Method to Produce Curcumin Oil-in-Water Nanoemulsions as Templates for Drug Carriers
Received: 14-Sep-2016 / Accepted Date: 05-Oct-2016 / Published Date: 12-Oct-2016 DOI: 10.4172/2155-952X.1000247
Abstract
Curcumin has a wide spectrum of biological and pharmacological activities, which could be better exploited in numerous medical applications if it were not for its poor water-solubility. In this study, we describe a new method to improve curcumin solubility, using a combination of paraffin oil and ethanol. Further, we describe a systematic and efficient procedure to develop a formulation and a scalable mixing method in order to produce curcumin loaded oilin- water nanoemulsions that can be used as nanocapsule templates with potential biomedical applications.
Keywords: Curcumin; Solubility; Nanoemulsion; Formulation; Mixing
249591Introduction
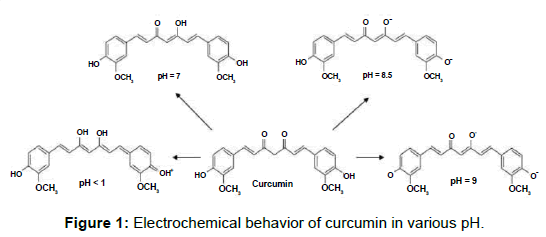
Turmeric is a spice obtained from the rhizomes of Curcuma longa [1]. Beside its culinary applications, it is used for health care, food preservation and as a yellow dye in textiles. Curcumin (1,7-bis- (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is a yellow colored pigment which makes up 2 to 5% of the spice [2]. Several studies have demonstrated that curcumin has a wide spectrum of biological and pharmacological properties such as antioxidant, antibacterial, anti-inflammatory [3] and anticarcinogenic activities [3,4]. It has also been claimed to be useful to prevent platelet aggregation, diabetes, rheumatism and Alzheimer´s disease [5]. However, on the one hand, curcumin is poorly soluble in water at neutral or acidic pH due to the hydrophobicity of the conjugated alkene chain and the absence of a strong polar group. On the other hand, at alkaline pH curcumin forms phenolate ions and gets degraded into compounds such as ferulic acid and vanillin, among others [6] (Figure 1). Poor water solubility, low absorption [7] and rapid metabolic elimination of curcumin are responsible for its low bioavailability and limit its medical applications. The low solubility of curcumin represents the major challenge to achieve suitable levels in plasma in order to produce its pharmacological effects [8].
The most common strategy used to overcome these problems is the encapsulation of curcumin into carriers such as nanoparticles. Several nano-encapsulating methods, designed to protect and improve the delivery efficiency of curcumin, have been used [9]. Curcumin has been formulated into polymeric nanoparticles that allows for an easy dispersion of the curcumin in aqueous media, self-emulsifying systems in a lipid-based dosage form that control the release of the compound or microemulsions for topic delivery of curcumin [10-12]. Vecchione et al. developed a nano emulsion of soy-bean oil using lecithin as surfactant; they coated it with functionalized chitosan capable of controlling the interaction with the intestinal barrier by easy deposition of functionalized biopolymers. In addition, they obtained the largest degree of bioavailability with 110 nm nano-emulsion coated with the highest degree of functionalized chitosan [13].
In this study, we describe a step by step procedure to improve curcumin dissolution and to manufacture a suitable carrier that can used as a template for curcumin loaded nanocapsules; emphasis is given to the practical applicability of the method herein described. We begin by improving curcumin dissolution in an oil matrix that is used to prepare oil-in-water nanoemulsions. The base nanoemulsion formulation is carefully selected using a systematic approach that aims to produce low interfacial tension systems using synergistic mixtures of nonionic surfactants that exclude the use of hazardous solvents [14]. The HLB value is used as the formulation parameter, and varying HLB is equivalent to changing the extent of surfactant molecules interaction with the aqueous and the oil phases at the interface. When the surfactants adsorb at the interface at the best possible packing, the interfacial tension tends to a minimum, thus facilitating the process of drop breakage during mixing [15-17].
The use of surfactant mixtures is a common practice in the industry and it has been extensively studied by many researches [18-20]. Combining two or more surfactants in a formulation may be beneficial, not only for the reduction of interfacial tension already mentioned (leading to smaller droplet sizes for the same energy expenditure) but for the decrease of the overall surfactant concentration required to produce stable emulsions, as opposed to single surfactant systems.
Using the aforementioned approach, a nanometric droplet size minimum is attained and the corresponding formulation is used to develop a mixing procedure using a high-shear, thin film-spinning device. The thin film-spinning (TFS) apparatus has impellers that spin a film of the fluid to the walls of a cylindrical vessel at very high speed, and under cavitation-free conditions. The resulting flow field induces a very high shear and droplets of the dispersed phase are fast and efficiently reduced in size. These apparatus can handle high throughputs; the small model used in this work may produce up to 40 kg/h using a 15 mL vessel and a 25 cm × 30 cm footprint.
This method describe a safe and scalable mixing procedure to generate a curcumin loaded stable nanoemulsion, in terms of droplet size, up to 10 months at room temperature. The nanoemulsions formation was evaluated using a semi batch and a continuous process; the best results were obtained using the semi batch method, although the continuous methods showed promising results. The produced model emulsion nanocarrier, stabilized by surfactants and co-surfactants, in which the paraffin oil drops contained dissolved curcumin, was characterized, showing good storage stability.
Materials and Method
Materials
The sample of curcumin used in this study was supplied by Sigma Aldrich (MKE, USA). Ethanol, methanol, acetic acid, analytical grade sodium dodecyl sulfate (anhydrous), paraffin oil, Tween 80 and Span 80 were purchased from Merck (MA, USA). Water was distilled using a Thermo Scientific Barnstead stationary still. It is worth noting that all the components are pharmaceutically acceptable for oral administration and fall under GRAS (Generally Regarded as Safe) category.
