Methamphetamine Use during Pregnancy, and Early Infant Development Using the Ages and Stages Questionnaire (ASQ-3) Assessment
Received: 03-Sep-2019 / Accepted Date: 04-Oct-2019 / Published Date: 11-Oct-2019
Abstract
Introduction: Methamphetamine use puts the woman at risk of disrupted parenting, infant removal and potential for poorer infant outcomes including preterm birth, low birth weight, congenital anomalies and neurodevelopmental impairments that persist into adulthood. Early identification in infants of risk factors related to methamphetamine exposure will facilitate timely and appropriate interventions during this critical developmental period. These risk factors for the infant include concerns such as exposure to methamphetamine and other drugs in utero, tobacco smoking, poorer socioeconomic factors, separation after birth, exposure to high maternal stress during pregnancy, and trauma. The evidence is clear that early detection and intervention results in improved long term outcomes.
Method: 115 pregnant women from 220 using Methamphetamine were recruited from July to December 2017 and were administered a structured questionnaire about their drug and alcohol use during each trimester of pregnancy. Basic demographic data on maternal and infant details were collected. The ages and stages questionnaire was administered at 4 and 12 months and included age-specific questions to assess infants. Written informed consent was obtained from all women prior to participation.
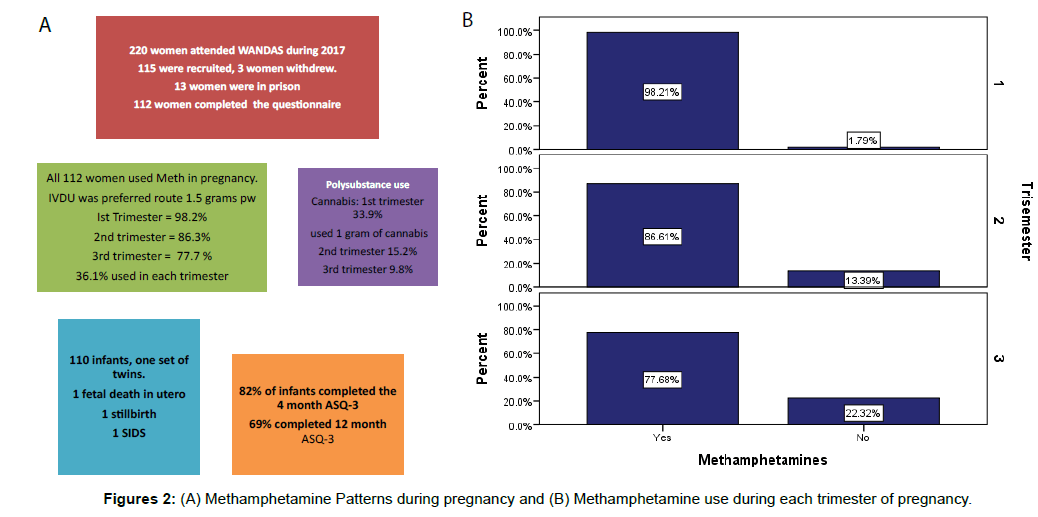
Results: 112 women completed the study. The majority (93%) of women were unemployed, used one gram of methamphetamine sometimes daily (50.9%) used it intravenously (67.9%) and smoked more than 10 cigarettes (tobacco) per day (87.5%). Polysubstance use was common (17.9%). Involvement with child protection and family support services was common throughout pregnancy and the postpartum period (53%). The social workers in the team manage child protection with the Department of Child Protection and Family Support (CPFS). They assess the protective factors of the parents and the risk factors associated with maternal drug use. Methamphetamine initiation occurred at a mean age of 13 years. During pregnancy, some women reported that they had overdosed as a result of their MA use (15.4%). Ages and Stages Questionnaire (ASQ-3) were completed on 82% (n=89) of infants at four months and 69% (n=75) at 12 months. Infants who scored in the problem range of at least one developmental area at four months accounted for 39.3% of the infants assessed compared to 49.3% at 12 months.
Conclusion: Our findings provide valuable insights regarding the use of methamphetamine in pregnancy. They highlight the complex needs of pregnant methamphetamine using women. Around 50% of infants were of concern in at least one developmental area suggesting that surveillance should be included in routine practice.
Keywords: Methamphetamine; Ages and stages screening; High-risk populations; Women and newborn drug and alcohol service
Introduction
In Australia during 2016-17, there were 47915 children were removed from maternal care because of drug and alcohol-related concerns [1], with Alcohol and Other Drugs (AOD) use also associated with high rates of child maltreatment [2]. The Adverse Childhood Experience Study (ACES) provided a comprehensive body of research that has shown the many linkages between childhood adversity and negative outcomes later in life [3-6] following maternal substance abuse and family and domestic violence [4,7,8]. The foundations of an infants’ early environment and experiences shape their brain development through their interactions of biological and psychosocial influences [6,9,10]. Early assessment of risk factors such as maternal mental health, psychosis and socio-economic disadvantage for infants prenatally exposed to methamphetamine, may impact on optimal neurological development for the infant across the lifespan [11,12].
