Metal Toxicity Taking New Forms-Iron Toxicity as an Emerging Threat to Aquatic Biome
Received: 15-Oct-2022 / Manuscript No. JFLP-22-79815 / Editor assigned: 17-Oct-2022 / PreQC No. JFLP-22-79815(PQ) / Reviewed: 31-Oct-2022 / QC No. JFLP-22-79815 / Revised: 04-Nov-2022 / Manuscript No. JFLP-22-79815(R) / Accepted Date: 07-Nov-2022 / Published Date: 11-Nov-2022
Abstract
Amongst the various appalling environmental hazards, metal toxicity is one such and is much of a consequential misfortune due to the other many more dreadful causatives or environmental threats like pollution, industrialisation, in humane usage of chemicals and like disasters. Heavy metal toxicity takes many forms out of which iron toxicity is the one which empanelled recently into the environmentalist’s list as it has been revealed that it has taken on to a more severe front in affecting the aquatic beings with its life and sustenance. As said by great human thinkers, every component of environment is crucial for ecosystem balance but, a mere mismatch of its quantity will have major ill-effects too. Iron is one such component whose content is important as a micronutrient but its excessiveness is direful. Hence a control over its usage stands valid.
Keywords
Toxicity; Bioaccumulation; Bio-magnification; Electrocoagulation; Contaminant; Sorption; Adsorption; Co-precipitation
Introduction
Aquaculture, the farming of aquatic organisms including fish, molluscs, crustaceans and aquatic plants [1] requires water to be good enough to sustain them, their generations and its physio-chemical and biological aspects. Water quality refers to the chemical, physical, biological and radiological characteristics of water [2] and that each of its component needs to be optimally maintained for wholesome goodness to sustain growth and survival of fishes as its medium is water. Therefore the overall performance of any aquaculture system or any water body is partly determined by its water quality [1]. In spite of tremendous growth in fisheries and aquaculture sector, the deteriorating waters however have affected their production and growth parameters along with physiological responses and behaviours. Thus the government and the private fish farmers are obliged to keep up the necessary water quality.
Out of the various characteristics of water, its chemical character is governed by the various chemical components dissolved in water in specific ratios. An imbalance in the ration will alter its chemical feature and toxicity is one of the major contributing factors to the same [3]. The longer exposure to heavy concentration of metals in water leads to toxicity whose affects are innumerable. Living beings, their habitat and the food web gets touched, ultimately accelerating towards bioaccumulation and bio-magnification. Many heavy metals have expressed their toxicity and iron is a recent addition [4]. This is therefore being investigated by scientists all over the globe to confirm and also to learn of the specific reactions that excessive iron is having on various life forms and that this article’s content will be in light of the so far found major milestones in iron toxicity to aquatic organisms and its mitigation strategy.
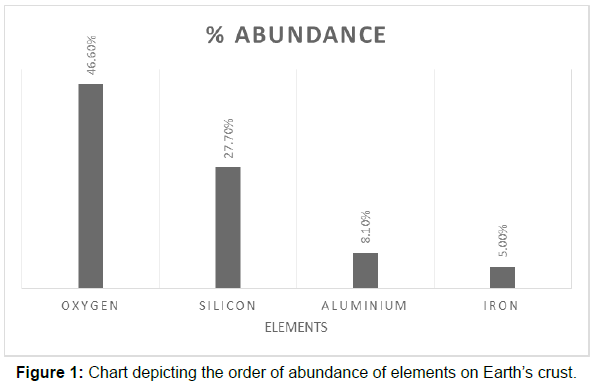
Iron is the fourth most abundant element on Earth’s crust and is an essential element for life. Due to its high abundance within Earth’s crust, it is ubiquitous and reaches significantly higher levels in water and sediments as compared to other trace metals [5, 6]. Although being an important metal ion for living beings, iron is said to become toxic once its concentration crosses 0.1 mg/l in water. Iron exists basically in five different oxidation states with ferrous and ferric as most common. In between the two, ferrous is more toxic (Figure 1).
