Research Article Open Access
Mice Infection by Methicillin-Resistant Staphylococcus Aureus from Different Colonization Sites in Humans Resulting in Difusion to Multiple Organs
Silva-Santana G1,2, Lenzi-Almeida KC1,3, Fernandes-Santos C4, Couto DS4, Paes-De-Almeida EC4 and Aguiar-Alves F1,2*1Department of Pathology, Fluminense Federal University (UFF), Niterói, RJ, Brazil
2Laboratory of Molecular Epidemiology and Biotechnology, Laboratory Academic Rodolfo Albino, Fluminense Federal University (UFF), Niterói, RJ, Brazil
3Department of Medicine, Federal University of Rio de Janeiro (UFRJ), Brazil
4Department of Basic Sciences, Fluminense Federal University (UFF), Nova Friburgo, RJ, Brazil
- *Corresponding Author:
- Giorgio Silva de Santana
Laboratory Academic Rodolfo Albino
Fluminense Federal University (UFF), Mário Viana, Santa Rosa, Niterói, RJ, Brazil
Tel: +55(21)2629-9569
E-mail: bio.sant@hotmail.com
Received Date: June 12, 2016; Accepted Date: June 29, 2016; Published Date: June 30, 2016
Citation: Silva-Santana G, Lenzi-Almeida KC, Fernandes-Santos C, Couto DS, Paes-De-Almeida EC, et al. (2016) Mice Infection by Methicillin-Resistant Staphylococcus Aureus from Different Colonization Sites in Humans Resulting in Difusion to Multiple Organs. J Clin Exp Pathol 6:283. doi: 10.4172/2161-0681.1000283
Copyright: © 2016 Silva-Santana G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Staphylococcus aureus is an infectious agent which can replicate in the blood, colonizing multiple organs and causing often infections which can lead to sepsis fatal. Studies show that nasal colonization by this organism can be cause of sepsis in hospitalized patients, where most of the patients' blood isolates are identical to those isolated from nasal cavities. The increase in number of infections caused by β-lactam antibiotics-resistant strains in hospital settings is cause of concern due to the conditions in which these patients are found, including immunosuppression conditions, in addition to the large level of virulence factors this organism carries. This study aims to identify pathological alterations, present in organs: heart, spleen, kidneys and lungs due to infection induced by methicillinresistant Staphylococcus aureus isolated from infections, as well as to nasal colonization in humans, using an experimental murine model. Six different groups of isolates were involved, ranging from methicillin-susceptible and resistant, and those carrying, or not, the Leucocidin Panton-Valentine gene. The isolates were inoculated intravenously via tail vein at a concentration of 1.0 × 107 CFU/ml and, after 72 hours, the organs were collected for histopathological analysis for inflammatory process identification. This analysis revealed significant differences in organ infections, regarding inoculated strains. Organ tissues presented the inoculated isolate, confirmed by PCR, as well as neutrophilic infiltrate predominance. However, resistance to methicillin, and presence of the Panton-Valentine leucocidin gene were not decisive factors for infection severity in mice.
Keywords
Infection; MRSA; Panton-valentine leucocidin; Staphylococcus aureus
Introduction
Staphylococcus Aureus is a gram-positive commensal bacteria colonizing human skin, nasal cavity and gastr ointestinal tract [1-3]. Approximately 30% of the human population has its nasal cavities colonized by this microorganism [3-6]. Colonization is asymptomatic in most individuals; however, depending on carrier conditions, it can cause infections in individuals from hospitals or community settings [2,3,7]. In a hospital environment, use of catheters, endotracheal intubation, medical implants, trauma, diabetes, immunosuppression, hemodialysis and peritoneal dialysis can promote infections by this microorganism [8,9], which is also associated with a variety of diseases, from superficial skin infections [2,10] to serious systemic infections [3,11], as well as, endocarditis, osteomyelitis, pneumonia and sepsis [2,10].
Infections caused by this microorganism are predominant in skin and soft tissues [3,11], with purulent lesions and abscesses presenting the microorganism associated with infiltration of neutrophiles in the lesion [3,12]. Human infections do not produce specific immune responses, and may be persistent, recurrent or chronic [3,13]. This microorganism is also skilled at evading the innate and adaptive immune response producing coagulases, capsular polysaccharides, adhesins, proteases and exoproteins which inactivate the complement system, and toxins which form pores in immune cells [14-16].
Infections caused by S. Aureus may be related to virulence factors carried by the strain carries, and especially, the kind of secreted toxin. Among the virulence factors are fibronectin-binding proteins A and B (FnBPA and FnBPB) and homologous, related to microorganism adhesion to host tissue and which, following adhesion can be internalized into various cell types such as neutrophiles [2,17,18]. Important polypeptides as such coagulase (Coa) and the von Willebrand factor binding protein (vWbp) can be associated to fibrinogen, fibronectin, thrombospondin and the von Willebrand factor, forming complex staphylotrombin and fibrin clots [3,12,19]. This activity performed by S. Aureus has been described in clinical renal abscess cases [3,20], also forming bridges for microorganisms to bind to platelet surface, reaching the bloodstream and therefore, adhere to the damaged heart valve surface. It is a major causative agent of endocardial infections and a major virulence factor in infective endocarditis pathogenesis [21].
