Research Article Open Access
Methanolic Extract of Clinacanthus nutans Leaves can Alter Adipocyte Cellularity, Inflammation and Acetyl Cholinesterase Activity in Male Obese Mice
Abdulwahid SJ1,2, Ebrahimi M3*, Goh Y3, Adeyemi KD4, Ismail H5 and Hashim Z11Department of Environmental and Occupational Health, Faculty of medicine and Health science, University Putra Malaysia, Malaysia
2Department of General Science, Faculty of Education, Soran Universiti, Kurdistan Region of Iraq, Iraq
3Department of Veterinary Preclinical Sciences, Faculty of Veterinary Medicine, University Putra Malaysia, Selangor, Malaysia
4Department of Animal Production, University of Ilorin, Ilorin, Nigeria
5Department of Biological Sciences, Bauchi State University, Bauchi State, Nigeria
- *Corresponding Author:
- Mahdi Ebrahimi
Department of Veterinary Preclinical Sciences, Faculty of Veterinary Medicine
Universiti Putra Malaysia, 43400, Serdang, Selangor, Malaysia
Tel: 0173821500
E-mail: mehdiebrahimii@gmail.com
Received date: March 17, 2017; Accepted date: April 07, 2017; Published date: April 14, 2017
Citation: Abdulwahid SJ, Ebrahimi M, Goh Y, Adeyemi KD, Ismail H, et al. (2017) Methanolic Extract of Clinacanthus nutans Leaves can Alter Adipocyte Cellularity, Inflammation and Acetyl Cholinesterase Activity in Male Obese Mice. J Obes Weight Loss Ther 7:336. doi: 10.4172/2165-7904.1000336
Copyright: © 2017 Abdulwahid SJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Background: The adverse effects of obesity on human health necessitate the development of effective interventions for the prevention and treatment of obesity. Mixture of natural products, including crude extracts and isolated pure natural compounds can cause a body weight reduction and prevent diet-induced obesity. Consequently, they have been widely utilized in treating obesity. Methods: A total of sixty imprinting control region (ICR) male mice (39.01 ± 1.03 g BW) were divided into six groups (10 per group) and randomly assigned to either a Normal diet (ND), High fat diet (HFD)+normal saline, HFD +Orlistat (15.9 mg/kg BW/day), HFD+CN500, 1000 and 1500 mg/kg BW/day, and fed daily for three weeks. Adipose cellularity was determined by histological methods (Haematoxylin and Eosin), whereas plasma lipid profiles were measured by using a Hitachi 902 automatic clinical analyzer, meanwhile cytokines with a Benchmark Plus Microplate Spectrophotometer (RT-2100C) and AChE was detected in muscle and heart by using acetylcholine as a substrate. Results: Mice supplemented with HFD+CN1000 and 1500 mg/kg had significantly lower adipocyte area, size, and diameter compared to those fed other diets. The mean adipocyte number of mice supplemented with C. nutans was significantly lower compared to the control groups. Mice fed HFD+CN1500 mg/kg had a greater adipocyte cell count compared with those supplemented with HFD+CN1000 and 500 mg/kg. Supplementation of C. nutans reduced plasma total cholesterol in mice. Diets had no effect on plasma lipid profile. High dose of methanolic extract have more ant obesity properties with better effects on adipocyte cellularity, cytokines, acetylcholinestrase than low dose. In addition, The C. nutans fed mice exhibited decrease but non-significant (P>0.05) in leptin, meanwhile the acetyl cholinesterase activity in the heart and muscle was greater in mice fed C. nutans compared with control groups. Conclusion: Methanolic extract of C. nutans leaves can preclude diet-induced obesity.
Keywords
Obesity; Clinacanthus nutans ; Snake grass; Adipose cellularity; IL6; Leptin; Acetyl cholinesterase
Abbreviations
CN: Clinacanthus nutans , ICR: Imprinting Control Region, ND: Normal Diet, HFD: High-Fat Diet, BW: Body Weight, TC: Total Cholesterol, TG: Triglycerol, HDL: High Density Lipoprotein, LDL: Low Density Lipoprotein, IL-6: Interlukin 6, AchE: Acetyl Cholinesterase
Introduction
Obesity is a medical condition characterized by dysregulation of lipid metabolism and excessive increase in adipose tissue mass [1]. Obesity is prevalent in both developed and developing countries [2] and it has deleterious effects on human health, which can cause reduced life expectancy [3,4]. Though the effects and extents of obesity are still controversial, it is well established that obesity has reached epidemic proportions across the world [5]. In [6] the World Health Organization (WHO) estimated that there were about more than 600 million people classified as obese and 1.9 billion overweight, including 41 million children under the age of five. The prevalence of obesity is on the increase in many parts of the world. There was an increase in the average annual change ranging from 0.2% to 18.5% in developed countries and 0.1% to 35.3% in developing countries [2]. The World Health Organization [7], recognized obesity as a disease in 2007. Nonetheless, recently overweight and obesity is the fifth leading cause of deaths worldwide [8]. To date, the complications related to obesity contribute to the 100,000 to 400,000 deaths per year [9]. Currently, diets high in calories, especially from carbohydrate, saturated fatty acids (SFAs), and long-chain omega-6 polyunsaturated fatty acids (PUFAs), and sedentary lifestyles lacking physical activity contribute significantly to this obesity [10]. Endogenous and exogenous agents that enhance preadipocyte proliferation or adipocyte hypertrophy have the capability to increase white adipose tissue (WAT) mass and the development of obesity [10]. The expansion of adipose tissue produces numerous bioactive substances, known as adipocytokines or adipokines, which may lead to the growth of various metabolic diseases through altered glucose, lipid homeostasis and inflammatory responses (Dandona et al.). It has been documented that the plasma concentration of inflammatory mediators, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), is increased in obesity and type 2 diabetes [11]. Treatment of obesity is beneficial because the weight loss reduces the hazard of mortality and morbidity [12].
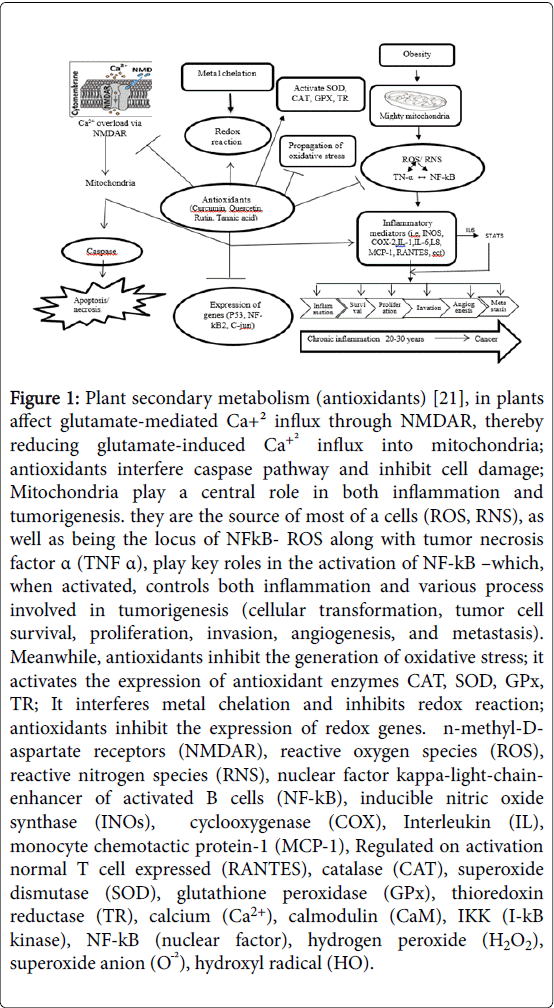
Recently, the Food and Drug Administration (FDA) has approved Orlistat and Sibutramine drugs, which both can induce serious side effects, such as palpitation and hypertension [12]. To ward off the undesirable side effects of these two drugs, dietary concepts look to play an important role in weight control programs [13]. Alternative medicine and adverse effects of conventional western medicine (WM) [14]. Consequently, a rapidly expanding field in therapeutics is in the use of natural supplements [15,16]. The utilization of plants in different traditional medicine of various cultures has been comprehensively documented [17]. The efficacy of medicinal plants could be attributed to the similarities between plant and mammalian biochemistry and molecular functioning, specifically, the many molecular signaling pathways that are preserved between the taxa and play roles in the synthesis of secondary metabolite within plants [18]. Medicinal plants contain polyphenols, which consist of tannins and flavonoids, which are widely found as secondary metabolites in plants [19]. However, is highly subjective and, therefore these phenolic substances derived antioxidant supplements [20], have been described to quench free radicals and prevent disease like anti-inflammatory, antihyperglycemic and anti-hyperlipidemic properties (Figure 1) [21].