Water bodies- both ground and surface water generally contain iron either from geogenic sources or from multiple points releasing effluents but its content nevertheless differs at different locations. For instance, as per [7, 8], the iron content in ground water at various locations inside Indian subcontinent varies and it is around 2-10 mg/l in West Bengal, upto 6 mg/l in Uttar Pradesh while the eastern state of Assam is highly contaminated with high concentrations of iron.
Enroute-Iron Toxicity
Excessive iron causes effects like inhibiting beneficial algae growth, stressing fishes due to acidic pH, colloid formation with organics thereby reducing oxygen transfer, causing Early Mortality Syndrome and incidence of soluble iron feeding Vibrio bacteria in aquatic organisms [9, 10].
According to scientists at Dryden Aqua, a Marine Biological company specialised in water treatment technology based in UK, the toxicity of iron depends mainly on the species and the size of the fish species concerned for the reason that the micron sized iron particles gets trapped in between the gill lamellae leading to irritation and secondary fungal and bacterial infections [11]. Also being a catalyst which promotes the free radical formation, these trapped molecules will form highly reactive free radicals which in turn oxidise and destructs the surrounding gill tissues hindering respiration and causing anaemic like conditions. However, sample evidences has not been found yet for this but once when the scientists used an iron sequester the fishes soon recovered, hence indirectly proving the phenomenon.
According to [11,12] a low temperature and presence of iron cause the occurrence of iron depositing bacteria like Acidithiobacillus sp. and Leptospirillum sp. which derive energy and multiply by oxidising ferrous ion to produce insoluble ferric ion covering gills as a colourless filamentous colony giving them an ultimate brown colour. The ferric ions produced are in fact also contributing to enhanced turbidity of water causing subsequent after effects.
Iron solubility depends on pH (lower the pH, higher the iron content), oxidation-reduction potential, temperature, oxygen and presence of substances to which it will bind like humic substances. Iron once absorbed into body causes lip peroxidation as well [13, 14]. Apart histopathological changes were found in the liver and kidney [15, 7].
Iron toxicity upon aquatic organisms
The effects of iron on aquatic organisms and their habitats are mainly indirect. Ionic forms of iron precipitates on biological and other surfaces besides disturbing normal metabolism and changing the structure and quality of benthic habitats and food resources. The combined direct and indirect effects decrease species biodiversity and abundance of periphyton, benthic invertebrates and fishes. Sorption and co-precipitation of metals by iron oxides decrease bioavailability and toxicity of water-borne metals or compounds like hydrogen sulphide, but may increase the dietary supply of metals causing toxic effects along food chain. Moreover the flux of iron is affected by seasonally varying physical, chemical and biological processes as well [16].
Molluscs are strong bio-indicators and accumulate heavy metals in their body because of their bottom feeding habit. Therefore studying the level of pollutants in molluscs and sediments helps to evaluate the margin of contamination and hazard to human population, nonetheless a study by [17] revealed that molluscs are comparatively more tolerant to iron concentration and also size and weight along with age is an important factor in determining bioaccumulation [18]
. In hatcheries, iron precipitation coat eggs or block gill surface and the water here is generally treated by gravity or mechanical aeration closely followed by sedimentation and sand filtration [19].