Methods and equipment
Formulation of emulsions: This study began with the evaluation of the formulation parameters required to produce stable oil-in-water emulsions to be used as carriers of curcumin in the oil droplets. In this sense, two non-ionic surfactant mixed in varying concentrations and proportions to yield different hydrophilic-lipophilic balances (HLB) were evaluated [21]. The surfactants evaluated were Tween 80 (T80) and Span 80 (S80). The first is a polyoxyethylene sorbitan monooleate hydrophilic emulsifier; the second is a lipofilic emulsifier consisting of sorbitan monooleate. As mentioned above, these two surfactants were mixed using different T80/S80 w/w ratios in order to obtain variable HLB values.
The total surfactant mixture concentration was also evaluated; the oil ratio in the emulsion varied in a relatively narrow range (12%-18% w/w) while the water content was constant (80% w/w). Table 1 shows the formulation parameters involved in this study. The screening procedure was as follows. First, the surfactants were homogenously mixed in the aqueous phase. Then, the organic phase was added into the aqueous solution and the mixture was stirred by hand to produce an emulsion; the resulting droplet size was measured and the rate of phase separation was monitored during one week after emulsification. This procedure led to the formation of oil-in-water emulsions, stable enough to be characterized. The formulation that allowed for the smallest droplet size and the most stable emulsion (least phase separation) was selected for the remaining tests.
Curcumin dissolution profile: Since curcumin has a poor solubility in the oil phase, ethanol was used to achieve the total dissolution of the target amount, 5 mg/mL (see section 3.2), in the oil phase. The dissolution profile of curcumin was carried out as follows. First, powdered curcumin was added into 15 mL centrifuge tubes containing the mixture of surfactants and liquid paraffin, selected as described in the previous section. Various amounts of ethanol calculated based on surfactant plus alcohol mass (0% ethanol-100% surfactants; 0.5% ethanol-99.5% surfactants, 1% ethanol-99% surfactants, 4% ethanol-96% surfactants and 6% ethanol–94% surfactants w/w) were also added to the mix in order to facilitate curcumin dissolution. Then, the tubes were heated at 50°C for 30 min and stirred overnight in a shaker at room temperature.
While the degree of curcumin dissolution could be easily determined by visual inspection of the tubes, it was decided that obtaining the curcumin partitioning between the oil and the water phases in the emulsion, was more informative. To this end, the content of 3 mL centrifuge tubes was mixed with water and stirred using a magnetic stirrer at 400 rpm, during 1 min, to produce an oil-in-water emulsion containing 64% w/w of water. Immediately, the emulsions were centrifuged at 10,000 g for 5 min, to induce phase separation. The curcumin content of both the oil separated phase and the precipitated solid were measured, the first by HPLC, the second by visible UV, as described in section 2.2.4. By this means, the undissolved curcumin could be determined as well as the curcumin that could have transferred to the water phase, by computing the difference between the total curcumin added to the system and the amount that ended in the oil phase and the solid precipitate.
| Group | Paraffin content (%w/w) | Water content (%w/w) | Surfactant content (%w/w) | Span 80(%w/w) | Tween 80(%w/w) | Particle size (µm) | HLB values |
|---|---|---|---|---|---|---|---|
| 1 | 16 | 80 | 4 | 2.24 | 1.76 | 78.9 | 9 |
| 16 | 80 | 1.84 | 2.16 | 108.3 | 10 | ||
| 16 | 80 | 1.48 | 2.52 | 53.6 | 11 | ||
| 16 | 80 | 1.12 | 2.88 | 58.6 | 12 | ||
| 16 | 80 | 0.76 | 3.24 | 75.5 | 13 | ||
| 2 | 14 | 80 | 6 | 3.36 | 2.64 | 125.5 | 9 |
| 14 | 80 | 2.76 | 3.24 | 85.8 | 10 | ||
| 14 | 80 | 2.22 | 3.78 | 34.2 | 11 | ||
| 14 | 80 | 1.68 | 4.32 | 56.2 | 12 | ||
| 14 | 80 | 1.14 | 4.86 | 67.9 | 13 | ||
| 3 | 12 | 80 | 8 | 4.48 | 3.52 | 127.9 | 9 |
| 12 | 80 | 3.68 | 4.32 | 17.9 | 10 | ||
| 12 | 80 | 2.96 | 5.04 | 16.6 | 11 | ||
| 12 | 80 | 2.24 | 5.76 | 51.6 | 12 | ||
| 12 | 80 | 1.52 | 6.48 | 77.9 | 13 |
Table 1: Formulation parameters used in formulation selection.
Emulsification procedure
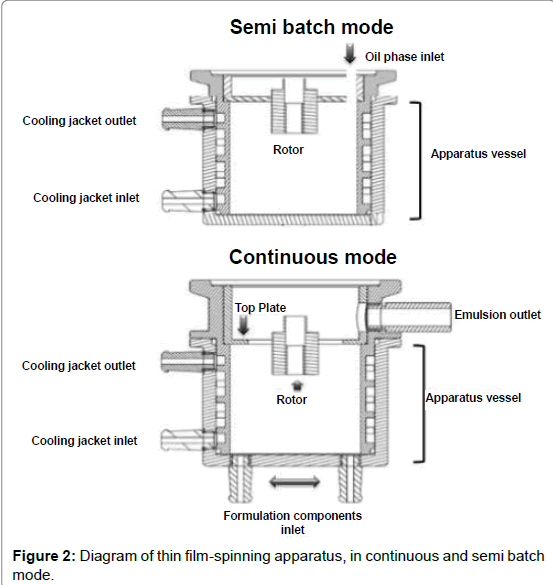
In order to prepare a carrier emulsion with a nano size distribution, a highly efficient thin film-spinning apparatus (TFS), manufactured by PRIMIX, model FILMIX 40-40, was used for the semi batch and continuous mixing protocols (Figure 2). Shows simplified diagrams of the TFS mixing vessel internals, either in continuous or semi batch mode. It essentially consists of a cylindrical vessel with a concentric impeller that rotates at very high speeds, up to 40 m/s at the impeller radius or peripheral speed. The fluid contained inside the vessel is centrifuged towards the vessel’s wall; the intense force field produces the fracture of one of the phases into the other, in this case, the oil phase, as determined by the formulation and composition of the system. At the top of the vessel, a fixed plate with a cutout aperture at the center contains the film. The film thickness depends on the size of the plate’s aperture: the larger the aperture, the thinner the film.
