Metabolomic Profiling of Electrolytes, Lipid and Steroids in Saliva during Menstrual Cycle
Received: 20-Sep-2022 / Manuscript No. ijrdpl-22-73998 / Editor assigned: 22-Sep-2022 / PreQC No. ijrdpl-22-73998 / Reviewed: 06-Oct-2022 / QC No. ijrdpl-22-73998 / Revised: 10-Oct-2022 / Manuscript No. ijrdpl-22-73998 / Accepted Date: 16-Oct-2022 / Published Date: 17-Oct-2022 QI No. / ijrdpl-22-73998
Abstract
During reproductive cycle, women’s do not show the consequent cyclical changes that would be an indicator of ovulation, therefore it is important to extend a method to identify the time of ovulation. Ovulation is such a decisive physiological process that its noninvasive detection based on salivary biochemicals has various advantages in human diagnosis. The present study is proposed to identify the ovulatory-specific biochemicals in saliva in order to identify ovulation phase. The rapid test and noninvasive study made in salivary biochemical’s as electrolytes, profile of lipids and putative hormones in saliva during pre-ovulatory, ovulatory and post-ovulatory phases of menstrual cycle. Saliva was collected from 120 menstruating women during preovulatory, ovulatory and postovulatory phases and electrolytes, lipid profile and hormones were analyzed. In particular electrolytes like sodium, potassium, lipids like total cholesterol, phospholipids, steroid hormones such as estrogen, progesterone and their derivatives were analyzed. Salivary electrolytes and lipid profiles in saliva increased significantly (<0.01) during the phase of ovulation at the time of LH surge while comparing to pre- and post-ovulatory phase also measured by ferning pattern. Estrogen and its derivative increased significantly (<0.01) during the ovulatory phase as compared to preovulatory phase, while progesterone and its conjugates increased significantly (<0.01) during post-ovulatory phase when compared to other two phases. As a conclusion, electrolytes, lipid profile and estrogen were found to be increased during ovulatory phase in normal menstruating women.
Keywords
Saliva; lipids; Electrolytes; Steroid hormones; Menstrual cycle
Introduction
Prediction of exact time of ovulation is important for infertility management in woman. The success of assisted reproductive technology (ART) is directly involved with the exact time of ovulation. Generally, the cyclic changes in gonadotropic hormones and steroid hormones levels in serum have been used as markers to predict ovulation. But the routine withdrawal of blood is stressful to the patient for the serum hormone analysis. In order to replace this existing invasive method, it is required to develop a reliable noninvasive method. Saliva collection is one of the easiest and least stressful body fluids to extract since it is noninvasive and stress-free. Hence, there is a need to develop salivary potential biomarkers for the prediction of ovulation on routine clinical practice. One way this has been achieved in recent years is by focusing more on the biochemical significance of saliva [1]. The main salivary glands (parotid, submandibular, and sublingual) create the majority of saliva, although the countless small labial, buccal, and palatal glands that line the mouth contribute a minor amount [2, 3]. Previous research has shown that ovarian hormones affect water and electrolyte balance [4]. Estrogen increases calcium up take and parathyroid activity in non-pregnant women [5, 6]. The parathyroid gland is widely known for maintaining calcium homeostasis, although the influence of the menstrual cycle on serum calcium is still debated [7]. Magnesium also plays a role in the menstrual cycle's basal metabolism, which varies entirely in the cycle [8]. According to these research, ovarian steroid hormones may affect calcium, magnesium, sodium, potassium, and inorganic phosphate metabolism at different stages of the menstrual cycle. The greater level of progesterone is related with its natriuretic action during the postovulatory phase due to a compensatory rise in aldosterone concentration [9]. And the higher level of progesterone is connected with its natriuretic impact [8].
