Short Communication Open Access
Metastable Intermediate Phase during Phase Transformation of Calcium Phosphates
Kazuo Onuma1* and Yuki Sugiura21National Institute of Advanced Industrial Science & Technology, Central 6, 1-1-1 Higashi, Tsukuba, Ibaraki 305-8566 Japan
2Department of Biomaterial, Faculty of Dental Science, Kyushu University, 3-1-1, Maidashi, Higashi, Fukuoka 812-0054 Japan
- Corresponding Author:
- Kazuo Onuma
National Institute of Advanced Industrial Science & Technology
Central 6, 1-1-1 Higashi, Tsukuba, Ibaraki 305-8566 Japan
Tel: +81-29-861-4832
E-mail: k.onuma@aist.go.jp
Received date: October 14, 2015; Accepted date: December 14, 2015; Published date: December 21, 2015
Citation: Onuma K, Sugiura Y (2015) Metastable Intermediate Phase during Phase Transformation of Calcium Phosphates. J Biotechnol Biomater 5:214. doi:10.4172/2155-952X.1000214
Copyright: © 2015 Onuma K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Phase transformation of calcium phosphates from amorphous to crystalline phases around neutral pH proceeds via direct structure conversion using non-ionic elementary units. This transformation inevitably forms metastable intermediate-structured phase(s) between the two end phases. In addition to conventional calcium phosphate phases appearing in the transformation, new unknown phases were observed. They did not correspond to simply poor crystalline materials of conventional phases and instead had particular structures. One was pseudo-OCP, which lacked the HPO4-OH layers in the conventional OCP structure.
Keywords
Phase transformation; Amorphous phase; Intermediate phase; OCP; HAP
Introduction
Calcium phosphates have been energetically investigated for more than half a century because of their important role not only in forming hard tissues such as bone and teeth but also being a frequent cause of calculus and arteriosclerosis [1,2]. One factor hindering a sound understanding of their formation mechanism is that they appear in several forms at room to body temperatures [3,4]. For example, amorphous calcium phosphate (ACP), dicalcium phosphate dihydrate (DCPD), β-tricalcium phosphate (β-TCP), octacalcium phosphate (OCP), and hydroxyapatite (HAP) form in weak acidic to weak basic calcium phosphate solutions. They precipitate and stably exist or transform into other more stable phases depending on the solution conditions. Transformation between each calcium phosphate [5-7] is of particular interest because the control of this process results in obtaining a desirable phase that can contribute to the design of calciumphosphate- based biomaterials with novel functions.
Two phase-transformation mechanisms have been proposed. One is solvent-mediated transformation, which is the simple dissolution and growth of materials depending on the difference in solubility. A representative example for calcium phosphate is the hydrolysis of DCPD and subsequent growth of OCP [8]. The other is structural conversion from metastable to stable phases as was revealed in situ using a light scattering technique [9,10]. If the concentrations of calcium and phosphate ions in the solution are sufficiently high, ACP first forms in the solution and transforms into other phases such as OCP and HAP. The transformation proceeds via direct conversion of an amorphous structure into crystalline ones. This kinetics is attributed to a common cluster unit present in both the amorphous and crystalline phases [11-15]. Posner's cluster [11] and its symmetric isomers [16] were proposed for this common cluster, and then more complex structures of clusters were found through experimental and computational investigations [17,18]. That the clusters can be decomposed into smaller ion pairs as the minimum unit was also proposed [19], indicating the need for more careful investigation of the elemental unit in the phase transformation of calcium phosphates.
Leaving aside the question about the structure of the minimum unit, we note that it is well accepted that phase transformation of calcium phosphates proceeds via non-ionic larger clusters (including ion pairs) as well as in calcium carbonate [20-22], which is another representative biomineral. One or more calcium phosphate phases among β-TCP, OCP, and HAP are known to appear during the transformation from ACP. This conventional scheme is, however, not sufficient to explain the transformation kinetics because it neglects the intermediate metastable phases that should appear during the transformation. These phases are naturally expected as long as the transformation proceeds via structural conversion from one stable phase to another stable phase. Several local free-energy minimums present during transformation between two phases, which suggests the formation of unknown calcium phosphates. Our recent investigations on this intermediate phase are presented in this short communication.