There is a substantial and growing body of research that identifies many inter-related risk factors for women and infants exposed to Methamphetamine (MA) during pregnancy [8,13,14]. These risk factors for the infant include concerns such as exposure to methamphetamine and other drugs in utero [13,15], tobacco smoking, poorer socioeconomic factors [16,17], separation after birth [18,19], exposure to high maternal stress during pregnancy, and trauma [20,21]. There is a 90% chance that infants and young children will experience delays in their language or social-emotional development if they are exposed to six risk factors in early childhood including substance use, poverty, family and domestic violence, single parent, poor nutrition, maltreatment or a mentally ill caregiver [3,6,19,22]. The evidence is clear that early detection and intervention including early access to antenatal care and support for maternal mental health and psychosocial support including referral to drug counselling and rehabilitation and intensive family support results in improved long term outcomes [23- 27]. There is considerable public health concern about the effects and impact of prenatal exposure to Methamphetamine (MA) in Western Australia (WA) [28]. According to the Australian National Drug Strategy Household Survey, in 2016 Methamphetamine (MA) became the drug of most serious concern to the general community because of its purity, price and availability [29] overtaking alcohol use.
The aim of this paper is to highlight the results of regular Methamphetamine (MA) using pregnant women attending Women and Newborn Drug and Alcohol Service (WANDAS) in order to prioritise early identification of infants at risk during the critically important first year of life.
Methods
The Women and Newborn Drug and Alcohol Service (WANDAS) is the state-wide referral centre for pregnant women with complex drug and alcohol use. WANDAS is a multi-disciplinary midwiferyled service which provides antenatal and postnatal care for pregnant women and infants, by specialized obstetricians, midwives, social workers, neonatologists, and psychiatric treatment and addiction specialist support during pregnancy and follows up of infants up to 3 months post-birth. Early access to antenatal care is seen as a priority and the women are seen every 2 weeks during pregnancy and weekly from 36 weeks until the birth of the infant.
Procedure
A structured questionnaire with tailored questions [29] was administered to 112 women with MA use in pregnancy and who consented to the study attending a single state-wide Women and Newborn Drug and Alcohol Service (WANDAS). Sampling was purposeful and chosen because all women who consented to the study were asked to participate, it is a very common method for difficultto- reach and drug-using populations. The main goal of purposive sampling is to focus on particular characteristics of a population that are of interest, which will best answer the research questions [30].
Eligible participants were aged 18 years to 45 years; had used any methamphetamine during pregnancy via any route of administration. Exclusion criteria were an intellectual disability, significant mental health issues affecting competence to understand and provide consent, and current treatment with methadone or buprenorphine for opiate dependence. Recruitment took place between July 2015 and December 2016. Women who had been accepted for antenatal care with WANDAS and identified MA as being their primary drug of choice were approached to participate. Signed informed consent was sought prior to involvement. A structured questionnaire was used from the 2010 National Drug and Alcohol Survey (DAS) [31], with tailored questions covering demographics history, past and current drug use patterns focusing particularly on patterns of MA use, motivations for MA use; mental health status; support services involved regarding use of MA and other substances (Questionnaire: Appendix 1).
Infants were assessed using the ASQ-3 assessment at 4 and 12 months when they returned for follow up [32]. A midwife blinded to MA drug history explained the questionnaire to parents or caregiver and assisted them in filling it out (Appendix 2). The ASQ-3 includes social competencies, including behaviours that when absent may indicate the presence of developmental outcomes requiring referral to specialist services [32]. The ASQ is a parent-completed or caregiver screening tool. It contains 30 developmental items organised into five domains: communication, gross motor, fine motor, problem-solving and personalsocial. The response choices for each item are “yes”, “sometimes” or “not yet”, which are scored as 10, for “yes”, 5 “for sometimes” and 0 for “not yet” respectively. The test is scored according to the domain tested and compared with an empirically derived screening cut-off score defined as >2.0 Standard Deviations (SD) below the mean [33]. The sensitivity of the ASQ (3rd edition) from 4 months to 5 years of age is 70–90% and specificity are 76–91% [34]. This measure has been validated against a number of standardised measures [12,32,35].
Data analysis
Descriptive statistics were calculated to describe the study sample and to provide summaries of the cohorts. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Western Australia [36]. Using the Australian Bureau of Statistics’ Index of Relative concept of Socio-economic Advantage and Disadvantage [16], the score corresponding to each mother’s address was determined. The Australian Bureau of Statistics Socio-Economic Indexes of Areas (SEIFA) data was drawn on as an indicator of the level of family disadvantage. The Australia-wide mean for the Index is 1000, with a score lower than this indicating relative disadvantage. Maternal methamphetamine use was classified: 1 gram of MA=10 points mild, (0-2 points per day) moderate (5 points a day) or heavy (10 or above points per day) during pregnancy.