Another virulence factor considered of great importance nowadays in S. Aureus infections comprises the Panton-Valentine leucocidin, having polymorphonuclear phagocytes as its main target. It binds to C5aR and C5L2 complement receptors on leukocytes [3,22], monocytes, and macrophages [14,23] forming pores in their membranes [3,24,25]. The lukS-PV and lukF-PV genes, responsible for this Leucocidin expression, are placed in the operon lukPV [14,26,27], mediated by lisogenic phages such as fSLT, among others [14,28,29]. These phages carrying genes responsible for PVL production have been more associated with strains possessing chromosomal cassette SCCmec IV present in methicillin-resistant strains associated with community environment (CA-MRSA) [30]. Clinically, PVL-producing strains have frequently been isolated in skin abscesses, soft tissue infections and necrotizing pneumonia [31,32].
It is estimated that 89% of S. Aureus isolated in hospital infections are MRSA [6]. The recommended antimicrobial therapy for infections caused by these isolates has been, so far, Vancomycin, Daptomycin and Linezolid [33-35]; however, Vancomycin-resistant strains (VRSA) have already been reported [36-38].
Based on this information, this study aimed to identify pathological alterations in animal organs: heart, spleen, kidneys and lungs in a murine experimental model due to infection by β-lactam resistant S. aureus isolated from human infections and nasal colonization.
Materials and Methods
This study was approved by the Ethics Committee on Animal Research of the Federal Fluminense University Dean of Research and Graduate Studies under registration number: 439/2013.
Thirty-five mice Swiss males were used, young adults six weeks of age, weighing approximately 34 g, assigned by NAL (The Fluminense Federal University, Laboratory Animal Core). All animals were kept in collective cages and ventilated, with five animals each group (number of animals needed to obtain the expected results, following the regulations of the Ethics Committee), receiving commercial diet and filtered water ad libitum, maintained in light-dark cycles, at temperature 21 to 22°C (± 2°C) and 50 to 55% humidity.
Groups were divided according to the phenotypic and genotypic characteristics of each bacterial strain under study, in addition to the sites which were isolated from humans (Table 1).
| Groups | Inoculum 1.0 × 107 CFU/ml |
Genes | N | |
|---|---|---|---|---|
| mecA | lukF-PV | |||
| 1 | MRSA: Sample isolated from nasal cavity | (+) | (-) | 5 |
| 2 | MRSA: Sample isolated from peripheral blood in patients affected by severe pulmonary infection (SCCmec IV) | (+) | (+) | 5 |
| 3 | MRSA: Sample isolated from nasal cavity | (+) | (+) | 5 |
| 4 | MRSA: Sample isolated in epithelialtissue(skin)injury | (+) | (+) | 5 |
| 5 | MSSA: Sample isolated from nasal cavity | (-) | (-) | 5 |
| 6 | MRSA: Sample isolatedfromperipheralblood in patientsaffectedbyinfectious endocarditis, courtesy of National Cardiology Institute of Laranjeiras, Rio de Janeiro - Brazil | (+) | (-) | 5 |
| 7 | Control | Sterile saline solution (0.9% NaCl) |
5 | |
Table 1: Division of groups according to the characteristics of the inoculated bacterial strains.
Microbiological samples used are part of the collection of the Federal Fluminense University - Molecular Epidemiology Laboratory and Biotechnology.
Procedures which could lead to anxiety and/or pain were conducted under inhalation anesthesia in a fume cupboard for saturation isoflurane Forane® (2-chloro-2-(difluoromethoxy)-1,1,1-trifluoroethane) [39,40].
Preparation of bacterial inoculum
The colonies were grown in TSB (Trypticase Soy Broth) for 24 hours, continuing tubes containing serial dilutions in sterile saline (0.9% NaCl) to acquire the concentration 1.0 x 107 CFU/mL. The animals were inoculated with 50 μL of bacterial suspension, except for the control group, inoculated only with sterile saline (0.9% NaCl) in the same volume as the other groups.
Method infection induction
The animals' tails were decontaminated with polyvinylpyrrolidoneiodine solution at 10%. Bacterial suspension was inoculated via lateral tail vein. The animals were kept in their cages, examined every six hours for 72 hours. After 72 hours, the animals remained alive were euthanized by inhalation in fume cupboard for saturation isoflurane Forane® (2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane).
Method for surgical organ extraction
Following euthanasia, trichotomy of the abdomen was performed, through vertical incision for heart, spleen, kidney and lung removal. The organs were stored in 10% formaldehyde with a pH between 6.0 and 7.0 for 48 hours and sent to the Antonio Pedro University Hospital anatomy pathology service.
Histological slide preparation
Samples of tissues of the heart, spleen, kidneys and lungs underwent a dehydration process, diaphanization and paraffin embedding. Subsequently, cuts were made with a 3 μm thickness using microtome (LAB-MR500); then these were fixed on slides and stained with hematoxylin & eosin. The slides were observed using an optical microscope (model LX 500) and photographed using a iVm 5000 camera through Pro 2.7 ProgRes program capture for inflammatory process histopathologic at description.
Survival time statistical analysis
Suvival time statistical analysis was used as animal interest mortality variable in different time slots, with monitoring every six hours after inoculation. Results were obtained using a log rank Mantel-Cox multiple comparison test to confirm results using the Mann-Whitney test. Software used was SPSS version 10.0, with statistical results to significance level α=0.05.
Results
All the animals succumbing before to 72 hours of experimental protocol, presented decreased appetite and reduced water intake, followed by reduction in mobility, aspects similar to cachexia, as well as isolation from other group components hours before death.