Figure 1: Plant secondary metabolism (antioxidants) [21], in plants affect glutamate-mediated Ca+² influx through NMDAR, thereby reducing glutamate-induced Ca+² influx into mitochondria; antioxidants interfere caspase pathway and inhibit cell damage; Mitochondria play a central role in both inflammation and tumorigenesis. they are the source of most of a cells (ROS, RNS), as well as being the locus of NFkB- ROS along with tumor necrosis factor α (TNF α), play key roles in the activation of NF-kB –which, when activated, controls both inflammation and various process involved in tumorigenesis (cellular transformation, tumor cell survival, proliferation, invasion, angiogenesis, and metastasis). Meanwhile, antioxidants inhibit the generation of oxidative stress; it activates the expression of antioxidant enzymes CAT, SOD, GPx, TR; It interferes metal chelation and inhibits redox reaction; antioxidants inhibit the expression of redox genes. n-methyl-Daspartate receptors (NMDAR), reactive oxygen species (ROS), reactive nitrogen species (RNS), nuclear factor kappa-light-chainenhancer of activated B cells (NF-kB), inducible nitric oxide synthase (INOs), cyclooxygenase (COX), Interleukin (IL), monocyte chemotactic protein-1 (MCP-1), Regulated on activation normal T cell expressed (RANTES), catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), thioredoxin reductase (TR), calcium (Ca2+), calmodulin (CaM), IKK (I-kB kinase), NF-kB (nuclear factor), hydrogen peroxide (H2O2), superoxide anion (OÖ¾²), hydroxyl radical (HO).
The complex pathogenesis of obesity indicates the need of different intervention strategies to confront this problem. Herbal supplements and diet-based therapies for weight loss are among the most common complementary and alternative medicine modalities [22]. The efficacies of medicinal plants in the treatment of obesity are highly variable and inconsistent in the published literature. It was surprising to observe dietary polyphenols are secondary metabolites of the plants have anorectic properties effecting a loss in body weight [23]. To address these concerns, supplementation of chlorogenic acid lowered the body weight gain and adipose weight compared to the high-fat diet HFD control group and caffeic acid group [24]. In chloroplast, thylakoids contain a hundred different membrane proteins, galactolipids and sulpholipids as well as various vitamins (A, E and K), thus a variety of bioactive compounds that could be responsible for the retardation of fat digestion [25], as well as the reduction in the size of the abdominal fat cell could be attributed to the bioactivities of the polyphenolic compounds [19,26]. Different plants contain a large variety of several components with different anti-obesity effects on body metabolism and fat oxidation, and for this reason have been investigated and reported to be useful in the treatment of obesity [27]. This justifies the need for additional studies using novel medicinal plants to give room for tailored decisions and informed choices in the utilization of medicinal plants for the treatment of obesity. One of the medicinal plants of interest is known as C. nutans . Lindau (Family: Acantheceae) commonly known as Sabah snake grass [28]. This plant is widely used for treating skin rashes, scorpion and insect bites, gastrointestinal complications, dysuria, menstrual function, anemia, jaundice, setting of fractured bones, diabetes mellitus, fever and diuretics [29]. Numerous bioactive have been demonstrated in C. nutans such as phenolics, flavonoids, sitosterol, stigmasterol, and chlorophyll derivatives [29]. In addition, The C-glycosidic flavones such as shaftoside, isoorientin, orientin, isovitexin, and vitexin have been found to be the major flavonoids in the leaves of C. nutans [30,31], although protocatechnic acid was one of the most abundant [29]. Nonetheless, despite the established polyphenols in C. nutans there is a dearth of information on the anti-obesity properties of the polyphenols. Despite all the known biological activities from previous work, emerging lay commendation and Malaysian newspaper reports indicated that C. nutans possesses weight loss (appetite suppression, fat and calorie burning and carb blocking properties) and lowers blood cholesterol levels effect. Nonetheless, these testimonies were not backed up by scientific evidence. We hypothesize that C. nutans would affect adipocyte cellularity, lipid profile and acetylcholinesterase activity in muscle and heart of diet-induced obese mice. Thus, the primary aim of this study was to determine the effects of C. nutans adipocyte cellularity, lipid profile and acetylcholinesterase enzyme activity in diet-induced obese mice.
Materials and Methods
Plant materials
Fresh leaves of C. nutans (Burm.f. ) Lindau was harvested at botanical garden in Sermpun II, UPM. Selangor, Malaysia. The botanical identify of C. nutans was characterized by the phytomedicinal herbarium, Institute of Bioscience, Universiti Putra Malaysia, Selangor (Voucher no. SK2942/15).
Preparation of extracts
The C. nutans leaves were freshly harvested and oven-dried at 40-45C. The leaves were ground to fine powder and sequentially soaked in methanol and distilled water, incubated for 72 h at room temperature with the gentle shaking, then filtered using filter paper (Whatman No. 1, Fitchburg, WI, USA), and extracted under compact pressure, by using a R-215 rotating evaporator (Buchi, Flawil, Switzerland), and the plants extracts were kept after dehydration and lyophilization by freeze drying machine (Labconco Free Zone 6Pluse Freeze Dry), at -20°C until all tests were performed.
Animals and ethical considerations
Sixty male ICR mice at three weeks of age were purchased and acclimatized for one week prior to the commencement of the trial. They were housed in an air-conditioned animal house with a 12 h light/12 h dark cycle at 22 ± 1°C and 50 ± 10% humidity. Two mice were housed per cage. The use and handling of animals in this study had been approved by the Animal Ethics Committee of Universiti Putra Malaysia (reference no UPM/IACUC/AUP-R083/2016).
Experimental design body weight monitoring and sample collection
To induce obesity, the mice were fed with high-fat diet (HFD) (Rodent Diet D12451 with 45% kcal from fat) (Research Diets, New Brunswick, NJ, USA) [32].
And mice were given with normal chow diet (Rodent Diet D12450B with 10% kcal from fat) (Research Diet, New Brunswick, NJ, USA). In the therapeutic strategy, the male ICR mice were fed the HFD for 16 weeks to induce obesity. Body weights were measured two times a week and housed in 2 mice/cage. The average food intakes were calculated every week. The sample concentrations, duration, and the complete experimental design are shown in (Tables 1 and 2).
| Group number | Group name | Oral feeding daily |
| 1 | HFD+distilled water | Positive control |
| 2 | ND +distilled water | Negative control |
| 3 | HFD+Orlistat | Positive control |
| 4 | HFD+CN 500mg/BW | Low concentration |
| 5 | HFD+CN1000mg/BW | Medium concentration |
| 6 | HFD+CN1500mg/BW | High concentration |
| CONTROL groups without C. nutans (HFD: High Fat Diet; ND: Normal Diet; HFD+Orlistat: High Fat Diet with orlistate). Whereas treatment groups: High Fat Diet(HFD) with the supplementation C.nutans (CN); HFD+CN 500: Low concentration C. nutans; HFD+CN1000: Medium concentration C.nutans; HFD+CN1500: High concentration of C. nutans | ||
Table 1: C. nutans experimental protocol.
| Nominal concentration | Actual concentration |
| ( mg/kg ) | (mg/ml ) |
| 500 | 19.5 (0.122) |
| 1000 | 39 (0.244) |
| 1500 | 58.5 (0.366) |
Table 2: Actual concentration of C. nutans.