The primary outcome measures were the scores on the ASQ- 3, which are age-adjusted numerical scores in multiple domains of functioning and which are used to place the infant in one of three categories: (i) meets developmental milestones, (ii) reason for concern, (iii) below expected milestones. ASQ-3 responses for each of the five domains (communication, fine motor, gross motor, and personal social and problem-solving skills) were converted to “pass”, “borderline” or “fail”, and total ASQ score was divided by five to give the mean. Statistical analyses were performed using SPSS statistical software (version 22.0, IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp). All hypotheses testing was two-sided and p-values <0.05 were considered statistically significant.
Results
Maternal characteristics
112 pregnant women participated in the study and gave birth to 113 infants. (Table 1) displays demographic information. The average age for the women in this study was 28.9 years at the time of the first antenatal visit. The women ranged from 17 to 41 years old. MA and other drug use initiation occurred at a mean age of 13 and the mean age of polysubstance was 16 years. This was the first pregnancy for 16% (n=18) of our cohort. The mean number of previous pregnancies was four. Family and domestic violence refers to violence, abuse and intimidation of women by a current or past intimate relationship [24] were high in our study with 86.6% (N=97) having experienced this.
| Maternal and Neonatal Data | Number (%)/mean (SD) | Followed up to 12 months | Lost to follow-up |
|---|---|---|---|
| Mothers n=112 | - | n=65 | n=44 |
| Age (yrs) | 29.6 (5.5) | 32.5 (5.7) | 31.9 (5.3) |
| Ethnicity | - | - | - |
| Caucasian | 50 (44.6 %) | 32 (50.0 %) | 17 (38.6%) |
| Aboriginal | 59 (52.7 %) | 32 (50.0 %) | 24 (54.5 %) |
| Other | 3 (2.7%) | 0 (0.0%) | 3 (6.8%) |
| Accommodation | - | - | - |
| Stable housing | 72 (64.3 %) | 44 (68.8 %) | 25 (56.8 %) |
| Homeless or staying in refuge | 27 (24.1 %) | 13 (20.3 %) | 13 (29.5 %) |
| Prison | 13 (11.6 %) | 7 (53.8 %) | 6 (46.2 %) |
| Index of relative socioeconomic advantage and disadvantage | 979.2 (72.1) | 992.2 (76.0) | 966.4 (62.3) |
| Methamphetamine use | - | - | - |
| Mild | 28 (25.0 %) | 17 (25.9 %) | 10 (22.9 %) |
| Moderate | 57 (50.9 %) | 32 (47.8 %) | 25 (61.0 %) |
| Heavy | 27 (24.1 %) | 16 (39.0%) | 9 (20.1 %) |
| Route of methamphetamine use | - | - | - |
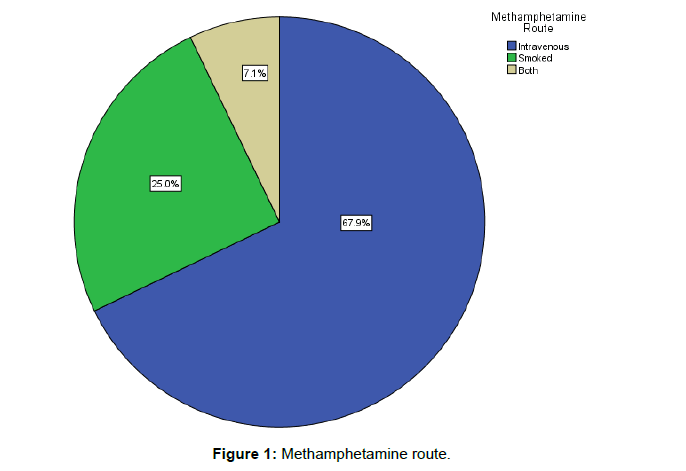
| Intravenous | 76 (67.9 %) | 44 (68.8%) | 30 (66.7 %) |
| Smoked | 28 (25.0 %) | 17 (25.4 %) | 11 24.4 %) |
| Both | 8 (7.1 %) | 4 (6.0 %) | 4 (8.9 %) |
| Temporal pattern of methamphetamine use | - | - | - |
| Cut down or ceased | 69 (63.9 %) | 42 (65.5 %) | 25 (61.0 %) |
| Sustained | 39 (36.1 %) | 22 (34.4 %) | 16 (39.0 %) |
| Alcohol during pregnancy | - | - | - |
| Yes | 34 (30.4 %) | 47 (70.1 %) | 29 (64.4%) |
| No | 76 (67.9 %) | 20 (29.9 %) | 14 (31.1 %) |
| Smoked during pregnancy | - | - | - |
| Yes | 98 (87.5 %) | 54 (84.4 %) | 40 (90.9 %) |
| No | 14 (12.5%) | 10 (15.6 %) | 4 (9.1 %) |
| Polysubstance use | - | - | - |
| Used ≥ 3 drugs (not including prescribed medications) | 20 (17.9 %) | 12 (18.7 %) | 8 (18.2 %) |
| Used < 3 drugs | 92 (82.1 %) | 52 (81.3 %) | 36 (81.8 %) |
| Infants n=110 | - | n=65 | n=44 |
| Sex | - | - | - |
| Male | - | 34(51%) | 26 (62%) |
| Female | - | 33 (49%) | 16 (38%) |
| Gestation | - | 37+6 (1.8) | 37+6(1.5) |
| Term/Preterm | 48 (73.8%)/17(26.2%) | 83 (75.5%)/27 (24.5%) | 34 (77.3%)/10(22.7%) |
| Birth growth parameter centile | - | - | - |
| Weight | 29th | 31st | 27th |
| Head circumference | 31.5th | 38th | 24.5th |
| Length | 29th | 31st | 21.5th |
| Birth Weight Group | - | - | - |
| Small for gestational age | 26(23.6%) | 13(20.0%) | 12(27.3%) |
| Appropriate for gestational age | 77(70.0%) | 51(78.5%) | 26(59.1%) |
| Large for Gestation | 7(6.4%) | 1 (1.5%) | 6(13.6%) |
| Child protection & family support | - | - | - |
| No involvement | 18 (16.5%) | 11(16.9%) | 7 (15.9%) |
| Involved but child remained in maternal custody | 58 (53%) | 34(52.3%) | 24(54.5%) |
| Child taken into CPFS custody | 33 (30.3%) | 20 (30.8%) | 13 (29.5%) |
Table 1: Demographic characteristics of the participants.