Table 2 shows some of apparatus settings and process parameters for either the semi batch or the continuous mixing modes: 1) surfactant concentration; 2) residence time during mixing; 3) peripheral speed and 4) top plate aperture diameter. In the semi batch mode, the continuous phase (water) was initially placed in the apparatus vessel and the dispersed phase (oil), containing the surfactants, was gradually added to the mixing vessel through the upper entry port. In the continuous mode, the aqueous phase and the oil phase were injected simultaneously into the apparatus vessel through the bottom entry ports. A peristaltic pump was used to control de flow rate of each phase: the higher the flow rate, the shorter the residence time.
Emulsions characterization
The droplet size of the initial formulations (see section 2.2.1) was measured by laser light scattering using a Mastersizer 2000Hydro 2000MU manufactured by Malvern instruments, UK. Measurements of droplet size distribution and polydispersity index of the emulsions containing curcumin (carrier emulsion) were carried out using a Microtrac Nanotrac TM 150 instrument that uses dynamic light scattering (DLS) to assess particle size. The latter has a 780 nm, 3 mW laser diode and a detector fixed in place. We report the volumetric mean, or D (4,3), as the representative parameter of the distribution. A Hitachi S-2360 N instrument was used to obtain images of the nanoemulsions by means of scan electron microscopy (SEM). The room temperature, long-term stability of the emulsions was evaluated by measuring the variation of droplet size as a function of storage time.
| Semi batch mode | ||||
|---|---|---|---|---|
| Top Plate | Speed (m/s) | Residence Time (s) | Vessel Volume | Surfactant (%w/w) |
| ---- | 10, 20, 30 | 3 | 15 mL | 8 |
| Continuous mode | ||||
| Top Plate | Speed (m/s) | Residence Time (s) | Q (ml/mim) | Surfactant (%w/w) |
| 5 mm | 20, 30 | 1 | 720 | 4, 6, 8 |
| 2 | 360 | 4, 6, 8 | ||
| 3 | 240 | 4, 6, 8 | ||
| 4 | 180 | 4, 6, 8 | ||
| 7 mm | 20, 30 | 1 | 720 | 4, 6, 8 |
| 2 | 360 | 4, 6, 8 | ||
| 3 | 240 | 4, 6, 8 | ||
| 4 | 180 | 4, 6, 8 | ||
Table 2: TFS settings evaluated using the semi batch and continuous protocol mode.
High Performance Liquid Chromatography (HPLC) analyses of the separated oil phase after emulsion centrifugation (see section 2.2.2) were carried out in an Agilent 1100 HPLC system equipped with a G1312A pump, a G1322A degasser and an injector. All operations were controlled by means of the ChemStation software (A.10.02 (1757)) and run on a Windows 2000 operating system. After centrifugation, 1.0 mL aliquots of supernatant were withdrawn and diluted to 50 mL with methanol, and then injected into the HPLC apparatus. Curcumin was separated from the oil phase on a Zorbax C18 column (4.6 mm × 75 mm, 3.5 μm) with a solvent mixture consisting of methanol/water (7:3) in an isocratic system that lasted 5 minutes. The flow rate was 1 mL/ min and the injection volume was 10 μl. The content of the undissolved curcumin, or solid precipitate after emulsion centrifugation (see section 2.2.2), was determined as follows. First, the solid was recovered and dissolved in 10 mL of ethanol. Then, the curcumin content was determined using an Agilent Cary 60 UV-Visible spectrophotometer, with 1 cm optical path and a wavelength of 423 nm.
Results and Discussion
Selection of the physicochemical parameters of emulsions formulation
In order to obtain a stable, nanosized carrier emulsion, an evaluation of a number of formulation parameters such as the HLB and surfactant concentration was performed (Table 1). Exploring these formulation variables allowed identifying the most convenient set of parameters in terms of emulsion stability and droplet size. An important feature of the method used is that two different, yet compatible surfactants are used to vary the HLB in a wide range. This is a semi-empirical approach that is based on well-known principles of formulation of surfactantoil- water systems and considerably reduces experimentation time; data interpretation is also facilitated.
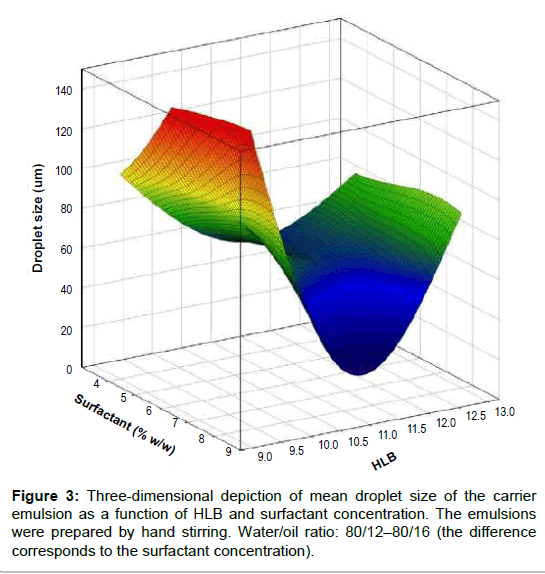
A three-dimensional depiction of the emulsions mean droplet diameter (volumetric mean), as a function of HLB and surfactant concentration, is displayed in (Figure 3). These data show a non linear dependence of droplet size with the latter parameters. The most hydrophobic surfactant mixture (HLB 9) and the most hydrophilic (HLB 13) produce the largest droplet sizes, regardless of the surfactant concentration. For the intermediate HLB values (10 to 12), the mean droplet size tends to decrease with surfactant concentration as expected; the smallest diameter (16.6 μm) is obtained for a HLB 11 and 8% of the surfactant mixture concentration. Presumably, this combination of physicochemical parameters is close to the so-called “optimum formulation”, in which the interfacial tension reaches a minimum thus facilitating droplet break-up. This formulation, HLB 11 and 8% surfactant mixture concentration, was selected to prepare the emulsions used to study the curcumin dissolution profile and to prepare the curcumin carrier nanoemulsion (section 3.3). At this stage of the study, a relatively low energy emulsification method was used (stirring by hand) to prepare the emulsions and to assess the effect of the formulation parameters; oil-in-water emulsions showing different mean droplet sizes and distributions were obtained. These variations are mainly the result of the physicochemical interactions between surfactant, oil and aqueous phase [22].