Methanolic extract of C. nutans was dissolved in distilled water while Orlistat was dissolved in ethanol 20 mg/ml. A total of sixty ICR male mice (39.01 ± 1.03) g body weight were divided into six groups (10 per group) and then were randomized to receive the normal diet (ND), and high-fat diet (HFD) control mice were treated with vehicle (normal saline), or Orlistat was used as positive treatments of obesity (15.9 mg/kg per day) [32] and HFD plus C. nutans (500, 1000 and 1500 mg/kg per day), daily for another three weeks and administered orally by the oral gavages. Body weight and daily food intake were measured twice per week. After twenty-one days, each mice was made to fast for overnight after the last treatment and then performed under Ketamine and Xylazine (1:10 v/v) anesthesia and sacrificed by exsanguinations of the jugular vein after blood sample collection. Blood samples were collected using syringes with 26-gauge needles and transferred into vacutainer tubes treated with ethylene diamine tetraacetic acid (EDTA, Becton Dickinson, Franklin Lakes, NJ). The blood samples were placed on ice then centrifuged at 3000 rpm for 15 min at 4°C to separate the plasma, Plasma samples were transferred into Eppendorf tubes and stored at -80°C until analysis. The gross morphology examination was performed and the absolute weight of livers and spleens were measured. The peri-epididymal fat, liver, heart and muscle were snap frozen in liquid nitrogen and stored at -80°C. Tissues were either fixed in buffered formalin or processed for histology. The experimental protocols were conducted in accordance with internationally accepted principles for laboratory animal use and care.
Adipocyte measurement
To determine the effects of treatment on adipocyte morphology, visceral and subcutaneous fat was excised immediately after euthanasia with some modified [19]. For analysis of adipocyte size, fat pads of mice from each group were fixed in formalin 10%, and then samples were fixed in Acetone 1:10 for 72 hours, processed by automated tissue processor (Leica, Germany). Molds were prepared by embedding in paraffin Wax and 5 μm thick sections were sliced by Leica tissue microtome. Finally, the slides were kept in an oven at 50°C then stained with Hematoxylin and Eosin. Sections were examined and pictures captured under a light microscope (Motic BA410:Imager ICC50). Adipocytes enumerated and their diameters measured using Image J software (National Institute of Health, USA). While mean an input image of the already prepared slide was taken from the camera attached to the microscope, the original colored input images were converted into grayscale images from the range starting from (0-255 pixel) and this area represents the white blood cells. The mean adipocyte diameter was calculated as an average of the diameters. To ensure the accuracy of measurement, three images of each animal were analyzed by three different investigators and averaged into a single measurement [33]. Measurements were obtained from three individual animals per group.
Plasma biochemical measures
Measurement of lipid profile: Plasma levels of total cholesterol, triglycerol and low-density lipoprotein (LDL) were determined in duplicate using a Hitachi 902 automatic clinical analyzer (Boehringer, Mannheim, Germany) and commercially available kits (Roche, Mannheim, Germany), high-density lipoprotein values were calculated by subtracting from (Cholesterol-LDL-Triglcerol/2.19).
Measurement of cytokines: Plasma interleukin (IL-6) and leptin concentrations were determined with mouse ELISA kits. Mouse ELISA kits (RayBiotech, USA) were used for IL-6 (sensitivity 2 pg/ml) and leptin (sensitivity 4 pg/ml). The assays were conducted in 96-well microplates according to the manufacturer’s instructions, with a Benchmark Plus Microplate Spectrophotometer (RT-2100C).
Preparation of cytosolic fraction of muscle and heart
Preparation of tissue homogenates: muscle and heart tissues were quickly dissected and frozen in dry ice. Frozen samples were weighed and transferred to 50 mL polypropylene tubes (Falcon; Becton Dickinson, Lincoln Park, NJ) containing 0.1 M cold phosphate buffer saline pH 8 (PBS, Sigma USA), (4°C) at a ratio of 4 mL buffer/g wet tissue, and added I μL cold phenylmethylsulfonyl fluoride (PMSF; Sigma–Aldrich UK). Samples were homogenized for 30 seconds with an electrical homogenizer (Polytron; Brinkmann Instruments, Westminster, NY). Differential centrifugation technique was used to prepare the subcellular organ cytosolic fractions according to the method of [34] with some modifications. Centrifuged at 15000 rpm for 20 minutes at 4°C to separate the cellular. The pellet was discarded and the supernatant was taken and used to determine AChE activity. The protein concentration of content of each cytosolic fraction from muscle and heart was determined by the method of Bradford [35].
Determination of acetylcholine esterase activity
The determination of acetyl cholinesterase (ATC) activity was conducted according to the method described by Ellman et al. [36] adapted for reading on a micro plates reader RT-2100C. The final concentration of the acetylcholine iodide (Sigma, Aldrich) substrate employed was 0.5 mM, while that of the 5,5 Dithio- bis (2-nitrobenzoic acid) DTNB (Sigma- Aldrich) color reagent was 0.067 mM in 0.1 M potassium phosphate buffer pH 8, for both tissues. Absorbance was determined in micro plate reader RT-2100C at 405 nm and the enzyme activity was expressed in μmol min1 mg1.
Statistical analysis
Data obtained from the Adipocyte, plasma biochemical, acetyl cholinesterase, were analyzed using the analysis of variance (ANOVA) procedure of the SAS software package, version 9.1 (SAS Institute Inc., Cary, NC). Significantly different mean values were further elucidated using Duncan’s test. The results were expressed as mean and pooled standard error of the means (SEM). Differences were considered to be statistically significant when <0.05. Therefore, we conducted univariate linear and nonlinear ANOVA with polynomial contrast. Besides linear trends, this method also examines quadratic (U-shaped) trends [37]. The linear contrast compared the lowest with the highest category and the quadratic compares both middle with the highest and the lowest categories together [38].
Results
Adipocyte cellularity
Based on the results obtained the area size, diameter and number of the adipocytes in male ICR obese mice. The data clearly indicated that the higher concentration of C. nutans had a significant effect on fat adipocytes cellularity.
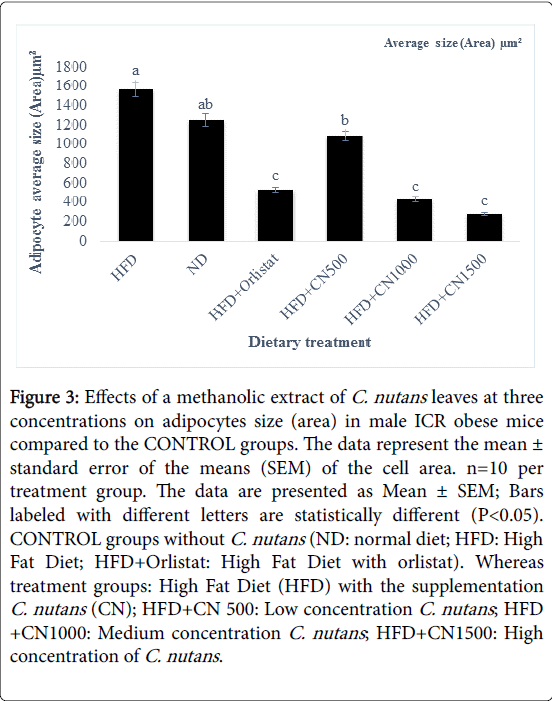
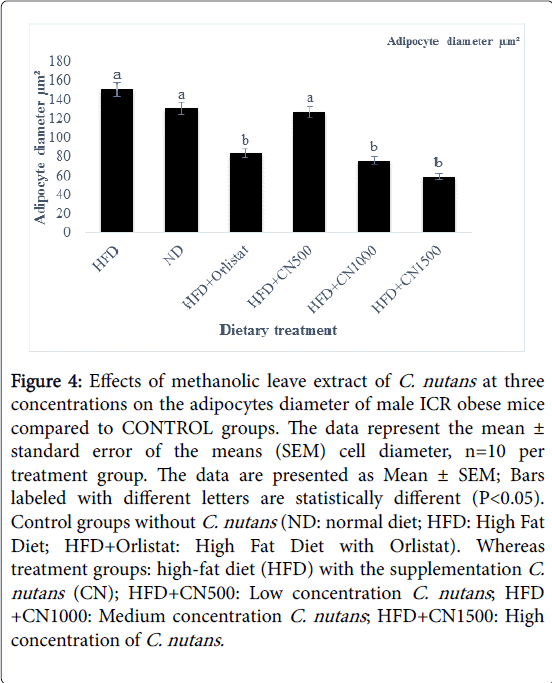
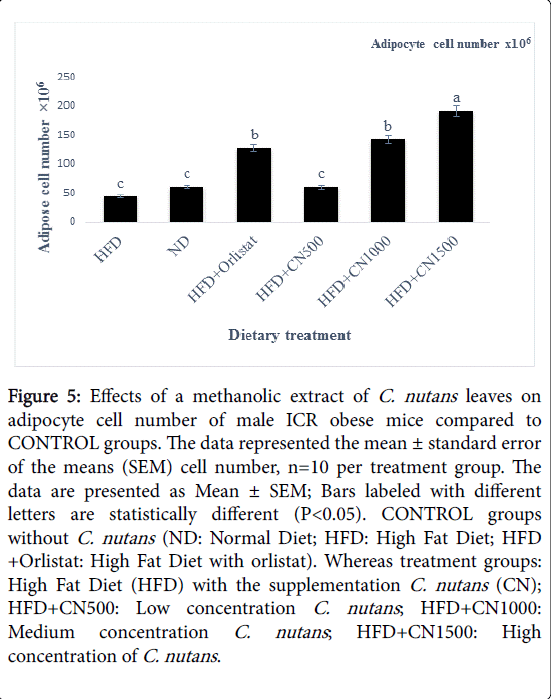
Treated groups HFD+CN1000 and HFD+CN1500 mg/kg have significantly (P<0.05) lowered the adipocytes area size and diameter compared to the HFD+500 mg group and HFD and ND CONTROL group are shown in (Figures 2-5) respectively. As the concentration of methanolic extract of C. nutans leaves increased, the adipocytes area size and diameter were decreased while cell numbers increased. Treatment with HFD+CN1500 mg/kg has the highest adipocyte cell count compared to the other treatment groups from HFD+CN500 and HFD+CN1000 mg/kg (Figure 5). The mean adipocyte number of C. nutans supplemented groups was significantly (P<0.05) higher compared to the HFD control groups.