Of the women enrolled in our study 52.7% (n=59) identified that they were aboriginal, 44.6% (n=50) of the women identified as being caucasian and 2.7% were of an ethnic background other than caucasian or aboriginal. Specifically, these women were Maori, Fijian, and Polynesian. The majority (93%) were neither employed nor in education. Just over half (55%) lived in an area of low socioeconomic status with 24.1% (n=27) reporting homelessness and 18.3% (n=20) had a prison sentence.
Patterns of maternal drug use
The majority of the women 69.6%% (n=78) reported heavy MA use (used up to 1.5 grams) and 67.9% (n=76) (Figure 1), injected almost daily. Just over one-third of the women 36.1% (n=39) did not reduce their MA usage over the course of their pregnancy. They used methamphetamine throughout each trimester. The most common pattern was daily or binge (repeated use MA to maintain their high until supply runs out 1-3 days usual pattern) use with partners (70.6%). The remaining two thirds 59% (n=69) either reduced or stopped using (Figure 2). The women reported that they reduced their use from 10 points to 3 points during their second and third trimesters. The women were referred to drug rehabilitation (49.5%) and were provided with support from the drug and alcohol counsellors (78%), and psychological medicine to assist them with reducing their drug use. Polysubstance use accounted for 17.9% (n=20) of the mothers in that they reported regularly using more than two different drugs, (excluding prescribed medications). These drugs included benzodiazepine, cannabis and alcohol. In the first trimester 12.5% of women reported using benzodiazepine and (33.9%) reported using cannabis. They reduced their benzodiazepine (5.4%) and cannabis (15.2%) use in the second trimester. In the 3rd trimester the women reported benzodiazepine use of (2.7%) and cannabis use (9.8%). The majority of the women (87.5%, n=98) were tobacco smokers and alcohol consumption was reported as 27% (n=30) of the women. Ten women (8.9%) reported using alcohol in the first trimester at least 2-3 times per week, 4 women drank at least 4 times per week and consumed at least 1 litre of spirits (3.6%), the women who attended rehabilitation for alcohol use reduced and ceased during the last trimester (15.2%). The women primarily used MA with their partners 70.6% (n=77). They reported heavy use during the first trimester 27 women (24.1%), and 57 women (50.9%) reported moderate use during each trimester of pregnancy. They used MA to block pain 47.1% (n=49) or reduce stress 39.4% (n=41) from early childhood trauma which included psychological sexual and physical abuse. The women built up a tolerance to MA and 43% (n=46) of the women reported MA being the most important thing in their life and craved the drug. Many of those who reduced in pregnancy 21.7% (n=15), reported that they still felt the compulsion 20.2% (n=21) to use the drug and rehabilitation and counselling did little to reduce the cravings 24.1% (n=27). The women who completed rehabilitation were referred by the team for a 7-day drug detox unit which is a closed unit. Midwifery care was provided to them by WANDAS midwives.
The trigger for them to cease was the women noticed a change in their behaviour and their mental health and often had uncontrolled outburst with partners and family 34.3% (n=36). When the women were asked about rehabilitation 49.5% (n=54) attended support services, the majority of them wanted to be able to keep their baby and the possibility of child removal was a concern. Most of the women reported that they lived from day to day 38.5%, (n=42) and made no plans for their future. Drug court attendance, 21.1% (n=23) counselling 78% (n=85) and urine drug screening 39.4% (n=43) was a constant part of the women’s lives. The majority of the women described feeling stigmatized 76.9% (n=83) and guilty 55% (n=60) about their drug use and worried about their children if they were not around.