Cholesterol produces steroid hormones as metabolic products. Fatty acids, which are crucial in the digestion, lubrication, and preservation of the oral mucosa, increase in concentration in the blood stream and saliva as cholesterol is broken down [10]. The possibility exists that these fatty acids and electrolytes might be associated with ovulation. As a result, analyzing lipids and electrolytes in saliva at different stages of the menstrual cycle may be useful in identifying potential salivary biomarkers for predicting ovulation. For fertility monitoring, salivary studies of female sex hormones have been employed.[11,12] Recent findings, however, suggest that these tests may have applications beyond the study of reproductive issues.[13] The bulk of estrogen in serum is bound to proteins, whereas the majority of estrogen in saliva is unbound, biologically active, and enters salivary glands by intracellular processes[14].The possibility exists that the level of estrogen and progesterone and their conjugates in saliva may be associated with the prediction of ovulation. We postulate that salivary biochemical parameters alter during different periods of the menstrual cycle based on these findings. The analysis of salivary biochemical parameters that are either decreased or increased during ovulatory period would be helpful in the identification of potential salivary biochemical marker to be used for the prediction of ovulation. To date, salivary biochemical markers that might be associated with ovulation have not been identified. Hence, in the present study, lipid profile, electrolytes and steroid hormones like estrogen and progesterone and its conjugates were analyzed in saliva collected during the menstrual cycles preovulatory, ovulatory, and post-ovulatory stages in order to identify potential salivary biochemical markers for the prediction of ovulation in women menstruating normally.
Materials And Methods
Collection of samples
Saliva samples were collected from 20 healthy and normally menstruating women (28+2 day’s cycle) aged 20-30 years from women’s hostel, Bharathidasan University. Daily oral body temperature measurements and ferning in saliva were used to determine the ovulation cycle. Saliva samples were obtained from all volunteers during the preovulatory (6-12 days), ovulatory (13-14 days), and postovulatory phases after their ovulatory periods were determined (15-26 days). Saliva was collected during various stages of the menstrual cycle, according to a protocol published elsewhere [12].In short, 10 ml of saliva was sampled between 8-9 am during each phase. No use of contraceptive pills or natural medicines was allowed from the participants during the study period. In order to reduce gingival bleeding, the participants were also encouraged to brush their teeth. At 4°C, saliva samples were centrifuged for 10 minutes at 5000 rpm. The supernatant was concentrated and then used immediately for the analysis of salivary biochemical constituents using standard protocol.
Salivary electrolytes assay
The quantification of various electrolytes in salivary samples were carried out following published protocol for calcium,[15], magnesium[16] , sodium[17] , potassium[18] and total phosphate[19] were analyzed using an atomic absorption spectrophotometer (Perkin and Elmer 2380).
Lipid profile assay
The lipid profile of saliva was carried out following the method of Folch et al. [21]. In summary, 1 mL saliva was well mixed in 2 mL of chloroform/methanol combination (2:1; v/v) containing 1 mL 0.9 percent sodium chloride. The samples were left to rest for 30 minutes. To make up the original volume, the bottom phase was separated and chloroform was added. This solution was dried on silica gel with vacuum desiccators, and the residue was cooked for 10 minutes in a boiling water bath. The mixture was then chilled to room temperature for 0.5 ml before being poured to a test tube with 5 ml vanillin reagent and 0.5 ml acid digest. The samples were vortexed well before being incubated at room temperature for 30 minutes. At 530 nm, the color was observed. Blank was made by combining 5ml of vanilla reagent with 0.5 ml distilled water as well as 0.5 ml acid digest. The cholesterol, triglycerides, phospholipids and lipoproteins were analyzed using commercial kits purchased from Boehringer-Mannheim Tests (Boehringer Mannheim, Mannheim, Germany). Preparative centrifugation of 10 ml saliva was carried out in a single step at 4 degrees Celsius and 40,000 rpm for 22 hours using a version of the gradient method published by Walton et al. [22]. The amounts of cholesterol [22], triglycerides [23], phospholipids [24], HDL-C, LDL-C, VLDL-C [25,26], and free fatty acids[27] were determined within each lipoprotein moieties using the techniques.