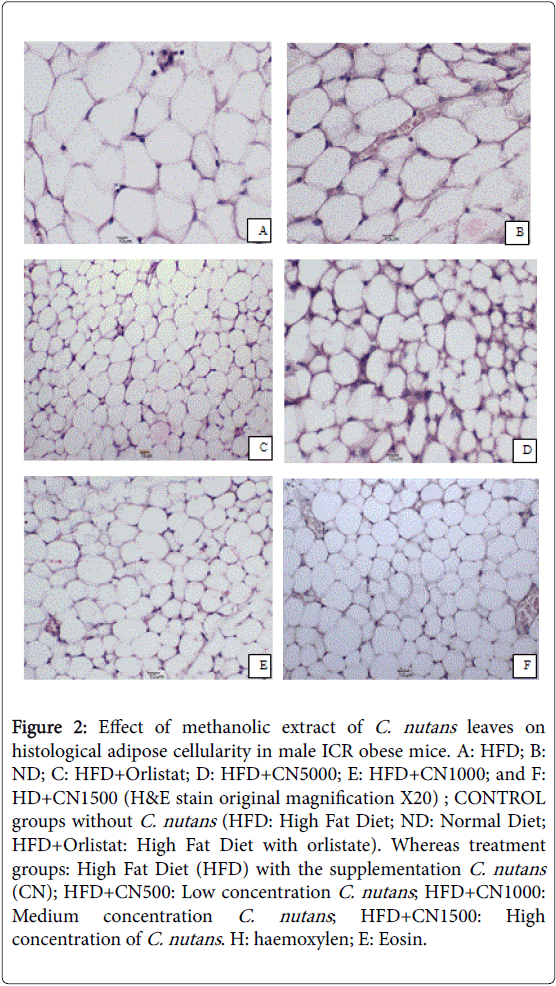
Figure 2: Effect of methanolic extract of C. nutans leaves on histological adipose cellularity in male ICR obese mice. A: HFD; B: ND; C: HFD+Orlistat; D: HFD+CN5000; E: HFD+CN1000; and F: HD+CN1500 (H&E stain original magnification X20) ; CONTROL groups without C. nutans (HFD: High Fat Diet; ND: Normal Diet; HFD+Orlistat: High Fat Diet with orlistate). Whereas treatment groups: High Fat Diet (HFD) with the supplementation C. nutans (CN); HFD+CN500: Low concentration C. nutans ; HFD+CN1000: Medium concentration C. nutans ; HFD+CN1500: High concentration of C. nutans . H: haemoxylen; E: Eosin.
Figure 3: Effects of a methanolic extract of C. nutans leaves at three concentrations on adipocytes size (area) in male ICR obese mice compared to the CONTROL groups. The data represent the mean ± standard error of the means (SEM) of the cell area. n=10 per treatment group. The data are presented as Mean ± SEM; Bars labeled with different letters are statistically different (P< 0.05). CONTROL groups without C. nutans (ND: normal diet; HFD: High Fat Diet; HFD+Orlistat: High Fat Diet with orlistat). Whereas treatment groups: High Fat Diet (HFD) with the supplementation C. nutans (CN); HFD+CN 500: Low concentration C. nutans ; HFD +CN1000: Medium concentration C. nutans ; HFD+CN1500: High concentration of C. nutans .
Figure 4: Effects of methanolic leave extract of C. nutans at three concentrations on the adipocytes diameter of male ICR obese mice compared to CONTROL groups. The data represent the mean ± standard error of the means (SEM) cell diameter, n=10 per treatment group. The data are presented as Mean ± SEM; Bars labeled with different letters are statistically different (P< 0.05). Control groups without C. nutans (ND: normal diet; HFD: High Fat Diet; HFD+Orlistat: High Fat Diet with Orlistat). Whereas treatment groups: high-fat diet (HFD) with the supplementation C. nutans (CN); HFD+CN500: Low concentration C. nutans ; HFD +CN1000: Medium concentration C. nutans ; HFD+CN1500: High concentration of C. nutans.
Figure 5: Effects of a methanolic extract of C. nutans leaves on adipocyte cell number of male ICR obese mice compared to CONTROL groups. The data represented the mean ± standard error of the means (SEM) cell number, n=10 per treatment group. The data are presented as Mean ± SEM; Bars labeled with different letters are statistically different (P< 0.05). CONTROL groups without C. nutans (ND: Normal Diet; HFD: High Fat Diet; HFD +Orlistat: High Fat Diet with orlistat). Whereas treatment groups: High Fat Diet (HFD) with the supplementation C. nutans (CN); HFD+CN500: Low concentration C. nutans ; HFD+CN1000: Medium concentration C. nutans ; HFD+CN1500: High concentration of C. nutans .
Plasma biochemical measures
Plasma lipids profile: The effects of methanolic extract of C. nutans leaves on plasma TC, TG, LDL and HDL for mice were reported in Table 3 The plasma TC, TG and HDL in the treatment C. nutans groups were slightly decreased compared to HFD CONTROL. There were no significant (P>0.05) differences noted for plasma TC, TG, LDL, and HDL between treatment and HFD and ND CONTROL groups.
| Lipid profile | HFD | ND | HFD+Orlistat | HFD+CN500 | HFD+CN1000 | HFD+CN1500 | SEM | P-Values | |
| Linear | Quad | ||||||||
| TC (mmol/L)n.s | 5.85 | 3.48 | 3.43 | 4.39 | 4.33 | 5 | 0.77 | 0.63 | 0.73 |
| TG. (mmol/L)n.s | 2.44 | 1.59 | 1.83 | 1.33 | 1.23 | 1.51 | 0.52 | 0.81 | 0.77 |
| L.D.L. (mmol/L)n.s | 0.75 | 0.5 | 0.61 | 0.61 | 0.79 | 0.88 | 0.25 | 0.474 | 0.88 |
| H.D.L (mmol/L)n.s | 3.99 | 2.25 | 1.98 | 3.18 | 2.98 | 3.44 | 0.61 | 0.77 | 0.67 |
| Values expressed as mean ± standard error of the means (SEM). There were no statistically significant differences between the measurements in different groups. n.s=no significant difference letter. The significant value was set at P<0.05; Control groups without C. nutans (ND: Normal Diet; HFD: High Fat Diet; HFD+Orlistat: High Fat Diet with Orlistat. Whereas treatment groups: High Fat Diet with the supplementation C.nutans. HFD+CN500: Low concentration C. nutans, HFD+CN1000: Medium concentration C. nutans; HFD+CN1500: High concentration of C. nutans | |||||||||
Table 3: Effects of oral administration methanolic leave extract of C. nutans on lipid profile in male ICR obese mice.