Neonatal characteristics
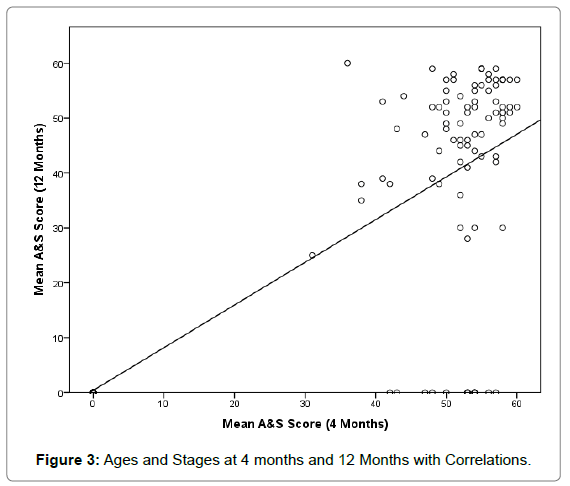
Of the live-born infants born to our study mothers, a quarter 24.8% (n=27) were born preterm (<37 weeks gestation at birth). There were 59 male infants 52.2% with 47.8% (n=54) female. There were two fetal deaths in utero, and one infant stillborn at 21 weeks following chorioamnionitis. The infants remained in hospital for 5 days post natal as per WANDAS guidelines in order to assess infant wellbeing and monitor for signs of drug withdrawal. Infants are assessed by a neonatologist daily while an inpatient and follow up to 3 months post-delivery. ASQ-3 screening of 82% (n=89) was completed on infants at four months and 69% (n=75) at 12 months (Table 2). Of those assessed 39.3% (35/89) of infants scored in the problem range of at least one developmental area at four months compared to 49.3% (n=37) at 12 months. A correlation between scores at four months was found r=0.79 (Figure 3). Thirteen infants 14.6% of infants scored in the problem range of socio-emotional concerns at four months compared to 25.3% (n=19) at 12 months (Table 2). The women lost to follow up were unable to be contacted; some carers for Child Protection and Family Support (CPFS) chose not to return the infant for follow up and were seen by a General Practitioner (GP). There was no statistically significant difference found between methamphetamine dose and birth weight, or head circumference during each trimester. The correlation between gestational age and MA dose; birth weight and meth dose was not significant. The only significant difference was found between apgar score and methamphetamine dose with the infants in our population having lower apgar scores 7 at 1 minute and 8 at 10 minutes (r=0.761). Infant who had been exposed to alcohol use during pregnancy are followed up by WANDAS and a referral pathway to child developmental service is available where the infant are assessed for Fetal Alcohol Spectrum Disorder (FASD) and therefore is not reported on in this paper.
| Domain | Fail, or borderline on 4 months ASQ (n=89) | Fail, or borderline on 12 months ASQ (n=75) |
|---|---|---|
| Gross motor | 19 (21.3%) | 18 (24%) |
| Fine motor | 8 (8.9%) | 11 (14.75%) |
| Communication/Language | 8 (9.0%) | 8 (10.7%) |
| Personal-Social | 7 (7.9%) | 8 (10.7%) |
| Problem solving and performance | 6 (6.7%) | 11 (14.7%) |
Table 2: Ages and stages (ASQ-3): Rates of developmental concerns on ASQ at 4 and 12 months.
Discussion
Our study findings highlight the complexities of MA use among pregnant women attending a specialist drug and alcohol service in Western Australia (WA). The women reported using MA as their primary drug of choice, their predominant pattern of use were daily up to 1.5 grams per week, mostly intravenously and combined with polysubstance use to manage their withdrawal symptoms; they had built up a tolerance to MA and found a strong compulsion to use despite the risk to themselves, their fetus and their newborn infant. Polysubstance use among our sample included alcohol, cannabis, tobacco smoking, and benzodiazepine, which is consistent with other Australian [37-39] and international [40,41] research involving MA. The frequent polysubstance use by our women placed additional risk in pregnancy with many requiring hospitalization for stabilization and early delivery. These admissions were for pregnancy-induced hypertension 28 (25.5%) of women were admitted for a period of a week at a time. Placenta praevia and ante-partum haemorrhage accounted for (12.7%) 14 women. Findings of our study are supported by other research which demonstrates that women who use illicit drugs suffer from a multitude of chronic life conditions [42,43]. These may include parental psychosocial risk factors socioeconomic disadvantage, psychiatric diagnosis violence and incarceration. We had an overrepresentation and removal of aboriginal children from families within our group which is a serious concern within Australia [44,45].
Many of the women reported a reduction in their MA use between the first and third trimester of pregnancy, suggesting recognition of potential harm for her unborn infant as the reason. This is consistent with past research [46,47]. A number of the women completed rehabilitation in an inpatient detox unit, attended counseling which was offered to all women. Thus, research indicates that pregnancy is a prime motivator to change and as such, the availability of services such as WANDAS which supports harm reduction strategies and stabilization to improve outcomes [48,49] are clearly needed. Currently, there is no substitution treatment for MA. The most common treatment is to use diazepam for acute withdrawal and add neuroleptics such as mirtazapine to reduce cravings and quetiapine to reduce cravings and manage withdrawal [50].
