Metaplastic Breast Cancer: Current Strategies for Diagnosis, Screening, Treatment, and Long-term Management
Received: 01-May-2023 / Manuscript No. jcd-23-98353 / Editor assigned: 04-May-2023 / PreQC No. jcd-23-98353 / Reviewed: 18-May-2023 / QC No. jcd-23-98353 / Revised: 23-May-2023 / Manuscript No. jcd-23-98353 / Published Date: 30-May-2023 DOI: 10.4172/2476-2253.1000180
Abstract
A malignancy called metaplastic breast cancer (MBC) is defined by the presence of two or more cellular types in its histology, typically a mixture of epithelial and mesenchymal cells. In comparison to invasive ductal carcinoma (IDC), MBC is uncommon, accounting for less than 1% of all breast malignancies. Other than having a lower frequency of lymph node metastases, MBC tumours have worse prognostic characteristics than IDC tumours. The best MBC therapy paradigm is unknown because of its low prevalence and pathological diversity. MBC has been regarded as a variation of IDC due to its rarity. Patients with MBC, however, had inferior outcomes while receiving comparable treatment plans. Recent studies have concentrated on the biological distinctions between MBC and IDC as well as potential new chemotherapeutic drug targets. This essay provides as a summary of recent research on methods for treating MBC patients in a multidisciplinary manner.
Cancer treatment, which uses a multidisciplinary approach and carefully takes into account each patient’s unique needs and preferences, begins with cancer diagnosis. Although screening tests are a crucial tool for cancer early detection, they can have some drawbacks. As opposed to treatment alternatives, which are based on the kind and stage of cancer as well as the patient’s general health, diagnostic tests provide conclusive information regarding the presence of cancer and its characteristics. Commonly utilised treatment modalities include surgery, radiation therapy, chemotherapy, and immunotherapy, either separately or in combination. For the best patient outcomes, it’s crucial to continue monitoring cancer after treatment to look for any indications of recurrence or the development of new tumours. An overview of the significance of cancer diagnosis, screening and diagnostic procedures, available therapies, and monitoring is provided in this review article.
Keywords
Metaplastic breast cancer; Diagnosis; Screening; Treatment; Long-term management; Pathology; Triple negative breast cancer
Introduction
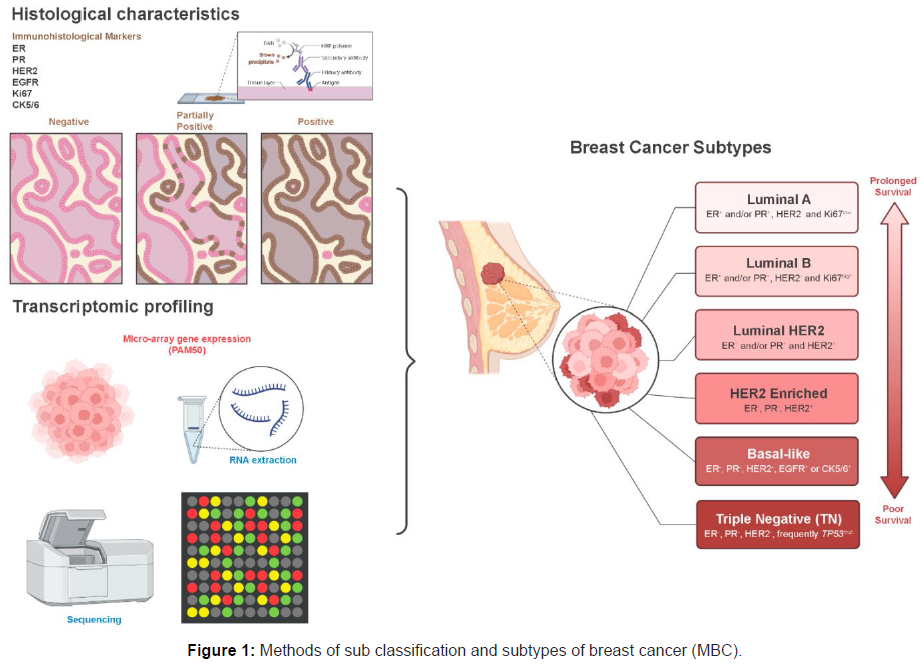
A rare form of breast cancer known as metaplastic breast cancer (MBC) is characterised by the presence of two or more cellular types in its histology, typically a mixture of epithelial and mesenchymal cells. MBC makes about 0.25 to 1% of all breast cancer diagnoses each year. The pathologic diagnosis of MBC is challenging because of its relative rarity and varied histologic appearance. MBC was recognised as a distinct pathologic entity by the World Health Organisation (WHO) in 2000. Since then, the prevalence of MBC has increased, most likely because to pathologists becoming more aware of the condition. In comparison to about 50% of patients with IDC, more than 70% of patients with MBC have illness that has reached stage II or higher according to the American Joint Committee on Cancer (AJCC). Patients with MBC experience worse results than those with IDC, with 5-year survival rates ranging from 49% to 68% (Figure 1) [1].