When the concentrations of calcium and phosphate ions were set to 5 mM in a weak acidic solution (pH = 6.5, 22ºC; reference solution), ACP immediately formed in the solution and transformed into OCP and subsequently precipitated within 2 h. However, when gold nanoparticles with immobilized carboxylic functional groups on their surface were added to the solution, less than a 1/1000 molar concentration of calcium or phosphate ions, an OCP-like intermediate phase precipitated prior to the formation of conventional OCP (experimental details are given elsewhere [23]).
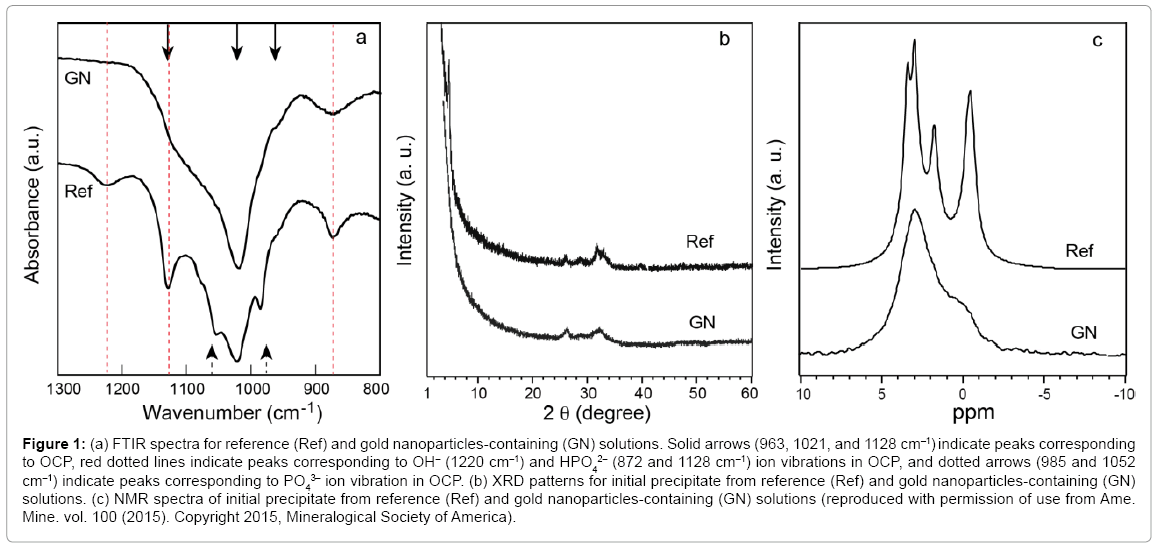
Figure 1a shows spectra obtained using in situ FTIR for the reference and gold nanoparticles-containing solutions before the initial precipitates were observed. Crystalline domains developed in the ACP. The spectra for both solutions showed typical absorption bands of OCP at 963, 1021, and 1128 cm–1, but the spectrum corresponding to the gold nanoparticles-containing solution (GN) lacked the band at 1220 cm–1, which corresponds to OH– vibration in the HPO4-OH layer of OCP crystal, although it was observed in the reference solution spectrum (Ref). The bands corresponding to HPO4 vibrations at 872 and 1128 cm–1 were also undeveloped in the GN spectrum.
Figure 1: (a) FTIR spectra for reference (Ref) and gold nanoparticles-containing (GN) solutions. Solid arrows (963, 1021, and 1128 cm–1) indicate peaks corresponding to OCP, red dotted lines indicate peaks corresponding to OH– (1220 cm–1) and HPO4 2– (872 and 1128 cm–1) ion vibrations in OCP, and dotted arrows (985 and 1052 cm–1) indicate peaks corresponding to PO4 3– ion vibration in OCP. (b) XRD patterns for initial precipitate from reference (Ref) and gold nanoparticles-containing (GN) solutions. (c) NMR spectra of initial precipitate from reference (Ref) and gold nanoparticles-containing (GN) solutions (reproduced with permission of use from Ame. Mine. vol. 100 (2015). Copyright 2015, Mineralogical Society of America).
The XRD pattern of the initial precipitate from the reference solution corresponded to that of OCP (Figure 1b-Ref) whereas that from the gold nanoparticles-containing solution (Figure 1b-GN) lacked the characteristic (100) diffraction of OCP (2O = 4.7°) although other peaks were observed. The 31P solid-sate NMR for the precipitate from the gold nanoparticles-containing solution (Figure 1c-GN) showed a broad main peak at 3.0 ppm (OCP P1 and P2/P4 sites) and shoulder peaks at 1.9 (OCP P3 site) and –0.4 ppm (OCP P5/P6 site), suggesting that the structure of this material was essentially similar to that of OCP.