ASQ-3 was used for the first time in our population to explore the risk of developmental delay [51]. We found a correlation between scores at 4 and 12 months. Developmental vulnerability may be due to a low level of education, pregnancy complications, APH, unemployment, environmental risks, such as homelessness, lack of safety from family and domestic violence, complex trauma and incarceration disrupted parenting, infant removal and potential for poorer infant outcomes including preterm birth, low birth weight, congenital anomalies and the effect of the drug directly on the developing brain [1,4,39]. There is some controversy in the literature about the developmental outcomes of infants born after methamphetamine use with some studies suggesting developmental delays were present where a large controlled study found few significant delays suggesting that the effect seen in other studies may be more due to socioeconomic status [15,52]. However animal models and Magnetic Resonance Imaging (MRI) of the brain of infants born after antenatal methamphetamine use have shown changes on the developing brain [53-55].
A recent study on the ages and stages questionnaires found that systematic screening for developmental delays using the ASQ-3 doubled the detection of developmental delays among infants in maternal care and or foster care [56]. The ASQ-3 is a validated screening tool used to identify infants and young children including aboriginal infants who may need further evaluation for possible developmental delays [12,32]. The women attending WANDAS experience significant socioeconomic disadvantage and have poorer health outcomes, are more likely to be aboriginal, present later for antenatal care and have higher rates of drug use and smoke tobacco. ASQ-3 screening that involves engagement of women addressing their concerns in addition to utilizing standardized developmental screening tools and effective communication from a strength-based outcome is the key to improving outcomes. The women may not realize what services are available to address their issues, and stigma may prevent them from answering a direct face-valid question about infant concerns [57]. This is a view supported by early childhood researchers in other Indigenous contexts [12,58,59].
Limitations
This was a single-site study from a specialist drug and alcohol service. The women self-reported on their drug use as previous studies have demonstrated that in the context of antenatal care this is as reliable as drug testing. The stigma associated with illicit drug use during pregnancy may have made some women reluctant to discuss their drug use or under-report their drug use. The ASQ-3 was completed by parents or caregivers who were provided with detailed instructions however there may have been inconsistencies. Some families or caregivers were difficult to follow up and infant removal reduced the follow-up rates.
Conclusion
This paper highlights the complexities of addiction to MA in pregnancy not only for the women but for their infants. In order to identify the risks services should consider the routine screening of infants to identify early concerns and formulate developmental care plans. Long term follows up that provides integrated services with intensive family resources to assist vulnerable families and infants is essential to identify developmental concerns in the infant.
Acknowledgment
WANDAS received no funding for this research; therefore we would like to acknowledge the support of the WANDAS team, Child Protection and Family Support (CPFS) and the women attending WANDAS who consented to be part of this study.
Conflict of Interest
There is no conflict of interest from any author in this study.
Ethical Considerations
Ethics approval was granted from the Women and Newborn Health Service Ethics and Governance Committee, University of Western Australia Human Ethics Committee, Department of Child Protection Ethics Committee and the Western Australian Aboriginal Health Ethics Committee.
References
- Taplin S (2017) Prenatal reporting to child protection: Characteristics and service responses in one Australian jurisdiction. Child Abuse Negl 65: 68-76.
- https://www.aihw.gov.au/getmedia/677d394f-92e1-4ad5-92b4-c13951b88968/12222.pdf.aspx?inline=true
- Tilson EC (2018) Adverse Childhood Experiences (ACEs) An Important Element of a Comprehensive Approach to the Opioid Crisis. N C Med J 79: 166-169.
- Taylor MF, Marquis R, Coall D, Wilkinson C (2017) Substance Misuse-Related Parental Child Maltreatment. J Drug Issues 47: 241-260.
- Vearrier D, Greenberg MI, Miller SN, Okaneku JT, Haggerty DA (2012) Methamphetamine: History, pathophysiology, adverse health effects, current trends, and hazards associated with the clandestine manufacture of methamphetamine. Dis Mon 58: 38-89.
- Walker SP, Wachs TD, Grantham-Mcgregor S, Black MM, Nelson CA, et al. (2011) Inequality in early childhood: risk and protective factors for early child development. Lancet 378: 1325-1338.
- Shonkoff JP (2012) Leveraging the biology of adversity to address the roots of disparities in health and development. Proc Natl Acad Sci 109: 17302-17307.
- Wright TE, Schuetter R, Sauvage L (2014) Methamphetamines and Birth Outcomes. Obstet Gynecol 123: 178S.
- Hackman DA, Farah MJ (2009) Socioeconomic status and the developing brain. Trends Cogn Sci 13: 65-73.
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, et al. (2006) The enduring effects of abuse and related adverse experiences in childhood. Eur Arch Psychiatry Clin Neurosci 256: 174-186.
- Shonkoff J, Duncan G, Yoshikawa H, Fisher P, Guyer B, et al. (2010) The foundations of lifelong health are built in early childhood. Massachusetts: National Scientific Council on the Developing Child, Harvard University.