Curcumin dissolution profile
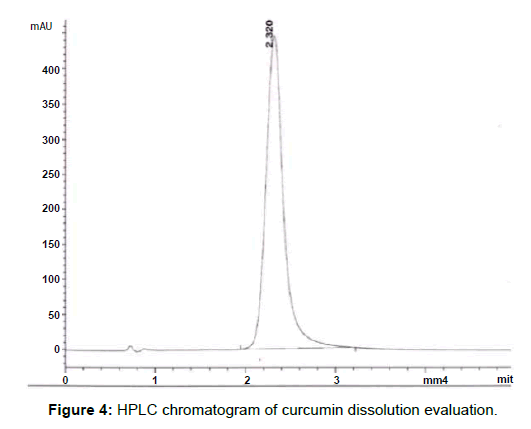
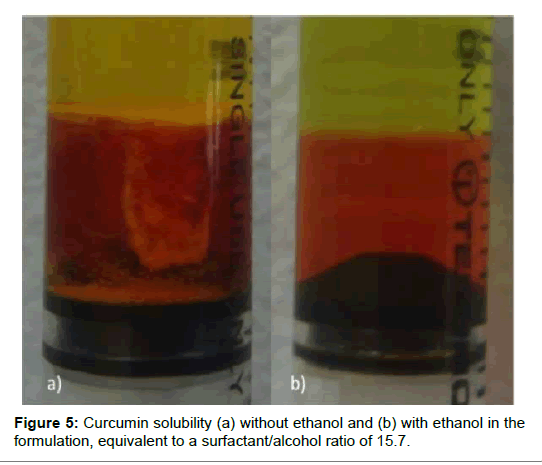
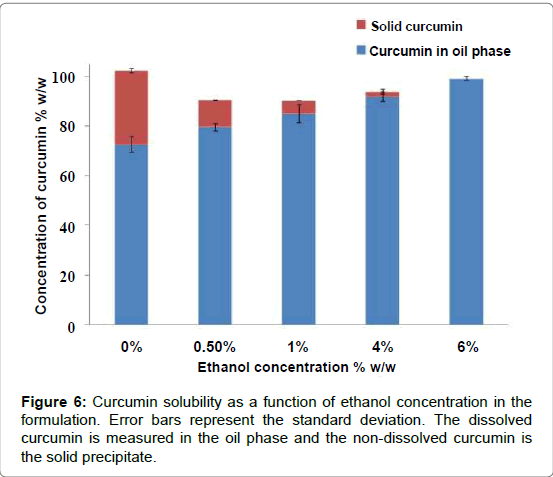
Several studies, dealing with curcumin absorption, plasma stability, bioavailability or pharmacokinetics, report dissolved curcumin in the formulations from 0.18 mg/mL to 18 mg/mL [23-26]; we decided to evaluate a constant curcumin concentration of 5 mg/mL (or target concentration) that is about in the middle range of the previous studies [27-29]. The curcumin content of both the oil separated phase and the precipitated solid were measured to obtain the curcumin partitioning between the oil and the water phases in the formulation. The concentration of curcumin was measured in the oil phase by HPLC and in the solid precipitate by visible UV; the extent of dissolution was computed from the known total amount of curcumin in the system. HPLC was used to measure the curcumin concentration in the emulsions at 423 nm since, in organic solvents, the low energy π–π* excitations of the chromophore group in the curcumin molecule, absorbs at this wavelength [6]. Further, the other components of the formulation do not absorb at 423 nm, which prevent unwanted interferences and errors in the quantification of curcumin. The chromatogram shown in presents a good reproducibility with a low tailing factor (Figure 4). The complete solubility of the active molecule in the oil phase ensures that the nanoemulsion can maintain the drug in solubilized form. If it were otherwise, there could be a risk of precipitation and loss of active component. It was verified that the target amount of curcumin could not be completely dissolved in the oil phase of the selected formulation and the excess material would settle at the bottom of the tube, as shown in (Figure 5a); according to the HPLC assessment, the curcumin dissolution rate was 72%. In order to overcome this problem, ethanol was added to the oil phase in increasing concentrations until complete curcumin dissolution was obtained, without changing the proportion of other components of the formulation, but the surfactant concentration; with the addition of 6% of ethanol, equivalent to a surfactant/alcohol ratio of 15.7 there was no visible solid curcumin, as shown in (Figure 5b). As mentioned before, the concentration of curcumin in the oil phase was measured by HLPC, while visible UV was used to determine the solid curcumin amount (Figure 6). Depicts the analysis results, computed as concentrations of solid curcumin and curcumin dissolved in the oil phase, relative to the total curcumin content in the emulsion, as a function of ethanol content. It can be observed that the fraction of solid curcumin decreases as alcohol content increases, while the curcumin concentration in the oil phase augments. It is worth noting that the amount of curcumin in the aqueous phase (which is the remaining percentage to complete 100%) also diminishes significantly and, at 6% of ethanol, very little curcumin remains in both the aqueous phase and the solid portion. This result is very significant since it means that the curcumin encapsulated in the oil droplets, or encapsulation efficiency, is very high (99%, within the experimental error), which validates the concept of using these nanoemulsions as curcumin carriers.