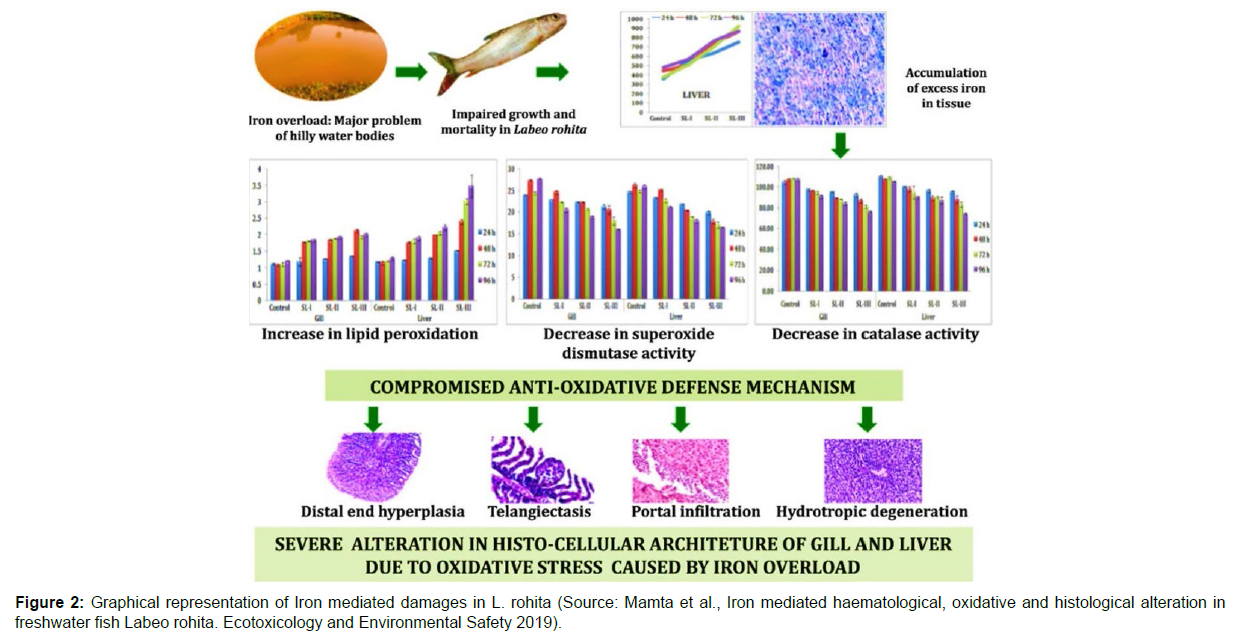
An investigation by [20] on iron toxicity in Labeo rohita identified and proved the haematological, histological and oxidative damages that iron cause on fishes and it was a successful effort to bring forth the negative impacts of iron in cultured species, as such data is scanty. Significant changes in leukocyte and erythrocyte count, haemoglobin, lipid peroxidation, antioxidant enzyme activity and tissue iron accumulation were observed and more noticeably the severity was found to grade up along the passing time and concentration as well indicating the necessity to address iron toxicity as a menace (Figure 2).
The Mitigation Strategies
Despite all the ill effects, iron is still considered a secondary contaminant (due to its negligible effect on human health) and is said to only affect the water taste [21, 22]. However, its removal is relevant in fisheries and aquaculture’s context aiming at proper fish yield and the various enlisted methods to reduce iron toxicity are as given in the passage below. Controlling the root causes for excessive iron in water will help lessen the impacts.
Ferrous iron is clear and dissolves in water, hence unidentifiable while once ferrous oxidises to ferric, it tends to solidify and gets easily removed through filtration. Oxidation, Sedimentation and Filtration are the three main basic steps involved in Iron removal. Either proper aeration or usage of oxidants is the important first step to facilitate oxidation. Filtration may be done by several ways including usage of single and double trickling filters. Apart the usage of activated carbon from bamboo is one another method mentioned by scientific studies [23].
Water purification system generally employs water softening, ion exchange, ozonisation and media filtration as the main treatment methods. In recent years, electrocoagulation has emerged as an effective strategy with efficiencies ranging between 90-100%, with the efficiencies depending on system conditions like pH, current, electrolysis time, size of electrodes, type of electrodes, coagulant dosage, velocity gradient and flux rate. Another popular method is adsorption-oxidation where ferrous iron gets adsorbed to media surface followed by its oxidation, well demonstrated for sand media by [24]. Several other media has also been tried successfully later with the same principle like for example using polythene bead media by [24].