Analysis of survival time after inoculation
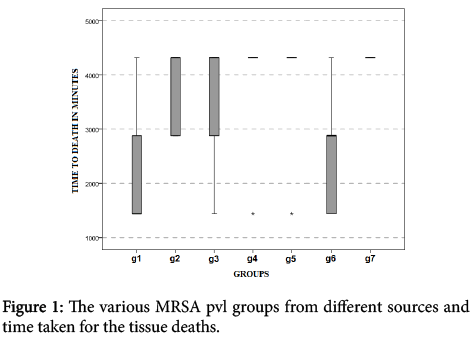
Upon Figure 1 evaluation, we observe existence of a statistically significant difference (p<0.05) between survival time of MRSA pvl (-) group, isolated from nasal cavity and control group inoculated with sterile saline (0.9% NaCl) (U=2.5; value-p=0.032), and MRSA pvl (-) group, isolated from peripheral blood in patients affected by infectious endocarditis and the control group inoculated with sterile saline (0.9% NaCl) (U=2.5; value-p=0.032). There were no other differences among the other peer groups.
However, after 72 horas the experimental protocol, noted in MRSA pvl (-) group, in isolated nasal cavity, four dead animals (80%); MRSA pvl (+) group, isolated from peripheral blood in patients affected by severe pulmonary infection, five animals dead (100%); MRSA pvl (+) group, isolated in nasal cavity, four dead animals (80%); MRSA pvl (+) group, isolated from injury in epithelial tissue (skin); four dead animals (80%); MRSA pvl (-) group, isolated in nasal cavity, one dead animal (20%); MRSA pvl (-) group, isolated from peripheral blood in patients affected by infectious endocarditis, five animals dead (100%); in control group, five animals living after 72 hours (Figure 1).
(g1) MRSA pvl (-) group, in isolated nasal cavity: three animals dead after 24 hours, a dead animal after 48 hours and one euthanized animal. (g2) MRSA pvl (+) group, isolated from peripheral blood in patients affected by severe pulmonary infection: two animals dead after 48 hours and three dead animals after 72 hours. (g3) MRSA pvl (+) group, isolated in nasal cavity: one animal dead after 24 hours, one animal dead after 48 hours, two animals dead after 72 hours and one animal euthanized.
(g4) MRSA pvl (+) group, isolated from injury in epithelial tissue (skin): one animal dead after 48 hours, three animals dead after 72 hours and one animal euthanized. (g5) MRSA pvl (-) group, isolated in nasal cavity: one animal dead after 24 hours and four animals euthanized after 72 hours. (g6) MRSA pvl (-) group, isolated from peripheral blood in patients affected by infectious endocarditis: two animals dead after 24 hours, two animals dead after 48 hours and one animal dead after 72 hours. (g7) control group: five animals euthanized after 72 hours.
Heart histopathology
The control group was presented within the normal heart range as described in the literature (Figure 2). MRSA pvl (-) group, isolated from nasal cavity, presented bacterial colonies with discreet neutrophilic inflammatory infiltrate, surrounded by necrotic areas (Figure 2). Conversely, MRSA pvl (+) group, isolated from peripheral blood in patients affected by severe pulmonary infection, displayed similarity to CG, but with neutrophilic infiltrate in pericardial fat and an outbreak of bacterial colonies in the left ventricle surrounded by necrosis (Figure 2).
MRSA pvl (+) group, isolated from nasal cavity, presented several outbreaks of bacterial colonies in the left ventricle, surrounded by neutrophilic infiltrate containing coagulation necrosis regions, cardiomyocytes in karyolysis with increased cytoplasmic acidophilia (Figure 2), aspect similarly found in the right ventricle. MRSA pvl (+) group, isolated in epithelial tissue (skin) injury, showed few outbreaks of ventricular neutrophilic infiltration and hyperemic capillaries. In the light of left ventricle there was a clot-containing area with red blood cells, and one with acidophilus materials and fibrin, associated with rich neutrophilic infiltrate and bacterial colonies arranged in clusters and diffuse between neutrophiles.
Some areas bore necrosis around the inflammatory infiltrate and neutrophile migration to pericardial fat plus several cardiomyocytes with intracytoplasmic vacuolar degeneration (Figure 2). Conversely, in MRSA pvl (-) group, isolated from nasal cavity, cardiac chambers showed several circumscribed bacterial colony outbreaks with intense neutrophilic infiltrate and fibrin, mainly in the ventricles (Figure 2).
Regarding, MRSA pvl (-) group, isolated from peripheral blood in patients affected by infective endocarditis, cardiomyocytes showed intracytoplasmic vacuolar degeneration, the right and left ventricles displayed several outbreaks of bacterial colonies and neutrophilic inflammatory infiltrate containing fibrin, a similar aspect seen in septum intraventricular (Figure 2).
(a) control group (400X): normal cardiomyocytes. (b) MRSA pvl (-) group, isolated from nasal cavity (400X): focus with neutrophilic inflammatory infiltrate and basophilic granular material (bacterial colony) (arrow), around eosinophilic region (necrosis) (asterisk). (c) MRSA pvl (+) group, isolated from peripheral blood in patients affected by severe pulmonary infection (400X): neutrophilic infiltrate with basophilic granular material (bacterial colony) between cardiac fibers (arrow), eosinophilic region (necrosis) (asterisk). (d) MRSA pvl (+) group, isolated from nasal cavity (400X): focus containing neutrophilic inflammatory infiltrate with granular basophilic material (bacterial colony) (arrow), around coagulation necrosis with cardiomyocytes in karyolysis and increased cytoplasmic acidophilia (asterisk). (e) MRSA pvl (+) group, isolated from epithelial tissue (skin) injury (400X): neutrophilic infiltrate with diffuse granular material (bacterial colony) (arrow), with necrosis around areas (asterisk). (f) MSSA pvl (-) group, isolated from nasal cavity (400X): heart chamber presenting inflammatory neutrophilic infiltrate (asterisk), bacterial colonies (arrow), fibrin (circle). (g) MRSA pvl (-) group, isolated from peripheral blood in patients affected by infectious endocarditis (400X): well-defined focus containing granular basophilic substance (bacterial colony) and neutrophile inflammatory infiltrate with fibrin (arrow), myocardium showing vacuolar degeneration intracytoplasmic (asterisk).