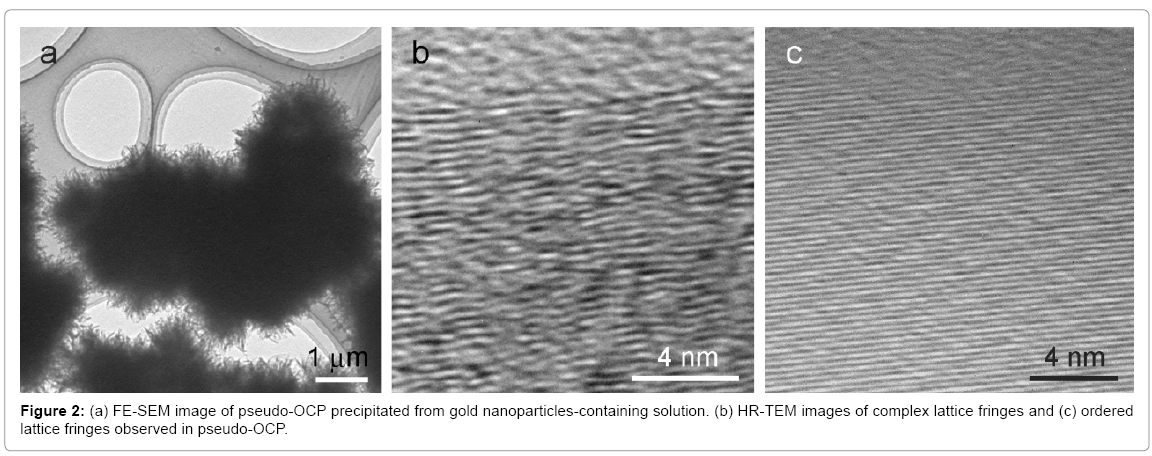
Figure 2a shows the macroscopic morphology of initial precipitates from gold nanoparticles-containing solution. HR-TEM images revealed the coexistence of complex lattice fringes (Figure 2b) and partially ordered fringes (Figure 2c) in the materials. We concluded from these findings that the material that initially precipitated in the gold nanoparticles-containing solution was not simply poor crystalline OCP but pseudo-OCP with a partially ordered structure. Since pseudo- OCP gradually changed to actual OCP with maturation in the solution, it was metastable intermediate phase. The OCP crystal structure along the a-axis consists of alternating apatite-like layers and HPO4-OH layers (corresponding to the center of hydrated layer in the previous OCP model) [8,24]. We simulated the XRD pattern based on the OCP structure and found that the lack of an HPO4-OH layer resulted in the XRD pattern of pseudo-OCP; i.e. disappearance of characteristic (100) diffraction. This model coincides well with the FTIR data. As described above, the appearance of an intermediate phase or phases is natural if the transformation from amorphous to crystalline phases proceeds via structural reconstruction using a common essential unit. Gold nanoparticles hindered formation of the particular structure in the crystalline phase during reconstruction, acting like a negative catalyst. The selection of the OCP or pseudo-OCP reaction route can be explained by the concept of pre-nucleation clusters and polymer induced liquid precursors (PILP) that the crystalline structure of each polymorph was controlled by the corresponding amorphous structure [25-29].
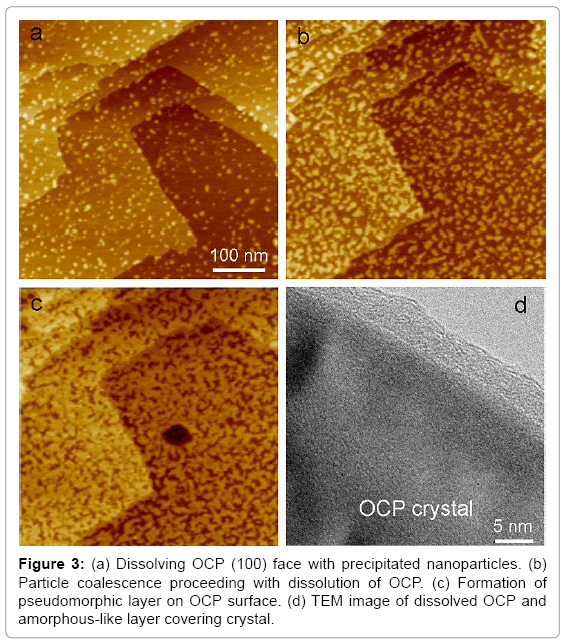
Phase transformation from OCP to HAP is an interesting topic because it relates to the formation mechanism of human tooth enamel [30]. Previous investigations using HR-TEM showed that HAP crystal epitaxially grew on the (100) face of OCP single crystal [30-33]. Owing to the structural resemblance between OCP and HAP crystals, oriented growth of HAP crystal on OCP has been thought to be a natural process. However, in situ AFM observations of OCP dissolution suggested the presence of an intermediate phase before the formation of HAP. When OCP single crystal was dissolved in pure or saline water (50 mM NaCl), dissolution steps and etch pits having the minimal step height (~2.0 nm) corresponding to the (100) orientation appeared on the surface. The synthesis of the OCP crystal and the AFM observation are explained elsewhere [34].