Unknown are the most effective MBC treatment plans. Despite mounting evidence that MBC is a unique entity that lies along the spectrum of basal-like breast tumours, management of MBC has generally matched that of IDC. The multimodality management of patients with MBC is summarised in this study using recent research and methods. Cancer is a complicated illness that can attack anybody component. It happens when abnormal body cells expand and divide out of control, resulting in a tumour or infiltrating nearby tissues. Based on the type of cell that is originally harmed [2], cancer can be divided into many forms, such as carcinoma, sarcoma, lymphoma, and leukaemia. The type and stage of the cancer, as well as the patient’s general condition, affect the treatment options and prognosis. For a successful course of therapy and the best possible patient results, early cancer detection is essential. Cancer screening tests are intended to find the disease in those who do not exhibit any symptoms. Mammography for breast cancer, a colonoscopy for colorectal cancer, and a Pap test for cervical cancer are a few examples of cancer screening tests. Screening tests have limits, such as the potential for false-positive and falsenegative results, yet they can identify cancer at an early stage when it is more curable (Figure 2) [3].
When a person exhibits the signs or symptoms of cancer, diagnostic tests are utilised to either confirm or rule out the presence of the disease. A biopsy, which entails the removal of a sample of tissue for examination under a microscope, is the most frequent diagnostic procedure for cancer. X-rays, CT scans, MRIs, and PET scans are examples of imaging tests that can offer further details on the location and severity of the malignancy. Blood testing can also be done to look for particular markers that could indicate malignancy. The type and stage of the cancer, as well as the patient’s general health and preferences, all influence the treatment options. Surgery is frequently used to remove malignant tissue, and for early-stage malignancies, it may be the only treatment necessary. Radiation therapy, which can be used alone or in conjunction with other therapies, uses high-energy X-rays or other types of radiation to kill cancer cells. Chemotherapy, which can be used either alone or in conjunction with other treatments, uses chemicals to destroy cancer cells. A more recent form of cancer treatment called immunotherapy encourages the immune system to attack cancer cells [4].
For the best patient outcomes, it’s crucial to continue monitoring cancer after treatment to look for any indications of recurrence or the development of new tumours. Blood tests, imaging studies, and routine physical examinations are all examples of follow-up care. The kind and stage of the cancer as well as the type of treatment received determine the frequency and length of follow-up care. In conclusion, determining a patient’s cancer diagnosis is a difficult procedure that includes screening tests for early detection, diagnostic testing for confirmation and treatment options that rely on the type and stage of the disease as well as the patient’s general health and preferences? To guarantee the best possible patient results, monitoring cancer following therapy is crucial. The greatest care for cancer patients can only be provided by a multidisciplinary approach [5].