Measurement of cytokines
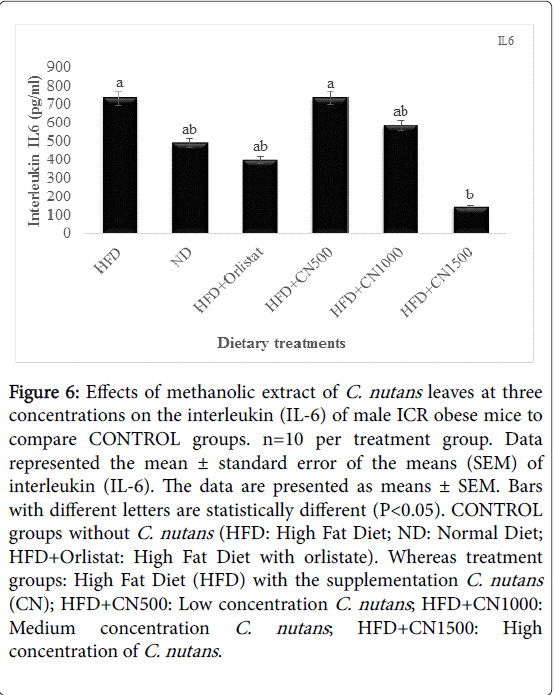
Interleukin IL6 and Leptin: The overall effect of C. nutans feeding on IL-6 level of obese mice is depicted in (Figure 6). It is evident that IL-6 was reduced by increasing concentration of dietary C. nutans HFD+CN1500 mg/kg significant (P<0.05) difference in linear. However, the IL-6 level of mice from the control and C. nutans dietary groups were no significantly (P>0.05) different.
Figure 6: Effects of methanolic extract of C. nutans leaves at three concentrations on the interleukin (IL-6) of male ICR obese mice to compare CONTROL groups. n=10 per treatment group. Data represented the mean ± standard error of the means (SEM) of interleukin (IL-6). The data are presented as means ± SEM. Bars with different letters are statistically different (P< 0.05). CONTROL groups without C. nutans (HFD: High Fat Diet; ND: Normal Diet; HFD+Orlistat: High Fat Diet with orlistate). Whereas treatment groups: High Fat Diet (HFD) with the supplementation C. nutans (CN); HFD+CN500: Low concentration C. nutans ; HFD+CN1000: Medium concentration C. nutans ; HFD+CN1500: High concentration of C. nutans .
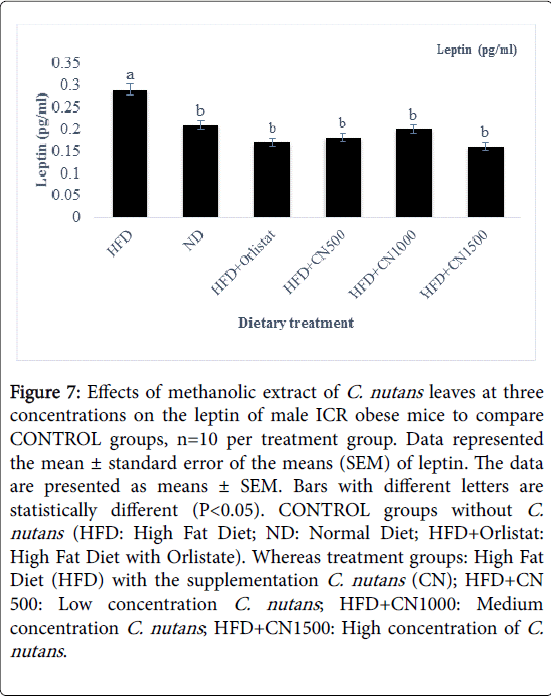
The leptin levels in mice serum were examined (Figure 7). The data showed that the C. nutans treated groups have significant lower leptin levels compared to CONTROL ND and HFD groups. As the concentration of C. nutans increased, the leptin level was decreasing.
Figure 7: Effects of methanolic extract of C. nutans leaves at three concentrations on the leptin of male ICR obese mice to compare CONTROL groups, n=10 per treatment group. Data represented the mean ± standard error of the means (SEM) of leptin. The data are presented as means ± SEM. Bars with different letters are statistically different (P< 0.05). CONTROL groups without C. nutans (HFD: High Fat Diet; ND: Normal Diet; HFD+Orlistat: High Fat Diet with Orlistate). Whereas treatment groups: High Fat Diet (HFD) with the supplementation C. nutans (CN); HFD+CN 500: Low concentration C. nutans ; HFD+CN1000: Medium concentration C. nutans ; HFD+CN1500: High concentration of C. nutans .
There were no significant (P>0.05) differences noted for the leptin plasma in HFD+CN 500, 1000 and 1500 mg/kg with HFD and ND control groups. The leptin level concentrations were the same across all treatment groups (P>0.05).
Determination of acetylcholinesterase activity
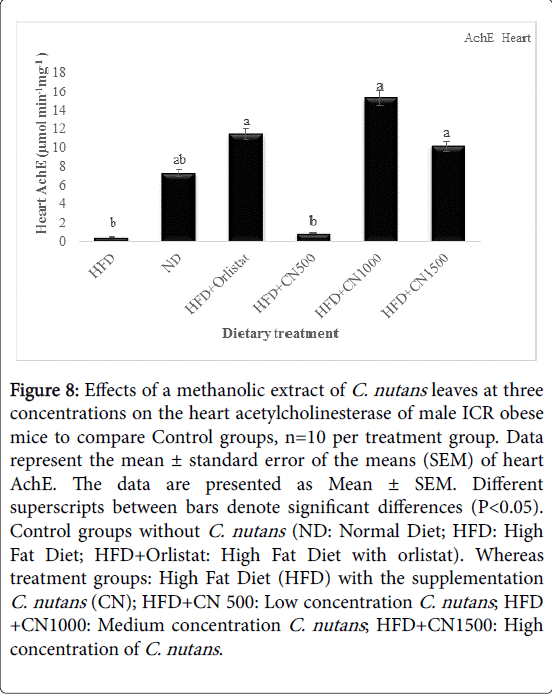
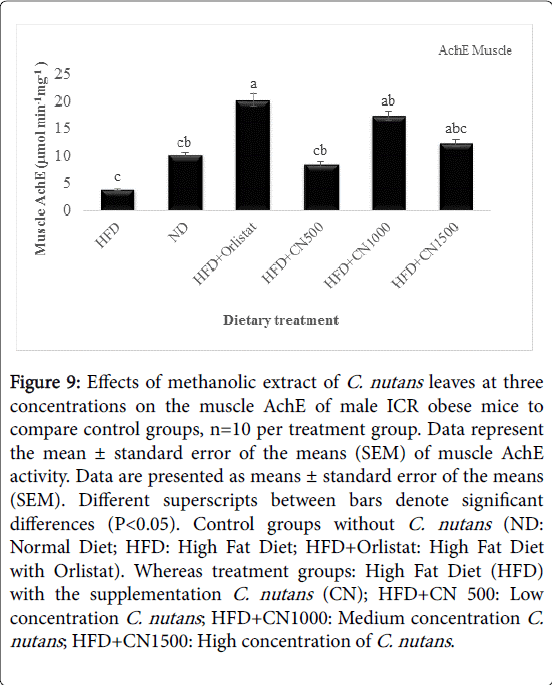
The activity of acetylcholinesterase (AchE) obtained from the mice heart and muscle at HFD500, 1000, and 1500 mg/kg was higher than the HFD and ND CONTROL group that showed in (Figures 8 and 9). However, there was no significant difference seen in the activity of the heart AchE activity between treatments and HFD and ND Control groups (P>0.05).
Figure 8: Effects of a methanolic extract of C. nutans leaves at three concentrations on the heart acetylcholinesterase of male ICR obese mice to compare Control groups, n=10 per treatment group. Data represent the mean ± standard error of the means (SEM) of heart AchE. The data are presented as Mean ± SEM. Different superscripts between bars denote significant differences (P< 0.05). Control groups without C. nutans (ND: Normal Diet; HFD: High Fat Diet; HFD+Orlistat: High Fat Diet with orlistat). Whereas treatment groups: High Fat Diet (HFD) with the supplementation C. nutans (CN); HFD+CN 500: Low concentration C. nutans ; HFD +CN1000: Medium concentration C. nutans ; HFD+CN1500: High concentration of C. nutans .