Extraction of samples and steroid hormones assay
Samples were ice covered soon after collection so as to breakdown the mucopolysaccharides. Samples (1 ml) were centrifuged at 4000 rpm for about10 min at 40C. The clear supernatant liquid was concentrated and used for determination of Estrone-1-glucuronide, pregnanediol- 3-glucuronide by immunoassay using Autodelfa kit purchased from Perkin-Elmer, Life sciences, Cambridge, UK. The estrogen and progesterone were analyzed using radioimmunoassay kit purchased from Diagnostics Systems Laboratories, Sinsheim, Germany. The sensitivity of estrogen, progesterone, E-1-G and Pd-3-G were 11pg/ml, 0.12ng/ml, 1-2 pg/ml, and 0.5-1.5 ng/ml, respectively. The intra-assay variation for progesterone, estrogen, E-1-G and Pd-3-G were 5.0pg/ ml, 5.2ng/ml, 1.8pg/ml and 1.0ng/ml, respectively. To avoid inter-assay variation, all samples were run in one assay.
Statistical analysis
Data are mean ± standard error of mean (SEM) of six replicates. Data are analyzed by one-way analysis of variance (ANOVA) using SPSS Software version 5.0 (SPSS Inc, Chicago, USA). A probability of p<0.01 was considered to be statistically significant.
Results
Salivary electrolytes levels during various phases of menstrual cycle
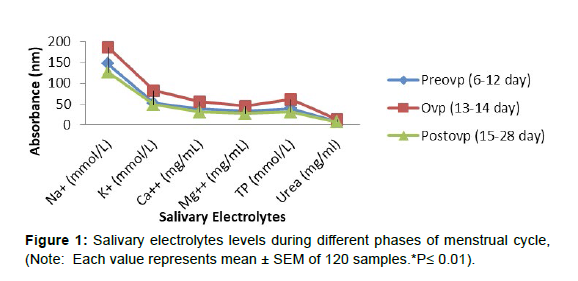
Figure 1 shows that during the ovulatory period, salivary electrolytes like calcium, magnesium, potassium, sodium, and inorganic phosphate levels increased (p 0.01) when compared to the preovulatory phase. However, when compared to the ovulatory phase, all salivary electrolytes, including urea, were considerably reduced (p 0.01) during the postovulatory phase, and the levels were comparable to the preovulatory phase.
Salivary lipid profile during various phases of menstrual cycle
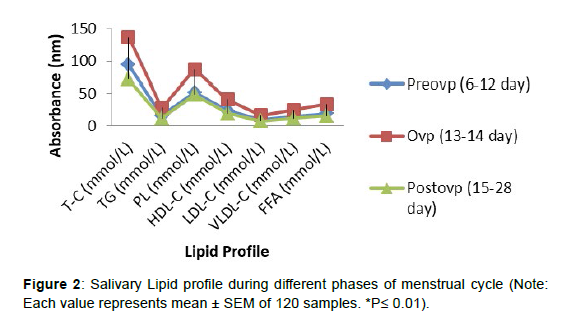
The salivary lipid profile during various stages of menstrual cycle is shown in Figure 2. During the ovulatory period, total cholesterol, triglycerides, phospholipids, HDL-C, LDL-C, and VLDL-C levels all increased significantly (p0.01) compared to the preovulatory phase. However, when compared to the ovulatory phase, all of these lipid profile elements in saliva decreased considerably (p0.01) during the postovulatory phase, and the levels were comparable to the preovulatory phase.