Millions of individuals worldwide are afflicted by the potentially fatal disease of cancer. It is brought on by the unchecked proliferation of abnormal cells, which can spread to other bodily parts and infect other tissues. Effective cancer therapy and management depend on early detection and precise diagnosis. In this post, we’ll examine the methods for screening, diagnosing, and monitoring cancer as they are right now. The practise of screening allows for the early detection of cancer. Cancer screening aims to spot the disease when it’s most treatable, this is in the early stages. For cancer screening, a variety of techniques are employed, including: Breast cancer and mammography: A low-dose X-ray of the breast tissue called a mammogram can be used to find anomalies such lumps or calcifications. Women over 50 or younger women with a family history of breast cancer are advised to get it [6].
For younger women who are sexually active and are between the ages of 21 and 65, it is advised. Colonoscopy for Colon Cancer: A flexible, lighted tube is inserted into the colon during a colonoscopy to look inside. It can spot polyps, which over time could turn cancerous. For people over the age of 50, it is advised. The PSA test, a blood test that detects the presence of prostate-specific antigen, is used to diagnose prostate cancer. Increased PSA levels may indicate prostate cancer. Men over 50 or younger are advised to use it [7].
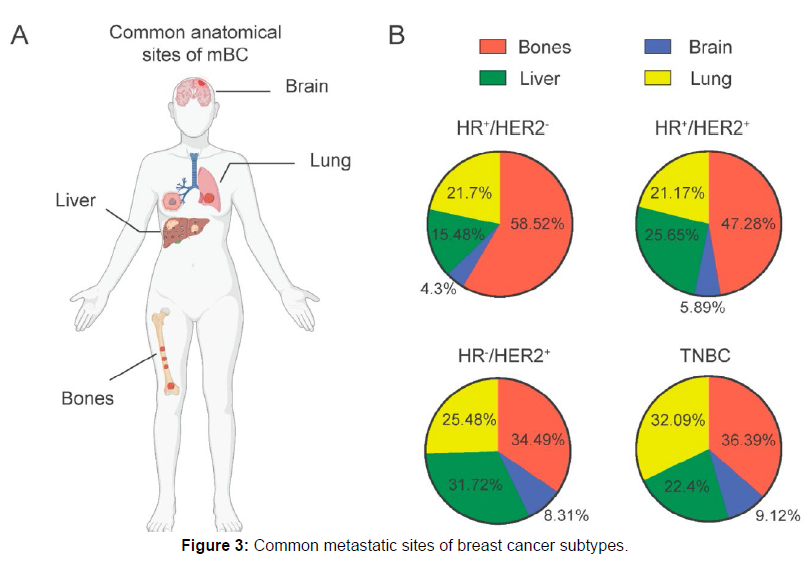
Breast cancer has a tendency to metastasize to specific anatomical sites, including the bone, brain, liver, and lung. This process involves a sequential cascade, starting with local invasion and migration through stromal connective tissues, followed by intravasation into the blood and lymphatic vessels. It ultimately leads to extravasation and infiltration into the tissue parenchyma of secondary organ sites. The metastatic spread is influenced by various factors, with molecular subtypes playing a significant role in determining the risk of spread to specific locations.
A recent study discovered that HR+ cancers have a higher frequency of metastasis to the bones, along with other subtypes. Conversely, HER- 2+ and TNBC subtypes are more commonly associated with brain metastasis. While lung and bone metastases can occur in all breast cancer subtypes, they are more frequently observed in HR+ cases, whereas liver metastasis is more prevalent in HER-2+ subtypes (Figure 3). The organotropism of metastatic breast cancer is influenced by intrinsic molecular and genomic characteristics, which extend beyond the scope of this review [8].
Materials and Methods
Additional diagnostic tests could be carried out if cancer is suspected as a result of screening results or symptoms. Finding the location, kind, and stage of the disease is the aim of the cancer diagnosis process. Cancer is diagnosed using a variety of techniques, including:
Imaging tests: Images of the inside of the body can be seen in great detail with imaging tests including X-rays, CT scans, MRI scans, and PET scans. They can identify tumours and aid in determining the cancer’s severity.