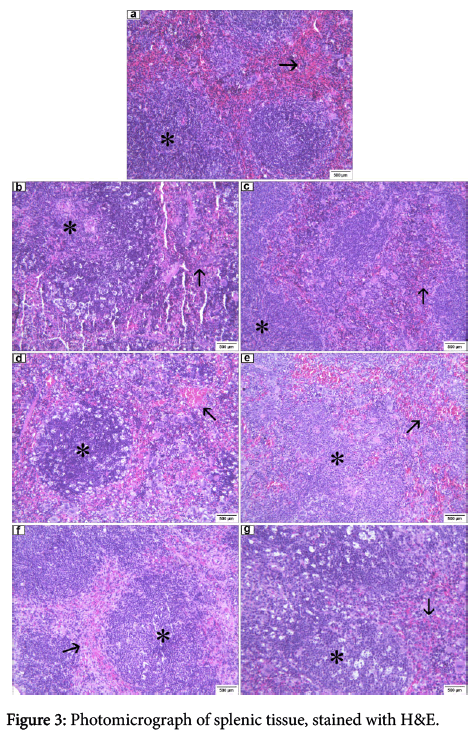
Splenic histopathology
In the control group inoculated with sterile saline (0.9% NaCl) spleens were normal in range according as described in the literature for mice, though the Malpighian follicles showed hyperplasia and capillaries hyperemic, the red pulp containing red blood cells and many megakaryocytes. Without any infectious process evidence (Figure 3), common features were seen in animals subjected to this type of inoculum. However, all the other groups inoculated with bacterial suspension, such as MRSA pvl (-), isolated from nasal cavity (Figure 3), MRSA pvl (+), isolated from peripheral blood in patients affected by severe lung infection (Figure 3) MRSA pvl (+), isolated from nasal cavity (Figure 3), MRSA pvl (+), isolated in epithelial tissue (skin) injury, MSSA pvl (-), isolated from nasal cavity and MRSA pvl (+), isolated from peripheral blood in patients affected by infectious endocarditis, presented the splenic tissue with the same characteristics: Malpighian hyperplastic follicles, containing numerous fragmented cells, basophilic granular materials (suggestive of bacterial colonies), the pulp red with red blood cells, and a great number of megakaryocytes, and hyperemic capillaries containing brown pigment (Figure 3).
(a) control group (100X): follicles Malpighian hyperplastic (asterisk), red polva with red blood cells and a great number of megakaryocytes (arrow). (b) MRSA pvl (-) group, isolated from nasal cavity (100X): hyperplastic Malpighian follicles with fragmented cells and basophilic granular substance (asterisk), red pulp with red blood cells and a great number of megakaryocytes (arrow), artifact presence. (c) MRSA pvl (+) group, isolated from peripheral blood in patients affected by severe pulmonary infection (100X): hyperplastic Malpighian follicles with fragmented cells and basophilic granular substance (asterisk), red pulp with red blood cells and a great number of megakaryocytes (arrow). (d) MRSA pvl (+) group, isolated from nasal cavity (100X): hyperplastic Malpighian follicles with fragmented cells and basophilic granular substance (asterisk), red pulp with red blood cells and a great number of megakaryocytes (arrow). (e) MRSA pvl (+) group, isolated from injury in epithelial tissue (100X): hyperplastic Malpighian follicles with fragmented cells and basophilic granular substance (asterisk), red pulp with red blood cells and a great number of megakaryocytes (arrow). (f) MSSA pvl (-) group, isolated from nasal cavity (400X): hyperplastic Malpighian follicles with fragmented cells and granular basophilic substance (asterisk), red pulp with red blood cells and a great number of megakaryocytes (arrow). (g) MRSA pvl (-) group, isolated from peripheral blood in patients affected by infectious endocarditis (100X): hyperplastic Malpighian follicles with fragmented cells and basophilic granular substance (asterisk), red pulp with red blood cells and a great number of megakaryocytes (arrow).