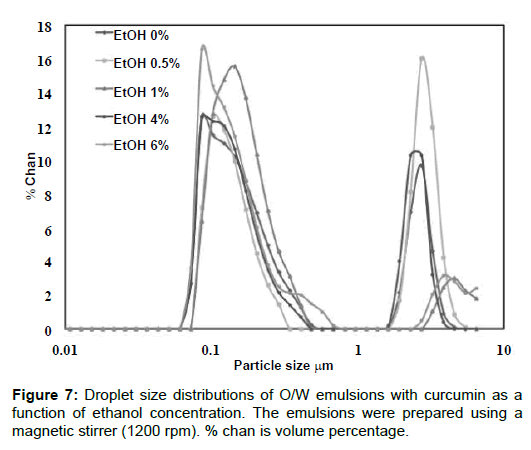
Co-surfactants are preferably short and medium-chain alcohols such as octanol, pentanol and hexanol, etc. and are often included in the formulation to reduce the interfacial tension and free energy to a minimum. For effective performance, it is important that the cosurfactant absorbs at the oil-water interfacial layer, allowing flexibility to take up different curvatures required to form nano drops [30,31]. Ethanol is usually described as a co-surfactant and can potentially alter the physicochemical balance in surfactant-oil-water systems and thus affecting droplet size (Figure 7). Shows the droplet size distribution of the emulsions as a function of ethanol content. The distributions are very similar despite the ethanol content; the distributions are bimodal and most of the droplet population lies in the range of 70 to 700 nm (Table 3). Shows some statistical parameters related to these distributions. There are large proportions of sub-micrometer sized droplet and most droplets are smaller than 10 μm, despite the fact a magnetic stirrer, i.e., a low energy emulsification set-up was used to prepare the emulsions [32].
Effect of mixing protocol in droplet size distribution
In this section, the results in terms of the droplet size distribution of the semi-batch and the continuous mixing protocols are shown. Table 2 displays the parameters that were evaluated: 1) a composition variable, surfactant concentration (only for the continuous protocol); 2) a time related variable, residence time or flow rate; 3) a mixing energy related parameter, peripheral speed, and 4) a geometric parameter, top plate aperture size (only for the continuous protocol).
Figure 8 depicts the droplet size distribution of emulsions produced using the TFS apparatus in a semi batch mode, as a function of peripheral speed. As expected, the droplet size distributions moved to lower sizes as peripheral speed was increased and bimodal distributions were obtained [33-36]. This last feature, bimodality, is commonly encountered in this type of mixing systems; in fact, droplet size distributions may shift to unimodality or bimodality depending on mixing and physicochemical conditions [37-40] . In the present case, it is seen that the modes of the most populated peaks correspond to the smaller droplets (all in the sub-micrometric range) and the least populated peaks tend to disappear as mixing speed increases. For the higher mixing speed (30 m/s), almost the entire population has affecting droplet size (Figure 7). Shows the droplet size distribution of the emulsions as a function of ethanol content. The distributions are very similar despite the ethanol content; the distributions are bimodal and most of the droplet population lies in the range of 70 to 700 nm (Table 3). Shows some statistical parameters related to these distributions. There are large proportions of sub-micrometer sized droplet and most droplets are smaller than 10 μm, despite the fact a magnetic stirrer, i.e., a low energy emulsification set-up was used to prepare the emulsions [32].
| Ethanol concentration %w/w | |||||
|---|---|---|---|---|---|
| Particle size information | 0 | 0.5 | 1 | 4 | 6 |
| Mean particle size peak 1, nm | 122 | 117 | 138 | 115 | 115 |
| Mean particle size peak 2, nm | 2440 | 2640 | 4380 | 2300 | 3780 |
| % of the volume of peak 1 | 75.6 | 58.0 | 90.1 | 71.7 | 87.2 |
Table 3: Droplet size and peakedness of curcumin loaded emulsions as a function of ethanol concentration using a magnetic stirrer (1200 rpm).
Effect of mixing protocol in droplet size distribution
In this section, the results in terms of the droplet size distribution of the semi-batch and the continuous mixing protocols are shown. Table 2 displays the parameters that were evaluated: 1) a composition variable, surfactant concentration (only for the continuous protocol); 2) a time related variable, residence time or flow rate; 3) a mixing energy related parameter, peripheral speed, and 4) a geometric parameter, top plate aperture size (only for the continuous protocol).
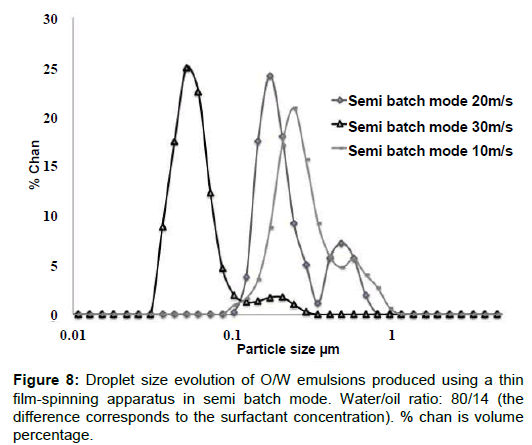
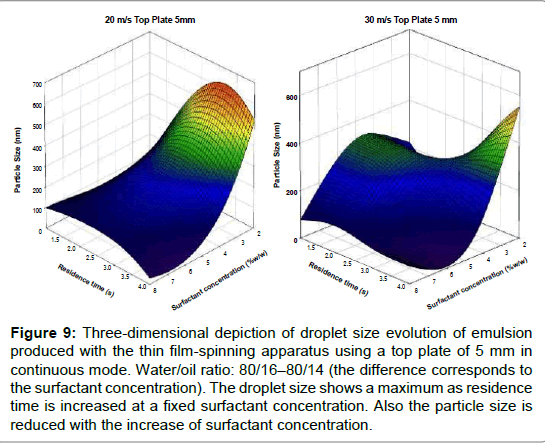
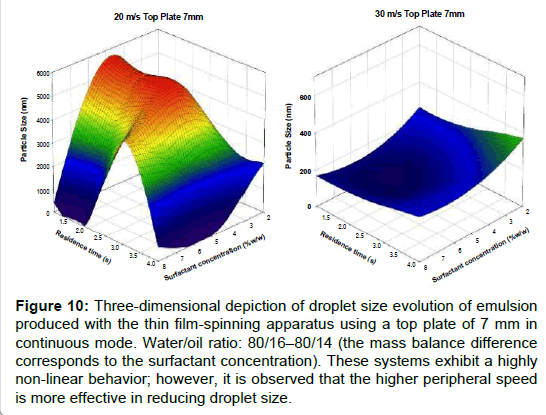
Figure 8 depicts the droplet size distribution of emulsions produced using the TFS apparatus in a semi batch mode, as a function of peripheral speed. As expected, the droplet size distributions moved to lower sizes as peripheral speed was increased and bimodal distributions were obtained [33-36]. This last feature, bimodality, is commonly encountered in this type of mixing systems; in fact, droplet size distributions may shift to unimodality or bimodality depending on mixing and physicochemical conditions [37-40] . In the present case, it is seen that the modes of the most populated peaks correspond to the smaller droplets (all in the sub-micrometric range) and the least populated peaks tend to disappear as mixing speed increases. For the higher mixing speed (30 m/s), almost the entire population has droplets below 100 nm in size. While for the semi-continuous protocol the surfactant concentration was kept constant, it was varied in the continuous protocol. Figures 9 and 10 show 3D maps of drop size as a function of surfactant concentration and residence time, for a top plate aperture of 5 mm (Figure 9) and a 7 mm top plate aperture (Figure10); two peripheral speeds (20 m/s and 30 m/s) were explored for each top plate.