Figure 9: Effects of methanolic extract of C. nutans leaves at three concentrations on the muscle AchE of male ICR obese mice to compare control groups, n=10 per treatment group. Data represent the mean ± standard error of the means (SEM) of muscle AchE activity. Data are presented as means ± standard error of the means (SEM). Different superscripts between bars denote significant differences (P< 0.05). Control groups without C. nutans (ND: Normal Diet; HFD: High Fat Diet; HFD+Orlistat: High Fat Diet with Orlistat). Whereas treatment groups: High Fat Diet (HFD) with the supplementation C. nutans (CN); HFD+CN 500: Low concentration C. nutans ; HFD+CN1000: Medium concentration C. nutans ; HFD+CN1500: High concentration of C. nutans .
Discussion
In the current study, our results from histological studies demonstrated that feeding mice with high-fat diet HFD could increase the weight of their abdominal fat and hypertrophy, due to excessive lipid storage within adipocytes [20].
Meanwhile, our results showed that the concentration of methanolic leaf extract of C. nutans had an obvious effect on the fat cell. The supplementation ofC. nutans reduced the size of the abdominal fat cell in mice. Similar to this study, Wu et al. [39] concluded that dietary mulberry anthocyanins (MACN) had an effect on both number and the size of adipocytes in mice adipose tissue [40]. The reduction in the size of the abdominal fat cell could be attributed to the bioactivities of the polyphenolic compounds in C. nutans [19,26]. This adipositymodulating effect of C. nutans extract could be attributed to various reasons. First, polyphenols could positively affect the uptake of lipids from the intestine by manipulating microbiota composition [41]. In addition, polyphenols could enhance oxidation in the liver, increasing the utilization of fatty acids as an energy source. In the adipose tissue, polyphenols inhibited adipocyte proliferation and triglyceride synthesis while stimulating lipolysis, resulting in a reduction in adipocyte size of male mice [41]. Reduction of mean adipocyte size is an effective approach to reduce body fat. Smaller mean abdominal adipocyte volume yields a smaller amount of fat action in mice [42]. This is in line with studies in rats where adipocyte size was increased by high-fat feeding independent of genetic factors, whereas an increase in number was enhanced by high-fat diet only in certain strains [43]. Hence, obesity significantly influences the adipocyte volume.
The effects of methanolic extract of C. nutans leaves on plasma TC, TG, LDL and HDL for mice are shown in Table 3. Triglycerides are the main lipids produced by the liver [44]. In other words, lipase is a major enzyme, which catalyzes the hydrolysis of lipids such as triglycerides and cholesterol, to be effectively transported within the body via HDL and LDL [45]. However, in the current study lack of significant differences in plasma TC, TG, HDL and LDL between HFD, ND, and Orlistat CONTROL and treated groups. Our finding is in agreement with findings in oral administration of star fruit (Averrhoa carambola) juice which did not effect on serum lipid profiles in Sprague-Dawley (SD) female normal rats [34]. Therefore, measuring these parameters can provide insight into the metabolic state of animals. The results of our experiment showed that the total cholesterol in mice supplemented with C. nutans was slightly lower than that of the control mice, and consequently, this observation is in agreement with the findings of Nakazato et al. [19] who reported that the plasma total cholesterol in rats supplemented with tea catechism was significantly lower than that of controls. Interestingly, the relation between plasma lipid profile and plant secondary compound content were further supported by polyphenol combined with pectin lowered rat plasma cholesterol [46], it is possible that tannin, another biologically active polyphenol in C. nutans [47], this finding could be related to the inhibition of the absorption of cholesterol from the intestine [48]. It is possible that the polyphenols decreased cholesterol absorption by forming insoluble precipitates of cholesterol [49] and decreasing bile acid-induced micellar solubility. Therefore, the hypocholesterolemic effect of the polyphenols could be due to decreased cholesterol absorption, enhanced cholesterol excretion and inhibition of cholesterol biosynthesis [50]. Although we observed that the highest concentration 1500 mg/kg was less effective than medium concentration 1000 mg/kg of crude methanolic leave extract of C. nutans on plasma lipid profile. The results of this study suggest that consuming high concentration of polyphenol plant secondary metabolism in the form of supplements could lead to an increased health risk [20,51]. To the best of our knowledge, this is the first time that crude methanolic leaf extract C. nutans has been evaluated for anti-obesity. Supplementation of crude methanolic leaf extract C. nutans had no effect on the serum lipid profile in mice. This observation could be due to the duration of the trial [19].
White adipose tissue produces a number of adipokines linked to inflammation. The circulating IL-6 is directly correlated with adiposity [52]. The release of IL-6 into the systemic circulation is greater in obese subjects and agrees with findings in mice fed a high fat diet [53]. This supports a possible novel role for IL-6 as a systemic regulator of lipid metabolism and body weight [53]. Accordingly, serum cytokine levels indicate that the inflammatory response was affected by the high-fat diets. This hypothesis is consistent with the current observations in which HFD+CN500 to 1500-fed mice had decreased IL-6 cytokine levels, indicating a lessened inflammatory response. Similar results were observed in mice supplemented with blueberries [54]. In addition, polyphenols, anthocyanin and catechin prevented inflammation by inhibiting nuclear factor kappa-light-chain-enhancer of activated NFB activity [55-57]. Indeed, as recently highlighted by de Melo et al. [58] showed that oleanolic acid a natural triterpenoid when administered daily (50 mg/L in drinking water), to HFD fed adult male Swiss mice for 15 weeks, they con firmed that the triterpene, oleanolic acid (OA) has been described to decrease the IL-6 production. Polyphenols, triterpene, linoleic acid and diterpene extracted and isolated from C. nutans leaves [30,31], exhibited anti-inflammatory properties [59]. Thus, the observed decreased in IL-6 production in mice supplemented with C. nutans could be attributed to the phenolic contents in C. nutans .
Leptin is a cytokine produced mainly by the white adipose tissue, which plays an important role in regulating lipid metabolism and energy homeostasis. In hypothalamus, leptin stimulates anorexigenic pathways and decrease food intake [60]. It is generally known that most obese animals exhibit high serum leptin concentrations [61]. Supplementation of C. nutans reduced serum leptin levels in mice. Similarly, supplementation of Blueberry juice or purified blueberry anthocyanins (1.0 mg/ml) in the drinking water were fed male C57BL/6J mice for a period of 72 days [40], and anthocyanin-rich grape-bilberry juice [62], reduced serum leptin levels in mice fed HFD. In addition, supplementation of thylakoid [63] and caffeic acid and chlorogenic acid [24] reduced serum leptin levels in mice. In the current study, Sakdarat et al. [64] have been reported that three chlorophyll derivatives (phaeophytins) were extracted from C. nutans leaves, it was expected that thylakoid from plant chloroplasts are useful to elevated the satiety hormone cholecystokinin, which lead to reduce leptin levels [63]. Reduced levels of leptin may suggest enhanced leptin sensitivity [65], which may stimulate fatty acid oxidation. Even thus, the present experiments have confirmed the ability to plant secondary metabolism of chlorophyll, caffeic acid, chlorogenic acid and polyphenol group which are available in C. nutans as well to decrease leptin levels and promote satiety signaling in healthy body [63].