- D’Aprano A, Silburn S, Johnston V, Robinson G, Oberklaid F, et al. (2016) Adaptation of the ages and stages questionnaire for remote aboriginal Australia. Qual Health Res 26: 613-625.
- Wouldes TA, LaGasse LL, Derauf C, Newman E, Shah R, et al. (2013) Co-morbidity of substance use disorder and psychopathology in women who use methamphetamine during pregnancy in the US and New Zealand. Drug Alcohol Depend 127: 101-107.
- Smith LM, Paz MS, LaGasse LL, Derauf C, Newman E, et al. (2012) Maternal depression and prenatal exposure to methamphetamine: neurodevelopmental findings from the infant development, environment, and lifestyle (ideal) study. Depress Anxiety 29: 515-522.
- Smith LM, Diaz S, LaGasse LL, Wouldes T, Derauf C, et al. (2015) Developmental and behavioral consequences of prenatal methamphetamine exposure: a review of the infant development, environment, and lifestyle (IDEAL) study. Neurotoxicol Teratol 51: 35-44.
- Fernandes NC, Sriram U, Gofman L, Cenna JM, Ramirez SH, et al. (2016) Methamphetamine alters microglial immune function through P2X7R signaling. J Neuroinflamm 13.
- Rebbe R, Mienko JA, Brown E, Rowhani-Rahbar A (2019) Child protection reports and removals of infants diagnosed with prenatal substance exposure. Child Abuse Negl 88: 28-36.
- Tsantefski M, Humphreys C, Jackson AC (2014) Infant risk and safety in the context of maternal substance use. Child Youth Serv Rev 47: 10.
- Burnette CE, Renner LM (2017) A Pattern of Cumulative Disadvantage: Risk Factors for Violence across Indigenous Women’s Lives. Brit J Soc Work 47: 1166-1185.
- O'Reilly R, Beale B, Gillies D (2010) Screening and intervention for domestic violence during pregnancy care: a systematic review. Trauma Violence Abuse. 11: 190-201.
- Judd F, Newman LK, Komiti AA (2018) Time for a new zeitgeist in perinatal mental health. Aust N Z J Psychiatry 52: 112-116.
- Heckman J, Pinto R, Savelyev P (2013) Understanding the mechanisms through which an influential early childhood program boosted adult outcomes. Am Econ Rev 103: 2052-2086.
- Coomber K, Mayshak R, Liknaitzky P, Curtis A, Walker A, et al. (2019) The Role of Illicit Drug Use in Family and Domestic Violence in Australia. J Interpers Violence 886260519843288.
- Bowen A, Duncan V, Peacock S, Bowen R, Schwartz L, et al. (2014) Mood and anxiety problems in perinatal Indigenous women in Australia, New Zealand, Canada, and the United States: A critical review of the literature. Transcult Psychiatry 51: 93-111.
- Torchalla I, Linden IA, Strehlau V, Neilson EK, Krausz M (2015) "Like a lots happened with my whole childhood": violence, trauma, and addiction in pregnant and postpartum women from Vancouver's Downtown Eastside. Harm Reduct J 12.
- Poole N (2006) Doing It All: Developing Integrated Support for Women Experiencing Mental Health, Trauma-related and Substance Use Problems. Centres of Excellence for Women's Health Research Bulletin. 5: 21-22.
- Fetherston JaL S (2018) Western Australian Drug Trends 2017. Findings from the Illicit Drug Reporting System (IDRS). Australian Drug Trend Series No 187.
- https://www.aihw.gov.au/reports/illicit-use-of-drugs/2016-ndshs-detailed/contents/table-of-contents
- Sandelowski M, Voils CI (2013) Quantitative research findings. Routledge International Handbook of Qualitative Nursing Research 347.
- https://www.aihw.gov.au/reports/illicit-use-of-drugs/2010-ndshs/contents/table-of-contents
- Squires J, Bricker D, Potter L (1997) Revision of a parent-completed developmental screening tool: Ages and Stages Questionnaires. J Pediatr Psychol 22: 313-328.
- Yoong T, McKenzie A, Grace R (2015) Is the Australian Developmental Screening Test (ADST) a useful step following the completion of the Ages and Stages Questionnaire (ASQ) on the pathway to diagnostic assessment for young children? Australian J Child Fam Health Nursing 12: 4-8.
- Schonhaut L, Armijo I, Schönstedt M, Alvarez J, Cordero M (2013) Validity of the ages and stages questionnaires in term and preterm infants. Pediatrics 131: e1468-e1474.
- Singh A, Squires J, Yeh CJ, Heo KH, Bian H (2016) Validity and reliability of the developmental assessment screening scale. J Fam Med Prim Care 5: 124-128.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, et al. (2009) Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377-381.
- Courtney KE, Ray LA (2014) Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug and Alcohol Dependence 143: 11-21.
- Thomas N, Bull M (2018) Representations of women and drug use in policy: A critical policy analysis. Int J Drug Policy 56: 30-39.