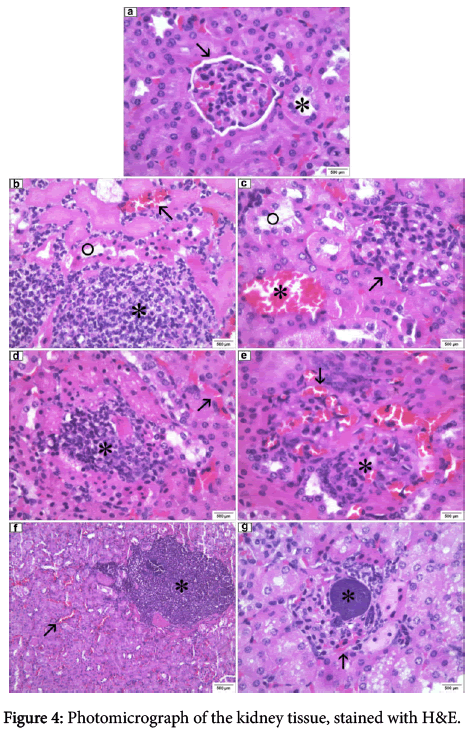
Renal histopathology
In the control group, inoculated with sterile saline (0.9% NaCl), both bore kidneys renal glomeruli and tubules with customary aspects, albeit, interstitial capillaries and hyperemic glomeruli (Figure 4). In MRSA pvl (-) group, isolated from the nasal cavity, both kidneys showed several circumscribed bacterial colony outbreaks in different sizes, some accompanied by neutrophilic inflammatory infiltrate and macrophages with interstitial capillaries and hyperemic glomeruli. Still, in the left kidney presence was observed of a coagulation necrosis area with surrounding tubular mononuclear and neutrophilic inflammatory cell infiltrate foci (Figure 4). On the other hand, MRSA pvl (+) group, isolated from peripheral blood in patients affected by severe pulmonary infection, both kidneys showed glomerular interstitial capillaries and hyperemic glomeruli with hemorrhagic foci in the cortical region. The glomeruli displayed hypercellularity and compression of Bowman space, and some tubules with lining epithelium necrosis (Figure 4). MRSA pvl (+) group, isolated from the nasal cavity showed bacterial colonies within the cortical region glomeruli, around neutrophilic inflammatory infiltration with macrophages, interstitial capillaries and glomerular hyperemia (Figure 4). In MRSA pvl (+) group, isolated from epithelial tissue (skin) injury in two kidneys, glomeruli showed hypercellularity with loss of Bowman's space, interstitial capillaries and hiperemic glomeruli and acute interstitial hyperemia. In the medullary region, near the renal pelvis presence was observed of inflammatory infiltrate with macrophages with no bacterial colonies (focal pyelitis) (Figure 4). In MRSA pvl (-) group, isolated from nasal cavity, the right kidney presented, several circumscribed outbreaks of bacterial colonies in different sizes in the cortical region, some accompanied by neutrophilic inflammatory infiltrate and macrophages, and interstitial capillaries and glomeruli with marked hyperemia. The renal pelvis also showed inflammatory infiltrate (pyelitis). Inside of the left kidney glomeruli presence was observed of bacterial colonies bearing neutrophilic infiltrate, extending around the entire structure, with serious and widespread hyperemia there was. Also had a triangular infarction area, with tubular coagulation necrosis in the cortical region adjacent the renal capsule (Figure 4). Conversely, MRSA pvl (-) group, isolated from peripheral blood in patients affected by infectious endocarditis in the right kidney, the pelvis showed macrophage infiltration and glomerular and interstitial hyperemia. Bacterial colonies were observed inside the left kidney glomerulus, with neutrophilic inflammatory infiltrate and hyperemia causing loss of Bowman's space (Figure 4). In the renal pelvis, an area with mononuclear cell infiltration, reaching the medullary region in ascending order (pyelonephritis), was also observed in the perivascular region (Figure 4).
(a) control group (400X): glomerulus with usual appearance (arrow), tubules with usual structure (asterisk). (b) MRSA pvl (-) group, isolated from nasal cavity (400X): hyperemic interstitial capillaries (arrow), basophilic granular substance foci (bacterial colonies) containing inflammatory neutrophilic infiltrate (asterisk), tubular coagulation necrosis (circle). (c) MRSA pvl (+) group, isolated from peripheral blood in patients affected by severe pulmonary infection (400X): glomerulus showing hypercellularity with compression of Bowman space (arrow), presenting tubular necrosis (circle), hyperemic interstitial capillaries (asterisk). (d) MRSA pvl (+) group, isolated from nasal cavity (400X): granular basophilic materials (bacterial colony) with neutrophiles infiltration (asterisk), hyperemic interstitial capilaries (arrow). (e) MRSA pvl (+) group, isolated from epithelial tissue (skin) injury (400X): glomeruli presenting hypercellularity with loss of Bowman space (asterisk), interstitial spaces and hyperemic glomeruli (arrow). (f) MSSA pvl (-) group, isolated from nasal cavity (100X): focus circumscribed basophilic granular substance (bacterial colonies) containing neutrophilic inflammatory infiltrate and macrophages (asterisk), hyperemic interstitial spaces (arrow). (g) MRSA pvl (-) group, isolated from peripheral blood in patients affected by infectious endocarditis (400X): hyperemia glomeruli (arrow), within the glomerulus basophilic granule presence foci containing neutrophilic inflammatory infiltrate and loss of Bowman space (asterisk).
Lung histopathology
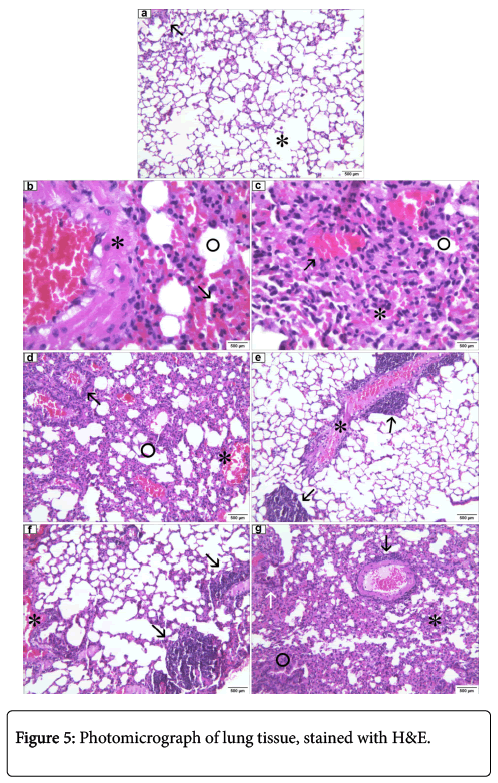
In the control group inoculated with sterile saline (0.9% NaCl), both the right and left lungs exhibited normal alveoli, mild hyperemia and lymphocyte outbreaks adjacent to bronchioles, normal aspect in mice (Figure 5).