Acetylcholinesterase (AchE) is an enzyme that hydrolyzes the neurotransmitter acetylcholine, producing choline and an acetate group [66]. Interestingly, it is clear that the brain both monitors and regulates the energy needs of the body. In turn, both carbohydrate and adipose stores are monitored by the brain using combinations of metabolic and neural signals from the periphery [67]. However, in the highly interconnected conditions of obesity and non-insulindependent diabetes mellitus, there appears to be a major resetting of normal homeostatic mechanisms [67]. Results of this study showed that the slightly higher activity of acetyl cholinesterase of muscle and heart in treatment HFD+CN500, 1000 and 1500 mg/kg compare to HFD and ND CONTROL groups. Similarly, de Nigris et al. [68] showed that regular pomegranate juice and pomegranate fruit extract were fed obese Zucker rats results indicated that acetylcholine (Ach) induced relaxation responses elevated compare to seed oil supplementation. Lau et al. [69] reported that oral administration of a methanol leaves extract of C. nutans increased acetyl cholinesterase activity in male normal mice. The observed elevation in acetyl cholinesterase activity might be due to the loss of fat in adipose tissue [70]. Nevertheless, in mice, an increase in acetylcholinesterase activity may be regarded as a general biomarker of plant secondary compound like polyphenol, flavonoids and alkaloids, which work as a natural antioxidant and anti acetylcholinesterase activity [69,71]. However, there was no significant difference seen in the activity of the heart AchE activity between treatments and control group (P>0.05). Meanwhile, all the plant extracts contained some level of inhibitory activity against acetylcholinesterase AchE and butylcholinesterase (BchE) [72,73]. Hence, it may be deduced from the current investigations that crude methanolic leaves extract of C. nutans leaf can be suitable sources of anti-cholinesterase and antioxidant compounds.
Conclusion
Despite the similar lipid profile in both groups, the changes in adipose cellularity and acetylcholinesterase activity in mice given C. nutans suggest that the polyphenols in C. nutans enabled the mice to cope with oxidative stress. The lower size of adipocytes in the adipose tissue of mice supplemented with C. nutans might due to the decrease in leptin and Interleukin IL-6 secretion and increase acetylcholinesterase activity. Accordingly, the results of this study also suggest that a weight loss agent did not cause any side effects. The best concentration was 1000 mg/kg BW for obese mice. These results could be extrapolated to obese humans, as has been done in the case of many obese animal models. Based on this, C. nutans might have beneficial effects against obesity.
Recommendations
The fractions of the plant extract might be repeated several times to isolate sufficient amounts of the plant secondary compound in order to test their bioactivities on the experimental animals.
Future surveys should concentrate on how to swap the herbs effectively from the in vivo animal models to in vitro cell line models, which can establish the adipose tissue efficiency of the plants.
Acknowledgment
The authors are very grateful to the Faculty of Veterinary Medicine and Faculty of Agriculture, Universiti Putra Malaysia.
References
- Ding L, Li J, Song B, Xiao X, Huang W, et al. (2014) Andrographolide prevents high-fat diet–induced obesity in C57BL/6 mice by suppressing the sterol regulatory element-binding protein pathway. J PharmacolExpTher 351: 474-483.
- Low S, Chin MC, Deurenberg-Yap M (2009) Review on epidemic of obesity. Ann Acad Med Singapore 38: 57-59.
- Kopelman PG (2000) Obesity as a medical problem. Nature 404: 635-643.
- Ahmad SI, Imam SK (2015) Obesity: A practical guide. Springer.
- Jeffery RW, Sherwood NE (2008) Is the obesity epidemic exaggerated? No. BMJ 336: 245.
- World Health Organization (2014) Global status report on non-communicable diseases. WHO, Geneva.
- World Health Organization (2007) Cancer control: Knowledge into action: WHO guide for effective programmes. WHO, Geneva.
- Amano SU (2013) Local macrophage proliferation in adipose tissue is a characteristic of obesity-associated inflammation: A dissertation. University of Massachusetts Medical School, USA.
- Zawawi N (2011) Effect of Strobilanthescrispus extract and individual polyphenols on lipolysis. University of Nottingham, USA.
- Chuang CC (2012) Grape polyphenols attenuate inflammation and insulin resistance in human adipocytes and obese mice.
- Dandona P, Aljada A, Bandyopadhyay A (2004) Inflammation: The link between insulin resistance, obesity and diabetes. Trends in Immunology 25: 4-7.
- Rudelle S, Ferruzzi MG, Cristiani I, Moulin J, Mac K, et al. (2007) Effect of thermogenic beverage on 24 hour energy metabolism in humans. Obesity 15: 349-355.
- MohdEffendy N, Mohamed N, Muhammad N, Naina MI, Shuid AN (2012) Eurycomalongifolia: Medicinal plant in the prevention and treatment of male osteoporosis due to androgen deficiency. Evidence-Based Complementary and Alternative Medicine 2012: 9.
- Wang J, Feng B, Xiong X (2013) Chinese herbal medicine for the treatment of obesity-related hypertension. Evidence-Based Complementary and Alternative Medicine 2013: 11.
- Bray GA (2000) A concise review on the therapeutics of obesity. Nutrition 16: 953-960.
- Kovacs EMR, Mela DJ (2006) Metabolically active functional food ingredients for weight control. Obesity Reviews 7: 59-78.
- Gurib-Fakim A (2006) Medicinal plants: Traditions of yesterday and drugs of tomorrow. Molecular Aspects of Medicine 27: 1-93.
- Kennedy DO, Wightman EL (2011) Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. AdvNutr 2: 32-50.
- Nakazato K, Song H, Waga T (2006) Effects of dietary apple polyphenol on adipose tissues weights in Wistar rats. Experimental Animals 55: 383-389.
- Boque N, Iglesia R, Garza AL, Milagro FI, Olivares M, et al. (2013) Prevention of diet induced obesity by apple polyphenols in Wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. MolNutr Food Res 57: 1473-1478.
- Aseervatham GS, Sivasudha T, Jeyadevi R, Ananth DA (2013) Environmental factors and unhealthy lifestyle influence oxidative stress in humans an overview. Environ SciPollut Res Int 20: 4356-4369.
- Barnes PM, Powell-Griner E, McFann K, Nahin RL (2004) Complementary and alternative medicine use among adults: United States, 2002. In seminars in integrative medicine, WB Saunders, USA, 2: 54-71.
- Kao YH, Hiipakka RA, Liao S (2000) Modulation of endocrine systems and food intake by green tea epigallocatechingallate 1. Endocrinology 141: 980-987.
- Cho AS, Jeon SM, Kim MJ, Yeo J, Seo KI (2010) Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food ChemToxicol 48: 937-943.
- Montelius C, Erlandsson D, Vitija E, Stenblom EL, Egecioglu EA (2014) Body weight loss, reduced urge for palatable food and increased release of GLP-1 through daily supplementation with green-plant membranes for three months in overweight women. Appetite 81: 295-304.
- Candiracci M, Justo ML, Castao A, Rodriguez-Rodriguez R, Herrera MD (2014) Rice bran enzymatic extractsupplemented diets modulate adipose tissue inflammation markers in Zucker rats. Nutrition 30: 466-472.
- Hasani-Ranjbar S, Larijani B, Abdollahi MA (2009) Systematic review of the potential herbal sources of future drugs effective in oxidant-related diseases. Inflamm Allergy Drug Targets 8: 2-10.
- Roosita K, Kusharto CM, Sekiyama M, Fachrurozi Y, Ohtsuka R (2008) Medicinal plants used by the villagers of a Sundanese community in West Java, Indonesia. J Ethnopharmacol 115: 72-81.
- Sarega N, Imam MU, Ooi DJ, Chan KW, MdEsa N (2016) Phenolic rich extract from clinacanthusnutans attenuates hyperlipidemia-associated oxidative stress in rats. Oxidative med cellular longevity 2016: 16.
- Tu SF, Liu RH, Cheng YB, Hsu YM, Du YC, et al. (2014) Chemical constituents and bioactivities of Clinacanthusnutans aerial parts. Molecules 19: 20382-20390.
- Alam A, Ferdosh S, Ghafoor K, Hakim A, Juraimi AS, et al. (2016) Clinacanthusnutans: A review of the medicinal uses, pharmacology and phytochemistry. Asian Pac J Trop Med 9: 402-409.
- Sung YY, Yoon T, Yang WK, Kim SJ, Kim DS, et al. (2013) The antiobesity effect of Polygonumaviculare L. ethanol extract in high-fat diet-induced obese mice. Evid Based Complement Alternat Med 2013: 626397.
- Khan S, Khan A, Khattak FS, Naseem A (2012) An accurate and cost effective approach to blood cell count. Int J Computer Applications 50: 1.
- Teh CC, Khoo ZY, Khursiah F, Rao NK, Chin JH (2010) Acetylcholinesterase inhibitory activity of star fruit and its effect on serum lipid profiles in rats. Int Food Res J 17: 987-994.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. BiochemPharmacol 7: 88-95.