At first glance, it is obvious that these systems exhibit a highly non-linear behavior, rather difficult to interpret. However, it is known that the production of emulsions is a multi-variable problem in which physicochemical and hydrodynamics variables intervene, often in conflicting ways [41-43]. For instance, droplet size shows a maximum as residence time is increased at a fixed surfactant concentration, as shown in Figures 9a and 9b, that correspond to a top plate aperture of 5 mm. In an attempt to explain the observed phenomenon, it could be said that increasing residence time should shift droplet size to smaller values but it can also produce an increase in temperature. The latter is a formulation variable that changes the HLB mixtures, pushing the formulation out of the “sweet spot” corresponding to the minimum droplet size (thereby increasing droplet size). However, temperature also decreases oil viscosity, thus facilitating droplet breakage. These two mechanisms may compete and produce the non-linear behavior observed.
Figure 9: Three-dimensional depiction of droplet size evolution of emulsion produced with the thin film-spinning apparatus using a top plate of 5 mm in continuous mode. Water/oil ratio: 80/16�80/14 (the difference corresponds to the surfactant concentration). The droplet size shows a maximum as residence time is increased at a fixed surfactant concentration. Also the particle size is reduced with the increase of surfactant concentration.
Figure 10: Three-dimensional depiction of droplet size evolution of emulsion produced with the thin film-spinning apparatus using a top plate of 7 mm in continuous mode. Water/oil ratio: 80/16�80/14 (the mass balance difference corresponds to the surfactant concentration). These systems exhibit a highly non-linear behavior; however, it is observed that the higher peripheral speed is more effective in reducing droplet size.
In any case, the influence of residence time is more intense at 20 m/s while the droplet size is less affected when the surfactant concentration is increased. It can also be observed that mixing at 30 m/s tends to produce smaller droplets than mixing at 20 m/s.
Figures 10a and 10b also depict droplet size as a function of residence time and surfactant concentration. In this case, the top plate aperture is 7 mm, which implies that the thickness of the spinning film is thicker than the film for a 5 mm aperture. Then again, the observed behavior is complex but some trends are similar to the ones obtained for the 5 mm aperture: the higher peripheral speed is more effective in reducing droplet size. It is also noteworthy that the set of variables, 20 m/s and 7 mm (Figure 10a), produce the larger droplet sizes of all the other sets.
It is out of the scope of this work to give a thorough interpretation of these results; nonetheless, an optimum set of mixing parameters, within the boundaries of the explored variables, can be selected. The best conditions for droplet size reduction, in continuous mode, correspond to 30 m/s mixing speed and 7 mm top plate aperture: most droplets are below 400 nm (volumetric mean).
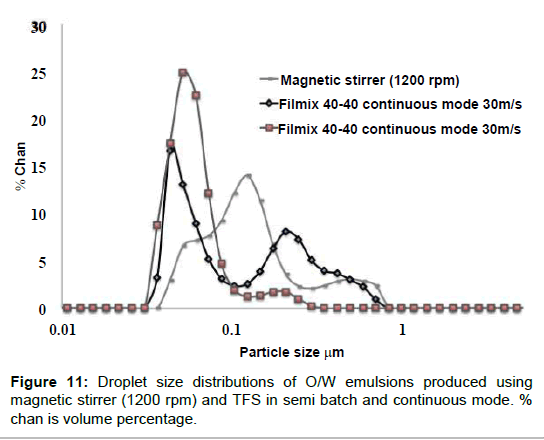
Figure 11 shows a comparison between the best results obtained, in terms of droplet size reduction, with three different procedures: mixing with a magnetic stirrer, mixing with the TFS apparatus in a semi-continuous fashion and mixing with the TFS apparatus continuously. All distributions are sub-micrometric but it is clear that the TFS apparatus produces globally smaller droplet sizes. However, the best results were obtained with the semi-continuous protocol; the distribution is quite narrow and most particles are smaller than 100 nm. For this reason, this procedure was selected to produce curcumin loaded nanoemulsions.
Curcumin loaded nanoemulsions
The carrier nanoemulsions were prepared adding curcumin and the co-solvent, ethanol, to the oil phase, previous to the emulsion formation. The latter was carried out using the TFS apparatus in a semi-continuous mode and characterized for droplet size and stability.