- Wu M, LaGasse L, Wouldes T, Arria A, Wilcox T, et al. (2013) Predictors of Inadequate Prenatal Care in Methamphetamine-Using Mothers in New Zealand and the United States. Matern Child Health J 17: 566-575.
- Arria AM, Derauf C, LaGasse LL, Grant P, Shah R, et al. (2006) Methamphetamine and Other Substance Use During Pregnancy: Preliminary Estimates From the Infant Development, Environment, and Lifestyle (IDEAL) Study. Matern Child Health J 10: 293-302.
- Wouldes TA, LaGasse LL, Huestis MA, DellaGrotta S, Dansereau LM, et al. (2014) Prenatal methamphetamine exposure and neurodevelopmental outcomes in children from 1 to 3 years. Neurotoxicol Teratol 42: 77-84.
- Pinto SM, Dodd S, Walkinshaw SA, Siney C, Kakkar P, et al. (2010) Substance abuse during pregnancy: effect on pregnancy outcomes. Eur J Obstet Gynecol Reprod Biol 150: 137-141.
- Broadhurst K, Mason C (2013) Maternal outcasts: raising the profile of women who are vulnerable to successive, compulsory removals of their children – A plea for preventative action. J Soc Welfare Fam Law 35: 291-304.
- Marsh CA, Browne J, Taylor J, Davis D (2015) Guilty until proven innocent? – The Assumption of Care of a baby at birth. Women Birth 28: 65-70.
- McDowall JJ (2016) Connection to culture by indigenous children and young people in out-of-home care in Australia. Commun Children Fam Aust 10: 5-26.
- Poole N, Schmidt RA, Green C, Hemsing N (2016) Prevention of fetal alcohol spectrum disorder: current Canadian efforts and analysis of gaps. Subst Abuse 10: 1-11.
- Gyllstrom ME (2010) Maternal Mental Health and Substance Use: An Examination of their Role in Pregnancy Health Behaviors and Birth Outcomes. Ph.
- McNeil R, Kerr T, Pauly B, Wood E, Small W (2016) Advancing patientâ€centered care for structurally vulnerable drugâ€using populations: a qualitative study of the perspectives of people who use drugs regarding the potential integration of harm reduction interventions into hospitals. Addict 111: 685-694.
- Miles M, Francis K, Chapman Y (2010) Challenges for Midwives: Pregnant Women and Illicit Drug Use. Aust J Adv Nurs 28: 83-90.
- Rawson RA, Ling W, Mooney LJ (2015) Clinical management: Metamphetamine. In: Galanter M, Kleber HD, Brady KT (eds) Substance Abuse Treatment. 5th edn. American Psychiatric Association, Arlington, VA.
- O'Connor A, Seeber C, Harris E, Hamilton D, Sachmann M, et al. (2019) Developmental outcomes following prenatal exposure to methamphetamine: A Western Australian perspective. J Paediatr Child Health.
- Dyk JV, Ramanjam V, Church P, Koren G, Donald K (2014) Maternal methamphetamine use in pregnancy and long-term neurodevelopmental and behavioral deficits in children. J Popul Ther Clin Pharmacol 21: e185-e196.
- Derauf C, Kekatpure M, Neyzi N, Lester B, Kosofsky B (2009) Neuroimaging of children following prenatal drug exposure. Semin Cell Dev Biol 20: 441-454.
- Roos A, Jones G, Howells FM, Stein DJ, Donald KA (2014) Structural brain changes in prenatal methamphetamine-exposed children. Metab Brain Dis 29: 341-349.
- Gorman MC, Orme KS, Nguyen NT, Kent EJ, Caughey AB (2014) Outcomes in pregnancies complicated by methamphetamine use. Am J Obstet Gynecol 211: 429.e1-e7.
- Jee SH, Conn AM, Szilagyi PG, Blumkin A, Baldwin CD, et al. (2010) Identification of socialâ€emotional problems among young children in foster care. J Child Psychol Psychiat 51: 1351-1358.
- Williams ME, Zamora I, Akinsilo O, Chen AH, Poulsen MK(2018) Broad Developmental Screening Misses Young Children With Social-Emotional Needs. Clin Pediatr 57: 844-849.
- Guhn M, Goelman H (2011) Bioecological theory, early child development and the validation of the population-level early development instrument. Soc Indic Res 103: 193-217.
- Staal IIE, van Stel HF, Hermanns JMA, Schrijvers AJP (2016) Early detection of parenting and developmental problems in young children: Non-randomized comparison of visits to the well-baby clinic with or without a validated interview. Int J Nurs Stud 62: 1-10.
Citation: O’Connor A, Harris E, Hamilton D, Sachmann M, Fisher C (2019) Methamphetamine Use during Pregnancy, and Early Infant Development Using the Ages and Stages Questionnaire (ASQ-3) Assessment. J Addict Res Ther 10:391.
Copyright: © 2019 O’Connor A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3295
- [From(publication date): 0-2019 - Apr 29, 2025]
- Breakdown by view type
- HTML page views: 2452
- PDF downloads: 843