During the dissolution, nanoparticles (~10 nm) suddenly precipitated due to the local increase in calcium- and phosphate-ion concentrations close to the surface, and they coalesced with each other, similar to the process in a liquid-like material (Figures 3a,b). OCP dissolution slowed during nanoparticle precipitation, but it never stopped. The precipitated phase covered the OCP surface without changing the morphology of the substrate; i.e., a pseudomorphic relationship was observed between the precipitated phase and OCP (Figure 3c).
After the pseudomorphic layer formed, the new phase started dissolving at a much slower rate than the substrate OCP. This phase should have lower solubility than OCP, and the corresponding phase is HAP in the conventional model. The behavior of this phase was, however, amorphous-like, and TEM observation revealed a nanometer-thick amorphous-like layer around the OCP crystal (Figure 3d). Complex lattice fringes in this layer similar to those found in the pseudo-OCP were also observed. The precipitated material is though to be amorphous-like pseudo-HAP, the intermediate phase formed prior to crystalline HAP formation, although a detailed characterization is needed.
The findings presented here indicate the presence of unknown metastable intermediate phases not previously investigated in full. As shown, adding appropriate impurities or catalysts can control the lifetime of these phases.
Acknowledgements
We thank Dr. M. Nagao for helping with the TEM observations.
References
- Berkovitz BKB, Boyde A, Frank RM, Holing HJ, Moxham BJ, et al. (1989) Handbook of Microscopy Anatomy.Teeth, Springer-Verlag, Berlin Heidelberg.
- Weiner S, Dove PM (2003) An Overview of Biomineralization Processes and the problem of the Vital Effect. In:Biomineralization, Reviews in Mineralogy and Geochemistry.Dove PM, De Yoreo JJ, Weiner S (Eds.), Mineralogical Society of America, USA.
- Elliot JC (1994) Studies in Inorganic Chemistry 18: Structures and Chemistry of the Apatites and Other Calcium Orthophosphates, Elsevier, Amsterdam.
- Wang L1, Nancollas GH (2008) Calcium orthophosphates: crystallization and dissolution. Chem Rev 108: 4628-4669.
- Termine JD, Eanes ED (1972) Comparative chemistry of amorphous and apatitic calcium phosphate preparations. Calcif Tissue Res 10: 171-197.
- Eanes ED, Meyer JL (1977) The maturation of crystalline calcium phosphates in aqueous suspensions at physiologic pH.Calcif Tissue Res 23: 259-269.
- Meyer JL (1983) Phase transformation in the spontaneous precipitation of calcium phosphate. Croat Chem. Acta 56: 753-767.
- Mathew M, Brown WE, Schroeder LW, Dickens B (1988) Crystal structure of octacalciumbis(hydrogenphosphate) tetrakis(phosphate) pentahydrate, Ca8(HPO4)4•5H2O. J CrystallogSpectro Res 18: 235-250.
- Onuma K, Oyane A, Tsutsui K, Tanaka K, Treboux G, et al. (2000) Precipitation kinetics of hydroxyapatite revealed by the continuous-angle laser light-scattering technique. JPhysChem B 104: 10563-10568.
- Tsuji T, Onuma K, Yamamoto A, Iijima M, Shiba K (2008) Direct transformation from amorphous to crystalline calcium phosphate facilitated by motif-programmed artificial proteins. Proc Nat AcadSci USA 105: 16866-16870.
- Posner AS, Betts F (1975) Synthetic amorphous calcium phosphate and its relation to bone mineral structure. AccChem Res 8: 273-281.
- Onuma K, Ito A (1998) Cluster growth model for hydroxyapatite. Chem Mater 10: 3346-3351.
- Kanzaki N, Treboux G, Onuma K, Tsutsumi S, Ito A (2001) Calcium phosphate clusters. Biomaterials 22: 2921-2929.