Control group (100X): normal alveoli (asterisk), focus lymphocyte (arrow). (b) MRSA pvl (-) group, isolated from nasal cavity (100X): sharp hyperemia (arrow), alveoli with edema (circle), thick caliber vessel congested presenting necrotic tissue with hyaline aspect (asterisk). (c) MRSA pvl (+) group, isolated from peripheral blood in patients affected by severe pulmonary infection (100X): atelectasis (asterisk), edema (circle), hyperemia (arrow). (d) MRSA pvl (+) group, isolated from nasal cavity (100X): light of the bronchial structure featuring lymphocyte infiltrate and hyperemia (arrow), edema (circle), acute hyperemia (asterisk). (e) MRSA pvl (+) group, isolated from epithelial tissue (skin) injury (100X): thick caliber vessel congested, presenting necrotic tissue with hyaline aspect containing inflammatory infiltrate (asterisk), granular basophilic foci (bacterial colonies) (arrow). (f) MSSA pvl (-) group, isolated from nasal cavity (100X): bronchial structure containing inflammatory infiltrate and acute hyperemia (asterisk), granular basophilic foci (bacterial colonies), containing lymphocyte infiltrate (arrow). (g) MRSA pvl (-) group, isolated from peripheral blood in patients affected by infectious endocarditis (100X): atelectasis area (asterisk), emphysema (circle), thick caliber vessel congested, presenting necrotic tissue with hyaline appearance with perivascular inflammatory infiltrate (black arrow), peribronchial inflammatory infiltrates (white arrow).
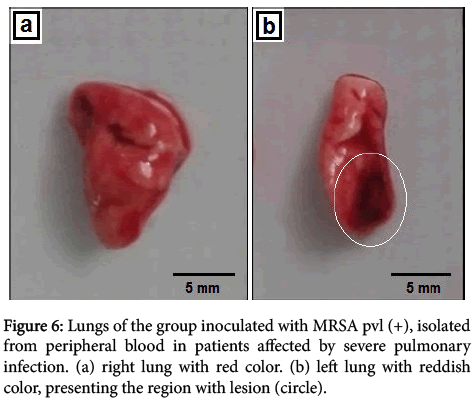
In MRSA pvl (-) group, isolated from nasal cavity, lung tissues showed several areas of atelectasis and some alveoli with edema, vessel bulk caliber congested showing necrotic tissue, diffuse and cute hyperemia through tissue and focus containing peribronchial lymphocytes (Figure 5). Yet, MRSA pvl (+) group, isolated from peripheral blood in patients affected by severe pulmonary infection in macroscopic noted the tissue with red color, the lower part of the left lung showing a blackish-colored lesion (Figure 6).
Figure 6: Lungs of the group inoculated with MRSA pvl (+), isolated from peripheral blood in patients affected by severe pulmonary infection. (a) right lung with red color. (b) left lung with reddish color, presenting the region with lesion (circle).
In left lung microscopy, we observe areas of atelectasis accompanied by lymphocytic infiltrate, mainly around the bronchi and bronchioles or adjacent pleura, hyperemia and some alveoli with edema (Figure 5). In MRSA pvl (+) group, isolated from nasal cavity, the left lung showed areas of emphysema near the periphery of lobes, with adjacent alveolar atelectasis and several areas of perivascular and peribronchial lymphocytic infiltrate; some alveoli also showed edema, severe and diffuse hyperemia. The lights of some bronchioles showed lymphocytic infiltration and hyperemia. In MRSA pvl (+) group, isolated from injury to epithelial tissue (skin), the left lung displayed emphysema and presented rich lymphocytic inflammatory infiltrate beyond peribronchiolar and perivascular macrophages and neutrophiles. The right lung displayed bacterial colony foci, thick-caliber vassel congested, presenting necrosis and inflammatory infiltrate (Figure 5). On the other hand, MRSA pvl (-) group, isolated from nasal cavity, in both lungs outbreaks of bacterial colonies were observed, surrounded by inflammatory infiltrate, and the presence of emphysema. The light bronchioles containing inflammatory infiltrates and severe hyperemia also bore inflammatory lymphocyte infiltrate and macrophages in the perivascular regions. In MRSA pvl (-) group, isolated from peripheral blood in patients affected by infective endocarditis, both the right lung and the left lung showed areas of atelectasis and others with emphysema, high-caliber vessel congested with necrosis in the vessel wall, accompanied by lymphocytic inflammatory infiltrate macrophages, and in the perivascular and peribronchial region. Only the right lung showed a lymphocytic focus peripleural fat.
Discussion
Decreased appetite with reduced water intake, followed by reduction in mobility are aspects similar to cachexia; in addition, isolation from other group components hours before death can be explained by the worsening of infection leading to compromising of organs and septicemia. Clinical signs of the disease are manifested in 2 to 3 hours, including decreased appetite, stooped posture, motion loss, diarrhea, dehydration and difficulty in breathing [3,12].
The only different point in the control group were found in the lungs, tissue hyperemia, which can be associated with the euthanasia method by anesthetic overdose through inhalation in closed campanula.