Salivary steroid hormones level during various phases of menstrual cycle
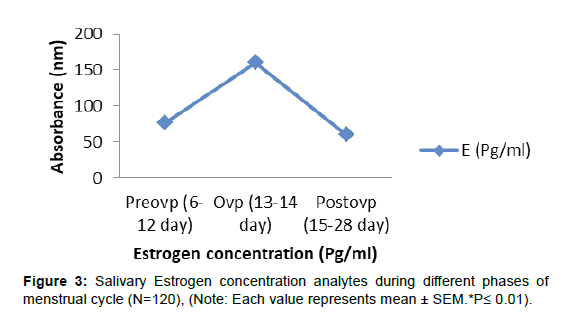
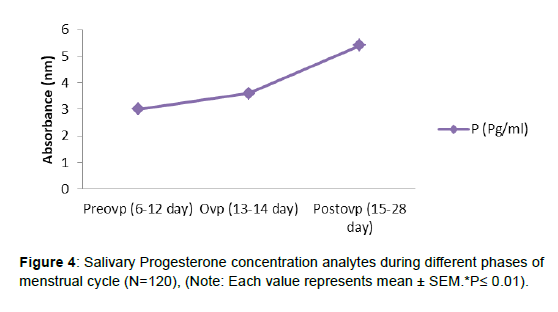
As indicated in Figure 3, as the menstrual cycle advanced from preovulatory to ovulatory and from ovulatory to postovulatory phases, salivary estrogen and its conjugate E-1-G levels decreased gradually. During the ovulatory phase, the levels of the other steroid progesterone and its conjugate P-3-G did not rise. In comparison to the preovulatory and ovulatory phases, there was a substantial (p0.01) rise in salivary progesterone and its conjugates during the postovulatory phase, (Figure 4, 5).
Discussion
The need for an accurate and noninvasive approach to predict the fertile period is growing. Despite significant progress in clarifying many intricacies of the physiologic processes of human reproduction over the last three decades, there is still no easy, reliable, affordable,and noninvasive technology for predicting and detecting the timing of ovulation. Biochemical elements of saliva appear to be the most effective ways of ovulation detection for therapeutic purposes. The current findings demonstrated that serum phosphorus levels in nine ovulating women did not change significantly throughout the menstrual cycle. A significant periovulatory surge in serum phosphorus was not discovered. These findings are consistent with those of other serum researchers. In contrast to Ben-Aryeh et al. [20] 's salivary phosphorus results, which demonstrated a substantial periovulatory surge of phosphorus in ovulating women, serum phosphorus concentrations in ovulatory and non-ovulatory women were stable. The findings of this and prior serum phosphorus studies, on the other hand, cannot be validly compared to salivary findings that looked at other physiological components. The fact that phosphorus in sputum peaks during the periovulatory phase yet serum phosphorus does not show any substantial oscillations suggests that phosphorus metabolism varies around ovulation. It's likely that active phosphorus release into saliva compensates for hormone-induced phosphorus changes. This theory has been advanced [21] and appears to be plausible because phosphorus, along with the intracellular messenger c-AMP, is implicated in a number of aspects of the menstrual cycle's regulation. Phosphorus appears to be controlled on its own by a number of feedback mechanisms and hormone systems, in addition to the above-mentioned regulating function in the menstrual cycle. According to Pitkin [24] parathyroid hormone (PTH) climbed continuously throughout the follicular phase (preovulatory), peaked at or just before LH surge, and then gradually decreased throughout the luteal phase (postovulatory). Calcium, on the other hand, appears to drop three to four days before ovulation before rising again. Estrogen is to blame for these changes; as estrogen levels rise preovulatory, PTHinduced hypocalcemia triggers PTH release, resulting in phosphouria [25]. Continuous phosphorus loss, according to the findings, should result in lower serum concentrations unless countered by another process. Because the results of this study reveal that serum phosphorus levels stay within normal ranges throughout the menstrual cycle, it's likely that the salivary gland or another regulator is in charge of maintaining appropriate blood phosphorus levels. The amount of sodium and other electrolytes in saliva and plasma during the menstrual cycle have not been studied. Total body water and intravascular volume remain unaltered since there is no correlation between changes in plasma and sodium and changes in weight, creatinine, urea, or albumin concentrations. As a result, it appears that sodium is lost in excess of water during the ovulation time. Because fluctuations in progesterone concentration during menstruation are unrelated to changes in plasma sodium, this hormone cannot be blamed for the shift between preovulatory and postovulatory phases. Increased levels of antidiuretic hormone in the postovulatory period could be one reason for the decline in salt content [26]. This change in saliva or plasma sodium may be related to many women who experience fluid imbalance during their premenstrual days. According to the current study, salivary electrolyte levels varied considerably depending on a woman's reproductive state. The study of the link between critical minerals