Blood Tests: Blood tests can find certain markers, such tumour markers or particular proteins, which may be linked to cancer. These tests, however, are not always accurate, and confirmation testing may be necessary [9].
The ideal treatment strategy can be created once cancer has been identified. The type and stage of the cancer, as well as the patient’s general condition, will all influence the therapy option. There are numerous cancer therapy options, including:
Surgery: The malignant tissue is frequently removed through surgery. For tumours in the early stages, it might be the only treatment necessary; for cancers in the later stages, it might be supplemented with other therapies [10].
Immunotherapy: A more recent form of cancer treatment called immunotherapy encourages the immune system to attack cancer cells. Following therapy, it’s crucial to keep an eye out for the patient’s symptoms of cancer recurrence or the development of any new cancers. Blood tests, imaging studies, and routine physical examinations are all examples of follow-up care [11].
Discussion
Cancer diagnosis is an essential part of cancer care since it serves as the foundation for deciding on the best course of therapy and keeping track of the progress of the patient. Diagnostic tests are used to confirm or rule out the presence of cancer in people who exhibit signs or symptoms of the disease, whereas screening tests are used to detect cancer in those who do not exhibit any symptoms. Surgery, radiation therapy, chemotherapy, and immunotherapy are all available as cancer treatments and may be used alone or in conjunction with other therapies [12].
MBC has mostly received similar surgical care to IDC. The surgical treatment for IDC changed from mastectomy to breast conservation therapy for the right patients with the announcement of the NSABP B-06 trial results. Even less extensive tumours may prevent breast conservation in people with smaller breasts if they have large tumours (5 cm) that are a relative contraindication to breast conservation therapy. These recommendations are especially crucial for MBC patients because they frequently have larger tumours than IDC patients do. As one may anticipate, a greater proportion of patients with MBC have mastectomy as opposed to lumpectomy as compared to those with IDC [13].
MBC histology shouldn’t bar breast conservation therapy in the right patients, despite the greater tumour size at presentation. Even after adjusting for known prognostic variables, Tseng and Martinez observed no difference in overall or disease-specific survival whether MBC patients underwent mastectomy or lumpectomy, The same is true for Dave et al., who discovered no differences in overall or disease-free survival between MBC patients following modified radical mastectomy or breast conservation therapy [14].
The approach to axillary staging of patients with IDC has changed, which is reflected in the evolution of lymph node staging for patients with MBC. Axillary lymph node dissection (ALND) has long been the standard procedure for axillary staging. Sentinel lymph node biopsy (SLNB), however, has mainly taken the role of ALND since it has a lower associated morbidity and is as accurate in detecting regional metastasis. All patients with a positive SLNB received a complete ALND prior to the American College of Surgeons Oncology Group (ACOSOG) Z0011 study. According to the findings of this experiment, however, complete ALND might not be advised in a small subset of women with early-stage breast cancers who are getting a lumpectomy and then whole-breast radiation. However, the Z0011 experiment did not specifically target MBC patients as a subset. The sensitivity and specificity of sentinel lymph node biopsy in MBC patients, as well as the requirement for full ALND in patients with sentinel lymph node metastases, are therefore uncertain [15].
In comparison to IDC, patients with MBC have reduced rates of axillary lymph node involvement, according to several studies. 22% of MBC patients, according to Tseng and Martinez, had axillary lymph node involvement. Further reported that nodal positive was more frequent in the carcinosarcoma form, showing axillary lymph node metastases in 22% of individuals with MBC versus 34% of those with IDC. Despite this, Beatty et al. showed that patients with MBC use SLNB and ALND equally compared to women with IDC. Women who had postmastectomy radiation therapy had a better survival rate compared to those who did not (i.e., initial tumours >5 cm and/ or four or more metastatic axillary nodes). It would be prudent to advise completion ALND for MBC patients undergoing mastectomy if they have documented sentinel lymph node metastasis because it is frequently the only way to document 4 lymph node metastases and because patients undergoing mastectomy were not included in the ACOSOG Z0011 trial [16].