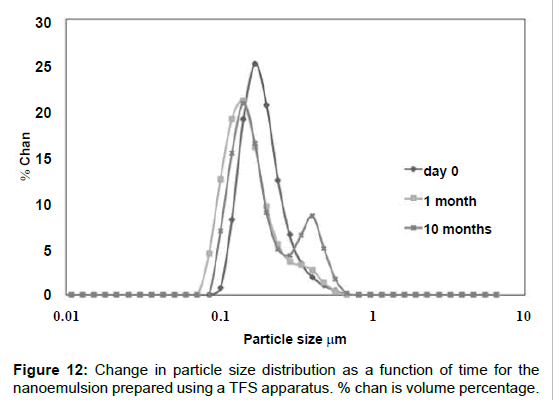
Figure 12 shows the droplet size distribution for a freshly prepared emulsion containing curcumin and ethanol. The last two components have a noticeable effect on droplet size as compared with the emulsions that did not contain curcumin and ethanol. While in the absence of these components most droplets were smaller than 100 nm (Figure 11, semi-continuous results), the curcumin loaded droplets shifted to larger values, although still in the sub-micrometric range; the distribution is quite narrow and unimodal.
The emulsion was also observed using a SEM; Figure 13 shows a microphotograph of an emulsion sample. It can be seen that the size of the oil droplets is very homogeneous and significantly smaller than 2 μm (reference scale in the protograph). In addition, Figure 12 depicts the evolution of the droplet size distribution as a function of storage time. The emulsion did not experience significant changes during the first month after preparation. After 10 months, the carrier emulsion experienced some coalescence as evidenced by the growth of a second mode corresponding to larger droplet sizes. This effect may be caused by Ostwald ripening, which is the main mechanism of nanoemulsion breakdown [44]. In addition, factors such as polydispersity or high surfactant concentration may have favored this phenomenon [42,45]. However, we think these emulsion samples were quite robust and, besides, the stability can be further increased by the use of a polymer to produce nanocapsular structures [46].
Conclusion
We prepared stable oil-in-water emulsions that can be used as carrier systems to deliver water insoluble or thermolabile compounds; the encapsulation efficiency of the active compound in the droplets is very high. In addition, a method to achieve an almost total dissolution of curcumin was developed. The use of co-surfactants facilitates the dissolution of curcumin into the paraffin oil drop. These nanoemulsions could be used as drug carriers or as templates/molds/guides to produce nanocapsules. This method might be applied in diverse fields related to medical or food industries due to the fact that it avoids the use of aggressive solvents like methanol, chloroform or dichloromethane.
Acknowledgement
The authors gratefully acknowledge the National Secretariat for Science, Technology and Innovation (SENACYT) of the Republic of Panama for financial support to Dr. Caballero-George through the incentive program of the National Innovation System (SNI) as well as through grant FIE10-08. Thanks are also due to IFARHU from the Panamanian government, which jointly with SENACYT gave a scholarship to Mr. Marín. The authors wish to thank Dr. Mavis Montero from the Universidad de Costa Rica, Ricardo Santamaria from INDICASAT AIP and Melissa Cuevas from Nano Dispersions Technology for technical assistance with SEM, HPLC experiments and sample preparation, respectively.
References
- Revathy S,Elumalai S, Benny M, Antony B (2011) Isolation, purification and identification of curcuminoids from turmeric (Curcuma longa L .) by column chromatography. J ExpSci2: 21-25.
- Himesh S, Sharan PS, Mishra K, Govind N, Singhai K (2011) Qualitative and quantitative profile of curcumin from ethanolic extract of Curcuma longa. Int Res J Pharm 2: 180-184.
- Teiten MH, Eifes S, Dicato M, Diederich M (2010)Curcumin-the paradigm of a multi-target natural compound with applications in cancer prevention and treatment. Toxins (Basel) 2: 128-161.
- Anand P, Kunnumakkara AB, Newman R, Aggarwal BB. Bioavailability of curcumin: Problems and promises.Mol. Pharm.4: 807-818.
- Aggarwal BB, Kumar A, Bharti AC (2003) Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res 23: 363-398.
- Jagannathan R, Abraham PM, Poddar P (2012) Temperature-dependent spectroscopic evidences of curcumin in aqueous medium: A mechanistic study of its solubility and stability. J PhysChem B116: 14533-14540.
- Sharma RA, McLelland HR, Hill KA, Ireson CA, Euden SA, et al. (2001) Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res7: 1894-1900.
- Zhongfa L, Chiu M, Wang J, Chen W, Yen W, et al. (2012) Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. CancerChemotherPharmacol69: 679-689.
- Prasad S, Tyagi AK, Aggarwal BB (2014)Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat1: 2-18.
- Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, et al. (2007) Polymeric nanoparticle-encapsulated curcumin (â??nanocurcuminâ??): A novel strategy for human cancer therapy. JNanobiotechnology 5: 3.
- Setthacheewakul S, Kedjinda W, Maneenuan D, Wiwattanapatapee R (2011) Controlled release of oral tetrahydrocurcumin from a novel self-emulsifying floating drug delivery system (SEFDDS).AAPS PharmSciTech12: 152-164.
- Liu CH, Chang FY (2011) Development and characterization of eucalyptol microemulsions for topic delivery of curcumin.Chem Pharm Bull (Tokyo)59: 172-178.
- Vecchione R, Quagliariello V, Calabria D, Calcagno V, De Luca E, et al. (2016) Curcumin bioavailability from oil in water nano-emulsions: In vitro and in vivo study on the dimensional, compositional and interactional dependence. J Control Release 233: 88-100.
- Anuchapreeda S, Fukumori Y, Okonogi S, Ichikawa H (2012) Preparation of lipid nanoemulsions incorporating curcumin for cancer therapy. JNanotechnol 2012: 1-11.
- Walstra P (1993) Principles of emulsion formation. ChemEngSci48: 333-349.
- Koshy A, Das TR, Kumar R (1988) Effect of surfactants on drop breakage in turbulent liquid dispersions. ChemEngSci43: 649-654.
- Sis H, Chander S (2004) Kinetics of emulsification of dodecane in the absence and presence of non-ionic surfactants. Colloids Surfaces A PhysicochemEng Asp235: 1-3.
- Golouba TP, Pughb RJ (2005) The role of the surfactant head group in the emulsification process: Binary (non-ionicâ??ionic) surfactant mixtures. J Colloid Interface Sci291: 256-262.