- Keppel G (1991) Design and analysis: A researcher's handbook. Prentice-Hall, Inc.
- Bewick V, Cheek L, Ball J (2004) Statistics review 9: One-way analysis of variance. Critical care 8: 130-136.
- Wu T, Qi X, Liu Y, Guo J, Zhu R, et al. (2013) Dietary supplementation with purified mulberry (MorusaustralisPoir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food chemistry 141: 482-487.
- Prior RLE, Wilkes SR, Rogers T, Khanal RC, Wu X, et al. (2010) Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J Agric Food Chem 58: 3970-3976.
- van der Heijden RA, Morrison MC, Sheedfar F, Mulder P, Schreurs M, et al. (2016) Effects of anthocyanin and flavanol compounds on lipid metabolism and adipose tissue associated systemic inflammation in diet-induced obesity. Mediators Inflamm 2016: 10.
- Zarrouki B, Pillon NJ, Kalbacher E, Soula HA, N'jomen GN, et al. (2010) Cirsimarin, a potent antilipogenic flavonoid, decreases fat deposition in mice intra-abdominal adipose tissue. Int J Obes (Lond) 34: 1566-1575.
- Heinonen S, Saarinen L, Naukkarinen J, Rodríguez A, Frühbeck G, et al. (2014) Adipocyte morphology and implications for metabolic derangements in acquired obesity. Int J Obes (Lond) 38: 1423-1431.
- Brodie BB, Butler WM, Horning MG, Maickel RP, Maling HM (1961) Alcohol-induced triglyceride deposition in liver through derangement of fat transport. Am J Clinical Nutr 9: 432-435.
- Norata GD, Pirillo A, Catapano AL (2006) Modified HDL: Biological and physiopathological consequences. Nutrition, metabolism and cardiovascular diseases 16: 371-386.
- Aprikian O, Duclos V, Guyot S, Besson C, Manach C, et al. (2003) Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats. J nutr 133: 1860-1865.
- Sekar M, Rashid NA (2016) Formulation, evaluation and antibacterial properties of herbal ointment containing methanolic extract of Clinacanthusnutans leaves. Int J Pharm Clin Res 8: 1170-1174.
- Sano M, Takenaka Y, Kojima R, Saito S, Tomita I, et al. (1986) Effects of Pu-Erh tea on lipid metabolism in rats. Chem Pharm Bull (Tokyo) 34: 221–228.
- AnandhBabu PV, Sabitha KE, Shyamaladevi CS (2006) Green tea extract impedes dyslipidaemia and development of cardiac dysfunction in streptozotocinâ┬?┬Édiabetic rats. ClinExpPharmacolPhysiol 33: 1184-1189.
- Ikeda I, Imasato Y, Sasaki E, Nakayama M, Nagao H, et al. (1992) Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats. BiochimBiophysActa 1127: 141-146.
- Holst B, Williamson G (2008) Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. CurrOpinBiotechnol 19: 73-82.
- Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116: 1793-1801.
- Wallenius V, Wallenius K, Ahrén B, Rudling M, Carlsten H, et al. (2002) Interleukin-6-deficient mice develop mature-onset obesity. Nature medicine 8: 75.
- Xie C, Kang J, Ferguson ME, Nagarajan S, Badger TM, et al. (2011) Blueberries reduce proâ┬?┬Éinflammatory cytokine TNFâ┬?┬Éα and ILâ┬?┬É6 production in mouse macrophages by inhibiting NFâ┬?┬ÉκB activation and the MAPK pathway. MolNutr Food Res 55: 1587-1591.
- Huang D, Li Y, Cui F, Chen J, Sun J (2016) Purification and characterization of a novel polysaccharide–peptide complex from ClinacanthusnutansLindau leaves. CarbohydrPolym 137: 701-708.
- Karlsen A, Retterstol L, Laake P, Paur I, Kjolsrud-Bøhn S, et al. (2007) Anthocyanins inhibit nuclear factor-κB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J Nutr 137: 1951-1954.
- Morrison M, van der Heijden R, Heeringa P, Kaijzel E, Verschuren L, et al. (2014) Epicatechin attenuates atherosclerosis and exerts anti-inflammatory effects on diet-induced human-CRP and NFκB in vivo. Atherosclerosis 233: 149-156.
- de Melo CL, Queiroz MG, Fonseca SG, Bizerra AM, Lemos TL, et al. (2010) Oleanolic acid, a natural triterpenoid improves blood glucose tolerance in normal mice and ameliorates visceral obesity in mice fed a high-fat diet. ChemBiol Interact 185: 59-65.
- Marquez-Martin A, De La Puerta R, Fernandez-Arche A, Ruiz-Gutierrez V, Yaqoob P (2006) Modulation of cytokine secretion by pentacyclictriterpenes from olive pomace oil in human mononuclear cells. Cytokine 36: 211-217.
- Jang EM, Choi MS, Jung UJ, Kim MJ, Kim HJ, et al. (2008) Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat–fed hamsters. Metabolism 57: 1576-1583.
- Haslam DW, James WPS (2005) Obesity, Lancet. Lancet 366: 1197-1209.
- Graf D, Seifert S, Jaudszus A, Bub A, Watzl B (2013) Anthocyanin-rich juice lowers serum cholesterol, leptin, and resistin and improves plasma fatty acid composition in fischer rats. PloS one 8: e66690.
- Kohnke R, Lindqvist A, Goransson N, Emek SC, Albertsson PA, et al. (2009) Thylakoids suppress appetite by increasing cholecystokinin resulting in lower food intake and body weight in highâ┬?┬Éfat fed mice. Phytotherapy Research 23: 1778-1783.
- Sakdarat S, Shuyprom A, Pientong C, Ekalaksananan T, Thongchai S (2009) Bioactive constituents from the leaves of ClinacanthusnutansLindau. Bioorg Med Chem 17: 1857-1860.
- Yamada T, Katagiri H, Ishigaki Y, Ogihara T, Imai J, et al. (2006) Signals from intra-abdominal fat modulate insulin and leptin sensitivity through different mechanisms: Neuronal involvement in food-intake regulation. Cell metabolism 3: 223-229.
- Tian Y, Zou B, Yang L, Xu SF, Yang J, et al. (2011) High molecular weight persimmon tannin ameliorates cognition deficits and attenuates oxidative damage in senescent mice induced by D-galactose. Food ChemToxicol 49: 1728-1736.
- Levin BE, Dunn-Meynell AA, Routh VH (1999) Brain glucose sensing and body energy homeostasis: Role in obesity and diabetes. A Am J Physiol 276: R1223-R1231.
- de Nigris F, Balestrieri ML, Williams-Ignarro S, D’Armiento FP, Fiorito C, et al.(2007) The influence of pomegranate fruit extract in comparison to regular pomegranate juice and seed oil on nitric oxide and arterial function in obese Zucker rats. Nitric oxide 17: 50-54.
- Lau KW, Lee SK, Chin JH (2014) Effect of the methanol leaves extract of Clinacanthusnutans on the activity of acetylcholinesterase in male mice. J Acute Disease 3: 22-25.
- Cazzola R, Rondanelli M, Russo-Volpe S, Ferrari E, Cestaro B (2004) Decreased membrane fluidity and altered susceptibility to peroxidation and lipid composition in overweight and obese female erythrocytes. J Lipid Res 45: 1846-1851.
- Bilal S, Khan AL, Waqas M, Shahzad R, Kim ID (2016) Biochemical constituents and in vitro antioxidant and anticholinesterase potential of seeds from Native Korean persimmon genotypes. Molecules 21: 893.
- Kamal AH, Komatsu S (2015) Involvement of reactive oxygen species and mitochondrial proteins in biophoton emission in roots of soybean plants under flooding stress. J Proteome Res 14: 2219-2236.
- Shah S, Shah SM, Ahmad Z, Yaseen M, Shah R, et al. (2015) Phytochemicals, in vitro antioxidant, total phenolic contents and phytotoxic activity of Cornusmacrophylla Wall bark collected from the North-West of Pakistan. Pak J Pharm Sci 28: 23-28.
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 4276
- [From(publication date):
April-2017 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 3372
- PDF downloads : 904