and ovarian hormone activity has gained popularity in recent years. It's unclear whether individual mineral distributions change in a similar or parallel way. Even though the coordinated sequence of hormonal changes that happens throughout a typical menstrual cycle is well established. Several studies have found evidence of phase-related changes in blood and salivary components during the menstrual cycle [24, 3, 27]. Furthermore, results show that blood calcium, magnesium, potassium, sodium [28] and inorganic phosphate change in different ways [21, 29]. When compared to the postovulatory phase, salivary sodium levels rise gradually but dramatically during the ovulatory and preovulatory periods. As a result, salt appears to be lost in excess of water during the ovulation phase. Previous study has found that serum calcium levels are highest during the ovulatory phase and lowest during the postovulatory phase [6], with some researchers reporting a small but significant difference during the preovulatory period [29]. The recent data, according to Ben Areyh et al. [20] shows rise in potassium and decrease in calcium throughout the mid-cycle phase. In pigeons, estrogen has been found to promote parathyroid activity [24] leading to a considerable increase in calcium intake [30] and a decrease in calcium disposal from the stomach [4]. In otherwise healthy women, increasing calcium levels in the blood can lead to increased bone density [30]. As a result, it's clear that estrogen alone, via parathyroid hormone regulation, increased blood calcium levels during the preovulatory and ovulatory phases; on the other hand, estrogen withdrawal causes a significant drop in bone calcium. During the postovulatory phase, estrogen levels rose, while calcium levels remained low. This is unlikely to be explained just by estrogen levels and parathyroid activity; progesterone is more likely to have played a role. A larger dosage of progesterone than estrogen during the postovulatory period can interfere with estrogen mimics. Because of its priming effect, estrogen is employed to promote progesterone activity and may not be involved in calcium uptake during the postovulatory period. The amount of magnesium in the blood during the menstrual cycle is a point of contention [24, 27]. Salivary magnesium levels are highest during ovulatory and preovulatory phases and lowest during postovulatory periods, according to our findings. The preovulatory estrogen peak appears to be linked to the ovulatory phase indicate on salivary magnesium [30]. It may be related to luteinizing hormone and follicle stimulating hormone peaks occurring at the same time; however the gonadotropin impact on mineral storage is most likely dependent on an active gonad [31]. Changes in the BMR (basal metabolic rate) may be linked to changes in plasma magnesium levels [7]. During the preovulatory stage, increased carbohydrate utilization is associated to higher BMR and oxygen demand. This carbohydrate consumption needs the presence of magnesium ions and oxidative enzymes, both of which have been found to be significantly increased during the postovulatory period [32, 33]. Although serum magnesium levels have been associated to Basal Body Temperature BBT during ovulation and the postovulatory phase of the cycle[33] it is unclear to say whether magnesium increases are a cause or a result of increased heat content. Despite the fact that hormone levels were not studied in this study, it appears that progesterone is to blame for the simultaneous rises in BBT and serum magnesium in a single day [33]. When the potassium and magnesium concentrations, as well as the flow rate, of ovulatory electrolyte values were compared to those of other indicated periods in the menstrual cycle, a decrease in potassium and magnesium concentrations, as well as a decrease in flow rate, were discovered. These changes in the menstrual cycle are most likely caused by hormonal fluctuations, particularly estrogen. Although hormone levels were not assessed in this study, it seems likely that progesterone is to account for the simultaneous elevations in BBT and serum magnesium in a single day [33]. Ovulatory electrolyte concentrations demonstrated a decrease in potassium and magnesium concentrations, as well as a decrease in flow rate, when compared to other indicated periods in the menstrual cycle. The hormonal oscillations in the menstrual