It has been demonstrated that screening tests are useful in lowering cancer incidence and mortality. For instance, mammography, a common breast cancer screening test, has been proven to cut breast cancer mortality in women over 50 by up to 40%. However, screening tests have drawbacks as well, such as false-positive results that might prompt pointless more testing and anxiety and false-negative results that can postpone the discovery of cancer [17].
When a person exhibits the signs or symptoms of cancer, diagnostic tests are utilised to either confirm or rule out the presence of the disease. The most accurate way to diagnose cancer is with a biopsy, which also reveals the kind and stage of the disease. Imaging studies can reveal more details about the location and size of the tumour, but they cannot conclusively rule out its existence. Blood tests can identify some potential cancer signs, but they are not always accurate and may call for additional testing for confirmation [18].
The type and stage of the cancer, as well as the patient’s general condition, all influence the available cancer treatments. Surgery is frequently used to remove malignant tissue, and for early-stage malignancies, it may be the only treatment necessary. Radiation therapy, which can be used alone or in conjunction with other therapies, uses high-energy X-rays or other types of radiation to kill cancer cells. Chemotherapy, which can be used either alone or in conjunction with other treatments, uses chemicals to destroy cancer cells. A more recent form of cancer treatment called immunotherapy encourages the immune system to attack cancer cells. Following treatment, it’s crucial to monitor cancer to look for any signs of recurrence or the development of new tumours. Blood tests, imaging studies, and routine physical examinations are all examples of follow-up care. The kind and stage of the cancer as well as the type of treatment received determine the frequency and length of follow-up care [19].
There is limited evidence that the typical breast cancer chemotherapy regimens used for IDC are also successful in treating MBC in women. Women with MBC who are undergoing adjuvant chemotherapy have a worse survival rate than stage-matched IDC patients. Nevertheless, 33%–86% of patients with MBC undergo chemotherapy, which is actually a twofold higher rate than that of patients with IDC. Retrospective investigations conducted at a single institution and genetic analysis indicates that these tumours are largely chemoresistant. According to a Mayo Clinic analysis, 33% of the 27 patients who were treated there during a 20-year period underwent chemotherapy. Ten different regimens were applied, and one partial response was obtained. The various genetic and nongenetic pathways in MBC that lead to phenotypically different subclones and intratumoral heterogeneity are likely the cause of this resistance to chemotherapy [20].
Conclusion
There is no “standard” treatment for every patient with MBC because of the disease’s rarity and variability. Traditional chemo- and hormonal therapies for IDC are ineffective against MBC and frequently associated with poorer survival, whereas histology specific novel chemotherapeutic strategies may offer a survival advantage. Surgical treatment and axillary staging are similar to those of IDC, with breast conservation therapy being appropriate for a select group of patients. While promising for the future, targeted medicines based on unique gene profile are not frequently used. Last but not least, adjuvant radiation should be seen as a component of multimodality therapy for patients with MBC, independent of the kind of surgery. The rarity of the disease makes it unlikely that clinical trials comparing current therapy for IDC in people with MBC will be carried out.
Acknowledgement
None
Conflict of Interest
None
References
- Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, et al. (2004) Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 10: 1243-1260.
- Siegel RL, Miller KD, Jemal A (2015) Cancer statistics. J Clin 65: 5-29.
- Austin JH, Yip R, D'Souza BM, Yankelevitz DF, Henschke CI, et al. (2012) International Early Lung Cancer Action Program Investigators. Small-cell carcinoma of the lung detected by CT screening: stage distribution and curability. Lung Cancer 76: 339-43.
- Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, et al. (2012) Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor micro emboli in patients with small-cell lung cancer. J Clin Oncol 30: 525-32.
- Shamji FM, Beauchamp G, Sekhon HJS (2021) The Lymphatic Spread of Lung Cancer: An Investigation of the Anatomy of the Lymphatic Drainage of the Lungs and Preoperative Mediastinal Staging. Thorac Surg Clin 31: 429-440.