However, mostly in Indian scenario, farmers use Potassium Permanganate solution to remove the excessive iron content quite inevitable in indigenous ponds, as the cheapest resort. Lime application to lower pH and alkalinity is also a common management measure against possible adverse effects of iron according to [19].
Iron can also be chemically oxidised with sodium hypochlorite or potassium permanganate but this is neither feasible on a broader scale nor they are healthy to aquatic beings. Hence there is a need for further innovations.
Among the innovative discoveries till date to treat iron [25] found that the farmers at Niger delta has been experimenting with bio treatment by adding ripe and unripe plantain peels to water to act as a buffer and the results were found to be worth quoting. It significantly reduced the iron content alongside improving pH. The farmers unable to afford the water treatment cost and also out of suffocation from the waste stream from plantain peels causing environmental perturbation has devised this useful strategy reports [25, 26].
In addition, [21, 27] reported through their research that bentonite a natural clay at 0.2 to 0.4 grams per litre in water effectively alleviate toxic iron levels in catfish. Also sodium citrate can be used which converts ferrous ion from aquaculture system to more bioavailable form for the plants to absorb. This because of having shorter shelf life is better replaced by EDTA. University of Idaho’s Aquaculture Research Institute has also revealed that feeding Vitamin C to rainbow trout has alleviated the negative effects of excessive iron in water.
Way Forward
Although iron is still a secondary contaminant due to its significance as an essential micronutrient, its effects on fishes cannot be neglected and the chances of its magnification through food web cannot be ruled out. Moreover since it is colourless and that its excessiveness cannot be quantified through mere observance, it is recommended that the farmers and stakeholders ensure water quality regularly to avoid massive fish kills. Also trying innovative natural remedies will do well or else under this banner many more chemical companies will flourish and the ecosystem crashes.
References
- Abd El-Hamed N (2014) Environmental Studies of Water Quality and It's Effect on Fish of Some Farms in Sharkia and Kafr El-Sheikh Governorates. Institute of Environmental studies & Research, Ain Shams University: Cairo, Egypt 1-141.
- Eruola AO, Ufoegbune GC, Awomeso JA, Abhulimen SA (2011) Assessment of cadmium, lead and iron in hand dug wells of llaro and Aiyetoro, Ogun State, South-Western Nigeria. Res J Chem Sci 1: 1-5.
- Bowman AB, Imperatore G (2002) Iron Overload: Too much of a good thing. McGraw -Hill Companies Inc.
- Dalzell DJB, MacFarlane NAA (1999) The toxicity of iron to brown trout and effects on the gills- a comparison of two grades of iron sulphate. J Fish Biol 55: 301-315.
- Davidson W (1993) Iron and manganese in lakes. Earth- Science Reviews 34: 119-163.
- Forstner U, Wittmann GTW (1979) Metalpollution in the Aquatic Environment. Springer-Verlag Berlin 1-486.
- Exley C, Chappel JS, Birchal JD (1991) A mechanism for acute aluminium toxicity in fish. J Theor Biol 151: 417–428.
- Khatri N, Tyagi S, Rawtani D (2017) Recent strategies for the removal of iron from water: A review. J Water Process Eng 19: 291- 304.
- Audrey Barucchi (2022) Iron in Aquaculture Ponds. Calix.
- Hyuha TS, Bukenya JO, Twinamasiko J, Molnar J (2011) Profitability analysis of small scale aquaculture enterprises in Central Uganda. Int J Fish Aquac 2: 271-278.
- Kesavan K, Murugan A, Venkatesan V, Vijay Kumar BS (2013) Heavy Metal Accumulation In Molluscs And Sediment From Uppanr Estuary, Southeast Coast Of India. Int J Mar Sci 29: 15-21.
- Kari-Matti V (1995) Direct and indirecr effects of iron on river ecosystems. Ann Zool Fennici 32: 317-329.