- Pichot R, Spyropoulos F, Norton IT (2009) Mixed-emulsifier stabilised emulsions: Investigation of the effect of mono-olein and hydrophilic silica particle mixtures on the stability against coalescence. J Colloid Interface Sci329: 284-291.
- Izquierdo P, Feng J, Esquena J, Tadros TF, Dederen JC, et al. (2005) The influence of surfactant mixing ratio on nano-emulsion formation by the pit method.J Colloid Interface Sci 285: 388-394.
- Salager J, Bullón J, Pizzin A,Rondón-González M, Tolosa L (2011) Emulsion formulation engineering for the practitioner. Encyclopedia of Surface and Colloid Science 2.
- Salager JL (1999) Formulación, Composición y Fabricación de EmulsionesparaObtenerlasPropiedadesdeseadas. Estado del Arte Parte A Introducción y Conceptos de FormulaciónFisicoquÃÂmica, New York 2.
- Kaminaga Y, Nagatsu A, Akiyama T, Sugimoto N, Yamazaki T, et al. (2003) Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthusroseus. FEBS Lett555: 311-316.
- Morimoto T, Sunagawa Y, Katanasaka Y, Hirano S, Namiki M, et al. Drinkable preparation of Theracurmin exhibits high absorption efficiency--a single-dose, double-blind, 4-way cross-over study. Biol Pharm Bull36: 1708-1714.
- Onoue S, Takahashi H, Kawabata Y, Seto Y, Hatanaka J, et al.(2010) Formulation design and photochemical studies on nanocrystal solid dispersion of curcumin with improved oral bioavailability. J Pharm Sci99: 1871-1881.
- Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, et al. (2011) Innovative preparation of curcumin for improved oral bioavailability.Biol Pharm Bull 34: 660-665.
- Gandapu U, Chaitanya RK, Kishore G, Reddy RC, Kondapi AK (2011)Curcumin-loaded apotransferrin nanoparticles provide efficient cellular uptake and effectively inhibit HIV-1 replication in vitro.PLoS One6.
- Leung MHM, Kee TW (2009) Effective stabilization of curcumin by association to plasma proteins: Human serum albumin and fibrinogen. Langmuir25: 5773-5777.
- Nguyen MH, Yu H, Kiew TY, Hadinoto K (2015) Cost-effective alternative to nano-encapsulation: Amorphous curcumin-chitosan nanoparticle complex exhibiting high payload and super saturation generation.Eur J PharmBiopharm96: 1-10.
- Agubata CO, Nzekwe IT, Obitte NC, Ugwu CE, Attama AA, et al. Effect of oil, surfactant and co-surfactant concentrations on the phase behavior, physicochemical properties and drug release from self-emulsifying drug delivery systems. J Drug DiscovDevDeliv 1: 1-7.
- Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, et al. (2007) Development and bioavailability assessment of ramiprilnanoemulsion formulation.Eur J Pharm Biopharm 66: 227-243.
- Sajjadi S (2006) Effect of mixing protocol on formation of fine emulsions. ChemEngSci61: 3009-3017.
- Liu C, Li M, Liang C, Wang W (2013) Measurement and analysis of bimodal drop size distribution in a rotor-stator homogenizer. ChemEngSci102: 622-631.
- Hall S, Cooke M, El-Hamouz A, Kowalski AJ (2011) Droplet break-up by in-line Silverson rotor-stator mixer.ChemEngSci66: 2068-2079.
- Steiner H, Teppner R, Brenn G, Vankova N, Tcholakova S, Denkov N (2006) Numerical simulation and experimental study of emulsification in a narrow-gap homogenizer. ChemEngSci61: 5841-5855.
- Vilasau J, Solans C, Gomez MJ, Dabrio J, Mujika-Garai R, et al. (2011) Influence of a mixed ionic/non-ionic surfactant system and the emulsification process on the properties of paraffin emulsions. Colloids Surfaces A PhysicochemEng Asp392: 38-44.
- Sánchez MC, Berjano M, Guerrero A, Brito E, Gallegos C (1998) Evolution of the microstructure and rheology of O/W emulsions during the emulsification process. Can J ChemEng76: 479-485.
- Laso M, Steiner L, Hartland S (1987) Dynamic simulation of liquid-liquid agitated dispersionsâ??I. Derivation of a simplified model. ChemEngSci42: 2429-2436.
- Laso M, Steiner L, Hartland S (1987) Dynamic simulation of agitated liquidâ??liquid dispersionsâ??II. Experimental determination of breakage and coalescence rates in a stirred tank.ChemEngSci 42: 2437-2445.
- Klink IM, Phillips RJ, Dungan SR (2011) Effect of emulsion drop-size distribution upon coalescence in simple shear flow: A population balance study.J Colloid Interface Sci 353: 467-475.
- Briceño M, Salager JL, Bertrand J (2001) Influence of dispersed phase content and viscosity on the mixing of concentrated oil-in-water emulsions in the transition flow regime. ChemEng Res Des79: 943-948.
- Salager JL, Antón RE, Briceno MI, Choplin L, Márquez L, et al. (2003) The emergence of formulation engineering in emulsion making - Transferring know-how from research laboratory to plant. PolymInt52: 471-478.
- MI B, RamÃÂrez M, Bullon J, Salager JL (1997) Customizing drop size distribution to change emulsion viscosity.RécentsProgrès en Génie des Procédés13: 303-309.
- Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ (2005) Nano-emulsions. CurrOpin Colloid Interface Sci10: 102-110.
- VandammeTF, Anton N (2010) Low-energy nanoemulsification to design veterinary controlled drug delivery devices.Int J Nanomedicine5: 867-873.
- Mora-Huertas CE, Fessi H, Elaissari A (2010) Polymer-based nanocapsules for drug delivery.Int J Pharm 385 : 113-142.
Citation: Marin E, Briceño MI, George CC (2016) Method to Produce Curcumin Oil-in-Water Nanoemulsions as Templates for Drug Carriers. J Biotechnol Biomater 6:247. DOI: 10.4172/2155-952X.1000247
Copyright: © 2016 Marin E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 17710
- [From(publication date): 12-2016 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 16207
- PDF downloads: 1503