- Wang CG, Liao JW, Gou BD, Huang J, Tang RK, et al. (2009) Crystallization at multiple sites inside particles of amorphous calcium phosphate. Cryst Growth Des 9: 2620-2626.
- Dey A, Bomans PHH, Muller FA, Will J, Frederick PM, et al. (2010) The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat Mater 9: 1010-1014.
- Treboux G, Layrolle P, Kanzaki N, Onuma K, Ito A (2000) Symmetry of Posner's cluster. JAmeChemSoc 122: 8323-8324.
- Yin X, Scott MJ (2003) Biological calcium phosphates and Posner's cluster. J ChemPhys 118: 3717-3723.
- Du LW, Bian S, Gou BD, Jiang Y, Huang J, et al. (2013) Structure of clusters and formation of amorphous calcium phosphate and hydroxyapatite: From the perspective of coordination chemistry. Cryst Growth Des 13: 3103-3019.
- Habraken WJEM, Tao JT, Brylka LJ, Friedrich H, Bertinetti L, et al. (2013) Ion-association complexes unite classical and non-classical theories for the biomimetic nucleation of calcium phosphates. NatCommun 4: 1507.
- Gebauer D, Völkel A, Cölfen H (2008) Stable prenucleation calcium carbonate clusters. Science 322: 1819-1822.
- Pouget EM, Bomans PH, Goos JA, Frederik PM, de With G, et al. (2009) The initial stages of template-controlled CaCO3 formation revealed by cryo-TEM. Science 323: 1455-1458.
- Gebauer D, Gunawidjaja PN, Ko JY, Bacsik Z, Aziz B, et al. (2010) Proto-calcite and proto-vaterite in amorphous calcium carbonates. AngewChemInt Ed Engl 49: 8889-8891.
- Sugiura Y, Onuma K, Nagao M, Yamazaki A (2015) The effects of immobilized carboxylic-functional groups on the dynamics of phase transformation from amorphous to octacalcium phosphate. Ame Mine 100: 1624-1632.
- Davies E, Duer MJ, Ashbrook SE, Griffin JM (2012) Applications of NMR Crystallography to Problems in Biomineralization: Refinement of the Crystal Structure and 31P Solid-State NMR Spectral Assignment of OctacalciumPhospahte. J Am ChemSoc 134:12508-12515.
- Gebauer D, Kellermeier M, Gale JD, Bergström L, Cölfen H (2014) Pre-nucleation clusters as solute precursors in crystallisation. ChemSoc Rev 43: 2348-2371.
- De Yoreo JJ, Gilbert PUPA, Sommerdijk NAJM, Penn RL, Whitelam S, et al. (2015) Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 349: aaa6760.
- Khouzani MF, Chevrier DM, Güttlein P, Hauser K, Zhang P, et al. (2015) Disordered amorphous calcium carbonate from direct precipitation. CrysEngComm 17: 4842-4849.
- Gower LB, Odom DJ (2000) Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J Cryst Growth 210: 719-734.
- Dai L, Douglas EP, Gower LB (2008) Compositional analysis of a polymer-induced liquid-precursor (PILP) amorphous CaCO3 phase. J Non-Crystalline Solids 354: 1845-1854.
- Chow LC, Eanes ED (2001) Octacalcium Phosphate. Monographs on Oral Science. Whitford GM, Karger, Basel, Switzerland.
- Miake Y, Aoba T, Moreno EC, Shimoda S, Prostak K, et al. (1991) Ultrastructural studies on crystal growth of enameloid minerals in elasmobranch and teleost fish. Calcif. Tissue Int 48: 204-217.
- Aoba T, Miake Y, Shimoda S, Prostak K, Moreno EC, et al. (1991) Dental apatites in vertebrates species: Morphology and chemical properties. Mechanisms and phylogeny of mineralization in biological systems, (Eds.) Nakahara H, Suga S, Springer-Verlag, Tokyo.
- Miake Y, Shimoda S, Fukae M, Aoba T (1993) Epitaxial overgrowth of apatite crystals on the thin-ribbon precursor at early stages of porcine enamel mineralization. Calcif Tissue Int 53: 249-256.
- Onuma K, Iijima M (2015) In situ atomic force microscopy observation of octacalcium phosphate (100) face dissolution in weak acidic solutions. J CrystProc Tech 5: 1-8.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15252
- [From(publication date):
December-2015 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 14316
- PDF downloads : 936