- Baker RTM, Martin P, Davies SJ (1997) Ingestion of sublethal levels of iron sulphate by African catfish affects growth and tissue lipid peroxidation. Aquatic Toxicology 40: 51-61.
- Lappivaara J, Kiviniemi A, Oikari A (1999) Bioaccumulation and subchronic physiological effects of water- borne iron overload on whitefish exposed in humic and nonhumic water. Arch Environ Contam Toxicol 37: 196–204.
- Playle RC, Wood CM (1989) Water pH and aluminium chemistry in the gill micro-environment of rainbow trout during acid and aluminium exposures. J Comp Physiol B 159: 539–550.
- Ohimain E, Angaye TCN & OKiongbo K (2014) Removal of Iron, Coliforms and Acidity from Ground Water Obtained from Shallow Aquifer Using Trickling Filter Method. J Envt Sci Eng 2: 549-555.
- Teristiandi N (2018) Freshwater Molluscs as Bioindicator of Fe and Mn Contamination on Lematang River, South Sumatera, Indonesia. E3S Web of Conferences 68: 01016.
- Jordaens K, Wolf HD, Vandecasteele B, Blust R, Backeljau T (2006) Associations between shell strength, shell morphology and heavy metals in the land snail Cepaea nemoralis (Gastropoda, Helicidae). Sci Total Env 363: 285–293.
- Boyd CE (2008) Iron important to pond water, bottom quality. Global Aquaculture Advocate.
- Singh MF, Barman AS, Devi AL, Devi AG, Pandey P (2019) Iron mediated hematological, oxidative and histological alterations in freshwater fish Labeo rohita. Ecotoxicol Environ Saf 170:87-97
- Romano N, Kumar V, Sinha AK (2021) Implications of excessive water iron to fish health and some mitigation strategies. Global Seafood Alliance.
- Ohimian E, Angaye TCN, Inyang IR (2014) Toxicological Assessment of Groundwater Containing High Levels of Iron against Fresh Water Fish (Clarias gariepinus). Am J Envt Prot 3: 59-63.
- Ujile AA, Joel FO (2013) Adsorption process of Iron (lll) from borehole water on activated carbon from Nigerian Bamboo. Int J Enfr Sci Tech 5: 1321-1331.
- Phadke, Asmita Anil (2014) Iron Removal Using Electro- coagulation Followed By Floating Bead Bed Filtration. LSU Master's Theses. 1256.
- Seiyaboh EI, Angaye TCN, Ogidi O (2017) The biotreatment of high iron containing water for aquaculture using ripe and unripe peels of plantain. MOJ Toxicol 3: 110-113.
- Machova SJ, Svobodova Z (2014) Fish kill caused by aluminium and iron contamination in a natural pond used for fish rearing: a case report. Veterinarni Medicina 59: 573-581.
- Teien Hch, garmo OA, Atland A, Salbu B (2008) Transformation of iron species in mixing zones and accumulation on fish gills. Env Sci Tech 42: 1780- 1786.
Google Scholar , Crossref , Indexed at
Google Scholar , Crossref , Indexed at
Google Scholar , Crossref , Indexed at
Google Scholar , Crossref , Indexed at
Google Scholar , Crossref , Indexed at
Google Scholar , Crossref , Indexed at
Google Scholar , Crossref , Indexed at
Google Scholar , Crossref , Indexed at
Google Scholar , Crossref , Indexed at
Google Scholar , Crossref , Indexed at
Citation: Vinod MS, Salimkumar AV (2022) Metal Toxicity Taking New Forms-Iron Toxicity as an Emerging Threat to Aquatic Biome. J Fisheries Livest Prod 10: 376.
Copyright: © 2022 Vinod MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1714
- [From(publication date): 0-2022 - Apr 26, 2025]
- Breakdown by view type
- HTML page views: 1383
- PDF downloads: 331