In cardiac tissue, conditions associated with increased severity were found in the groups: MRSA pvl (+), isolated from the nasal cavity, showing 80% mortality within 72 hours of infection; in MRSA pvl (+) group, isolated from skin lesions, reaching 80% mortality in 72 hours, in the presence of large numbers of bacterial colonies in intraventricular clot characterizing septicemia; and in MRSA pvl (-) group, isolated from peripheral blood in patients affected by infective endocarditis with mortality of 100% of animals within 72 hours. Importantly, even if a strain does not produce PVL, the fact of having been a patient in isolation affected by infective endocarditis may be associated with more severe infections in cardiac tissue resulting in the high number of deaths among animals.
In renal tissue, all groups showed bacterial colonies; however, group MRSA pvl (+) group, isolated from skin lesions, presented focal pyelitis, and MRSA pvl (-), isolated from peripheral blood in patients affected by infectious endocarditis presented ascending pyelonephritis. They are acute urinary tract infections, associated with bacterial colonization; the pyelonephritis focus comprises an interstitial suppurative inflammation in small abscesses which coalesce with the evolution and are usually located at poles. The ascending form reaches the pelvis of the kidney, the collector system, and almost all renal structures, including tubules and interstitium; however, glomeruli are the exception; if the infection progresses it can cause tubular necrosis, affecting the glomeruli [41]. The presence of this type of infection in kidney tissue may have been one of the important factors associated with mortality of 80% of the animals within 72 hours in MRSA pvl (+) group, isolated from skin lesions, and 100% in the group inoculated with bacterial strain MRSA pvl (-), isolated from peripheral blood in patients affected by infectious endocarditis within 72 hours. MRSA pvl (+) group, isolated from the nasal cavity presents foci with bacterial colonies intraglomerular featuring septicemia, which could be explained by animal 80% mortality within 72 hours.
In the splenic tissue, similarity found in infections and the presence of fragmented cells, characteristic of apoptosis, may be associated with low PVL production by colonizing bacterial strains found in this tissue, because of the low PVL production promoting apoptosis by binding to mitochondrial membrane, resulting in the release of oxygen in the reactive form [42,43].
For a possible histopathological analysis of the lungs, the animals can be infected with 2.0 × 108 CFU/ml of S. Aureus [3,44]. However, in our study severe inflammatory processes in the concentration 107 CFU/mL were identified. The groups where the inflammation displayed greater severity were as follows: MRSA pvl (+), isolated from peripheral blood in patients affected by serious lung infection, causing 100% mortality after 72 hours of observation, MRSA pvl (+), isolated in epithelial tissue (skin) injury with 80% animal mortality after 72 hours, and MRSA pvl (-) group, isolated from peripheral blood in patients affected by infectious endocarditis, achieving 100% animal mortality after 72 hours of observation. All strains responsible for causing greater damage to the lung tissue were derived from infections, including those isolated from lung infections, showing that as the more harmed tissue.
A study conducted by Wandenburg et al. (2007) exhibited, cell infiltration of the immune system and destruction of alveolar architecture at lung histopathology, after a 24-hour challenge using a bacterial concentration of 2.0 × 108 CFU/ml. However, after 72 hours, the infection is cleared, showing the lungs only displaying an residual inflamation of the alveolar walls [3,44]. Similar inflamatory aspects were observed in our study, but after 72 hours we did not have infection resolution, presenting the acute form.
Conclusion
The results showed that the presence of methicillin resistance and Panton-Valentine Leucocicdin production were not decisive factors in increased severity of organ tissue infections in a murine model, because, in kidney MRSA pvl (-) strains were responsible for septicemia signs, and lungs MRSA pvl (-) strains were responsible for serious infections. However, the strains isolated from human infections had a higher inflammatory response in lung tissue in the murine model, when compared to strains isolated from nasal cavity in healthy people.
More than one factor may be related to mortality, because, in most groups, infections extended from severe forms containing bacterial colonies in multiple organs.
Acknowledgement
We would like to thank Research Support Foundation of the State of Rio de Janeiro (FAPERJ), Program to supporting research - UFF, Pathology Program (Fluminense Federal University) and Coordination for the Improvement of Higher Level Personnel (CAPES) for the financial support to this study.
Compliance with Ethical Standards
The study was approved by the regional ethical review board at Federal Fluminense University (number 439/2013).
References
- Fournier B, Philpott DJ (2005) Recognition of Staphylococcus aureus by the innate immune system. ClinMicrobiol Rev 18: 521-540.
- Sharma-Kuinkel BK, Zhang Y, Yan Q, Ahn SH, Fowler VG (2013) Host gene expression profiling and in vivo cytokine studies to characterize the role of Linezolid and Vancomycin in Methicillin-resistant Staphylococcus aureus (MRSA) murine sepsis model. PLoS ONE 8: e60463.
- Kim HK, Missiakas D, Schneewind O (2014) Mouse models for infectious diseases caused by Staphylococcus aureus. J Immunol Methods 410: 88-99.
- von Eiff C, Becker K, Machka K, Stammer H, Peters G (2001) Nasal carriage as a source of Staphylococcus aureusbacteremia. Study Group. N Engl J Med 344: 11-16.
- Chambers HF, Deleo FR (2009) Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7: 629-641.
- Amalaradjou MA, Venkitanarayanan K (2014) Antibiofilm Effect of Octenidine Hydrochloride on Staphylococcus aureus, MRSA and VRSA. Pathogens 3: 404-416.
- Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, et al. (2008) Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001-2004. J Infect Dis 197: 1226-1234.
- Miller LG, Quan C, Shay A, Mostafaie K, Bharadwa K, et al. (2007) A prospective investigation of outcomes after hospital discharge for endemic, community-acquired methicillin-resistant and susceptible Staphylococcus aureus skin infection. Clin Infect Dis 44: 483-492.