- Shamji FM, Beauchamp G, Sekhon HJS (2021) The Lymphatic Spread of Lung Cancer: An Investigation of the Anatomy of the Lymphatic Drainage of the Lungs and Preoperative Mediastinal Staging. Thorac Surg Clin 31: 429-440.
- Bastos AU, Oler G, Nozima BH, Moyses RA, Cerutti JM, et al. (2015) BRAF V600E and decreased NIS and TPO expression are associated with aggressiveness of a subgroup of papillary thyroid microcarcinoma. Eur J Endocrinol 173: 525-540.
- Zoghlami A, Roussel F, Sabourin JC, Kuhn JM, Marie JP, et al. (2014) BRAF mutation in papillary thyroid carcinoma: predictive value for long-term prognosis and radioiodine sensitivity. Eur Ann Otorhinolaryngol Head Neck Dis 131: 7-13.
- Ito Y, Yoshida H, Maruo R, Morita S, Takano T, et al. (2009) BRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinic pathological features and disease-free survival of patients. Endocrine journal 56: 89-97.
- Stanojevic B, Dzodic R, Saenko V, Milovanovic Z, Pupic G, et al. (2011) Mutational and clinico-pathological analysis of papillary thyroid carcinoma in Serbia. Endocrine journal 58: 381-393.
- Sahpaz A, Onal B, Yesilyurt A, Han U, Delibasi T, et al. (2015) BRAF(V600E) Mutation, RET/PTC1 and PAX8-PPAR Gamma Rearrangements in Follicular Epithelium Derived Thyroid Lesions- Institutional Experience and Literature Review. Balkan Med J 32: 156-166.
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics CA Cancer J Clin 69:7-10.
- Rashid FA, Fukuoka J, Bychkov A (2020) Prevalence of BRAFV600E mutation in Asian series of papillary thyroid carcinoma-a contemporary systematic review. Gland Surg 9: 1878-1900.
- Pyo JS, Kim DH, Yang J (2018) Diagnostic value of CD56 immunohistochemistry in thyroid lesions. Int J Biol Markers 33: 161-167.
- Ahn D, Park JS, Sohn JH, Kim JH, Park SK, et al. (2012) BRAFV600E mutation does not serve as a prognostic factor in Korean patients with papillary thyroid carcinoma. Auris Nasus Larynx 39: 198-203.
- Mond M, Alexiadis M, Fuller P J, Gilfillan C (2014) Mutation profile of differentiated thyroid tumours in an Australian urban population. Intern Med J 44: 727-734.
- Nasirden A, Saito T, Fukumura Y, Hara K, Akaike K, et al. (2016) In Japanese patients with papillary thyroid carcinoma, TERT promoter mutation is associated with poor prognosis, in contrast to BRAF (V600E) mutation. Virchows Arch 469: 687-696.
- Lupi C, Giannini R, Ugolini C, Proietti A, Berti P, et al. (2007) Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab 92: 4085-4090.
- Jo YS, Li S, Song JH, Kwon KH, Lee JC, et al. (2006) Influence of the BRAF V600E Mutation on Expression of Vascular Endothelial Growth Factor in Papillary Thyroid Cancer. J Clin Endocrinol Metab 91: 3667-3670.
- Abrosimov A, Saenko V, Rogounovitch T, Namba H, Lushnikov E, et al. (2007) Different structural components of conventional papillary thyroid carcinoma display mostly identical BRAF status. Int J Cancer 120: 196-200.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Citation: Magaón F (2023) Metaplastic Breast Cancer: Current Strategies for Diagnosis, Screening, Treatment, and Long-term Management. J Cancer Diagn 7: 180. DOI: 10.4172/2476-2253.1000180
Copyright: © 2023 Magaón F. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2211
- [From(publication date): 0-2023 - Nov 25, 2025]
- Breakdown by view type
- HTML page views: 1884
- PDF downloads: 327