration of Petroleum Degrading Marine and Estuarine Microorganisms by Most Probable Number Method. Canadian Journal of Microbiology 12: 234-248.
- HoltJG,KriegNR,SneathPHA,StaleyJT,WilliamsST(1994) Bergery’sManualof Determinative Bacteriology. 9th edn, Williams and Wilkins, Baltimore.
- APi (2009) BioMerieuxsa 69280 Marcy l’Etoile, France.
- John DM, Whitton BA, Brook AJ (2002) The freshwater Algal Flora of the British Isles an Identification Guide to Freshwater and Terrestrial Algae. Cambridge: Cambridge University Press,UK, pp: 39-43.
- Rippka R (1989) Methods in enzymology. California, San Diego, USA.
- Department of Petroleum Resources (DPR) (2002) Environment Guidelines and Standards for the Petroleum Industry in Nigeria (EGASPIN). Revised edition, December 2011. Journal of Environmental Sciences and Resource Management 3: 135-140.
- Akpor OB, Igbinosa OE, Igbinosa OO (2007) Studies on the Effect of Petroleum Hydrocarbon on the Microbial and Physico-Chemicals Characteristics of Soil. AJB 6: 1939-1943.
- Amund OO, Igiri CO (1990) Biodegradation of Petroleum Hydrocarbon under Tropical Estuarine Conditions. World Journal of Microbiology and Biotechnology 16: 118-121.
- Perez-Armendariz B, Mauricio-Gutierrez A, Jimenez-Salgado T, Tapia-Hernandez A, Santiesteban-Lopez A (2013) Emulsification of Hydrocarbons using Biosurfactant Producing Strains Isolated from Contaminated Soil in Puebla, Mexico. Biodegradation-Engineering and Technology 1: 1-5
- De Oteyza TG, Grimalt JO, Llirós M, Estevez I (2006). Microcosm experiments of oil degradation by microbial mats. Science of the Total Environment 357: 12-24.
- American Standard Testing and Materials (ASTM) (1995) Water and Environmental Technology. Vol. I and II.
- Wackett TM (1996) Co-metabolism: is the emperor wearing any clothes? Curriculum Opinion in Biotechnology 7: 311-316.
- Ogbulie JN, Ifeanyi VO (2006) Microcystis flo-aquae and Chaetophora tuberculosa Algae Endowed to Degrade Hydrocarbon. Journal of Science, Engineering & Technology 13: 7095-7096.
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 13113
- [From(publication date):
July-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 11999
- PDF downloads : 1114