- Kallen AJ, Mu Y, Bulens S, Reingold A, Petit S, et al. (2010) Health care-associated invasive MRSA infections, 2005-2008. JAMA 304: 641-648.
- Olsen RJ, Kobayashi SD, Ayeras AA, Ashraf M, Graves SF, et al. (2007) Lack of a major role of Staphylococcus aureus Panton-Valentine leukocidin in lower respiratory tract infection in nonhuman primates. Am J Pathol 176: 1346-1354.
- Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339: 520-532.
- Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, et al. (2009) Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J 23: 3393-3404.
- Lessa FC, Mu Y, Davies J, Murray M, Lillie M, et al. (2010) Comparison of incidence of bloodstream infection with methicillin-resistant Staphylococcus aureus between England and United States, 2006-2007. Clin Infect Dis 51: 925-928.
- Karauzum H, Adhikari RP, Sarwar J, Devi VS, Abaandou L, et al. (2003) Structurally designed attenuated subunit vaccines for S. aureusLukS-PV and LukF-PV confer protection in a mouse bacteremia model. PLoS ONE 8: e65384.
- Nizet V (2007) Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. J Allergy ClinImmunol120: 13-22.
- Tristan A, Ferry T, Durand G, Dauwalder O, Bes M, et al. (2007) Virulence determinants in community and hospital meticillin-resistant Staphylococcus aureus. J Hosp Infect 65: 105-109.
- Zimbelman J, Palmer A, Todd J (1999) Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J 18: 1096-1100.
- Crémieux AC, Dumitrescu O, Lina G, Vallee C, Côté JF, et al. (2009) Panton-valentine leukocidin enhances the severity of community-associated methicillin-resistant Staphylococcus aureus rabbit osteomyelitis. PLoS One 4: e7204.
- Friedrich R, Panizzi P, Fuentes-Prior P, Richter K, Verhamme I, et al. (2003) Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature 425: 535-539.
- Cheng AG, DeDent AC, Schneewind O, Missiakas D (2011) A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol 19: 225-232.
- Siboo IR, Cheung AL, Bayer AS, Sullam PM (2001) Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect Immun 69: 3120-3127.
- Spaan AN, Henry T, van Rooijen WJ, Perret M, Badiou C, et al. (2013) The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell Host Microbe 13: 584-594.
- Kaneko J, Kamio Y (2004) Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes.BiosciBiotechnolBiochem 68: 981-1003.
- Panton PN, Valentine FCO (1932) Staphylococcal toxin. Lancet 222: 506-508.
- Chambers HF (2005) Community-associated MRSA--resistance and virulence converge. N Engl J Med 352: 1485-1487.
- Pédelacq JD, Prévost G, Monteil H, Mourey L, Samama JP (2000) Crystal structure of the F component of the Panton-Valentine leucocidin. Int J Med Microbiol 290: 395-401.
- Miles G, Movileanu L, Bayley H (2002) Subunit composition of a bicomponent toxin: staphylococcal leukocidin forms an octamerictransmembrane pore. Protein Science 11: 894-902.
- Narita S, Kanekoa J, Chibaa JI, Âmontb YP, Jarraudc S, et al. (2001) Phage conversion of Panton-Valentine leukocidin in Staphylococcus aureus: molecular analysis of a PVL-converting phage, phiSLT. Gene 268: 195-206.
- Diep BA, Gill SR, Chang RF, HaiVan T, Chen JH, et al. (2006) Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. Lancet 367: 731-739.
- Diep BA, Otto M (2008) The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol 16: 361-369.
- Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, et al. (2002) Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359: 753-759.
- Evangelista SS, Oliveira AC (2015) Community-acquired methicillin-resistant Staphylococcus aureus: a global problem. Rev Bras Enferm 68: 128-135, 136-43.
- Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, et al. (2002) Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 34: 1481-1490.
- Arbeit RD, Maki D, Tally FP, Campanaro E, Eisenstein BI; Daptomycin 98-01 and 99-01 Investigators (2004) The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis 38: 1673-1681.
- Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, et al. (2011) Clinical practice guidelines by the infectious diseases society of america for the treatment of Methicillin-Resistant Staphylococcus aureus infections in adults and children. Clinical Infectious Diseases 52: 285-292.
- Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, et al. (2003) Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med 348: 1342-1347.
- Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, et al. (2003) Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 302: 1569-1571.
- Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, et al. (2005) Methicillin-Resistant Staphylococcus aureus disease in three communities. The New England Journal of Medicine 352: 1436-1444.
- Kiedrowski MR, Kavanaugh JS, Malone CL, Mootz JM, Voyich JM, et al. (2011) Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS ONE 6: e26714.
- Lima JBA, Skare TL, Malafaia O, Ribas-Filho JM, Michaelis T, et al. (2011) Sepsis inducing syndrome of multiple organ dysfunction: an experimental study in rats. ABCD Arq Bras Cir Dig 24: 95-102.
- Kumar V, Abbas AK, Fausto N (2004) Robbins &Cotran: Pathologic basis of disease. 7ª Ed. Rio de Janeiro: Elsevier p: 1040-1046.
- Genestier AL, Michallet MC, Prévost G, Bellot G, Chalabreysse L, et al. (2005) Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. JClin Invest 115: 3117-3127.
- Boyle-Vavra S, Daum RS (2007) Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Laboratory Investigation 87: 3-9.
- BubeckWardenburg J, Patel RJ, Schneewind O (2007) Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun 75: 1040-1044.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14738
- [From(publication date):
June-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 13641
- PDF downloads : 1097