cycle, particularly estrogen, are most likely to blame for these changes in the menstrual cycle. Low density lipoprotein (LDL), high density lipoprotein (HDL),total cholesterol, triglycerides, phospholipids and free fatty acids all increase significantly during the menstrual cycle, with peak estradiol levels during ovulation matching to considerable elevations in total cholesterol, LDL,HDL, phospholipids, triglycerides, and free fatty acids. Estrogen has been demonstrated in rat models to reduce hepatic lipase activity while increasing LDL receptor activation, altering circulating lipoprotein levels. It's improbable that the observed variations in plasma cholesterol are due to a 17 percent reduction in caloric consumption during ovulation [34]. While in the ovulatory and preovulatory phases of the menstrual cycle, some researchers [35, 36] noticed increased total and LDL cholesterol concentrations. The mechanisms underlying month-to-month variations in saliva lipids are unknown. Estrogen-induced changes in cholesterol metabolism have been blamed in part for these differences. Lower salivary triglycerides and hepatic lipase activity may be responsible for the greater saliva HDL cholesterol levels in the preovulatory period, contrary to previous observations [36, 37]. The gonadal hormones are engaged not only in biomolecule excretion, but also in female behavior. Estradiol levels were shown to be higher during the menstrual cycles preovulatory and ovulatory stages. This study is unique in that it collects data on a range of lipid and lipoprotein measurements, as well as sex hormones, from well-characterized patients in a metabolically controlled environment during the preovulatory and postovulatory stages of the menstrual cycle. These data back up our theory that a lower cholesterol and lipoprotein profile during the postovulatory phase of the menstrual cycle is linked to a decreased risk of coronary heart disease than during the preovulatory phase [38]. Exogenous hormones, on the other hand, have been reported to cause considerably greater changes in lipid and lipoprotein levels in premenopausal women in studies examining these effects [37]. Throughout the menstrual cycle, endogenous levels of estradiol, progesterone, and other hormones are consistent. There are significant differences in the length of the menstrual cycle between and among women, in addition to differences in the underlying hormone patterns [39]. The length of a woman's menstrual cycle varies from one woman to the next and even from month to month for the same woman. Controlling these variations is critical because they make it difficult to investigate changes in cholesterol and lipoprotein levels over the menstrual cycle and are likely to account for some of the discrepancies in earlier findings [40, 36]. Between the preovulatory and ovulatory phases of the menstrual cycle, total cholesterol, triglycerides, phospholipids, free fatty acids, and lipoprotein levels all increased significantly. The fact that ovulation timing is difficult to predict.[41] Fluctuations in lipid and derivative levels between menstrual cycles must be taken into account in premenopausal women's screening and medical surveillance, especially those with borderline values. In the long run, however, such fluctuations may be clinically significant. The current investigation reveals that in free fatty acids determination anticipated with progesterone and estrogen have important effects on fat cell metabolism;[36,37,38] little is known about their role in modulating lipolysis in humans. Progesterone and estrogen and surely have major effects on the distribution of fat deposition in humans. This impact could be through either change in storage (lipoprotein lipase) [36,40] or release of lipolysis[35,37].Insulin is a major regulator of lipolysis in resting humans[42] and the failure to observe differences in insulin- stimulated glucose disposal in different phases of menstrual cycle prior to ovulation.[43] Finally, the findings show that sex steroid hormone alters while in the menstrual cycle have a considerable impact on overnight after absorptive free fatty acid flow or hypoinsulinemia response. The normal menstrual cycle's periodic fluctuations in estrogen and progesterone production appear to have modest, if any, influence on human adipose tissue lipolysis. Some studies have revealed that estrogen and progesterone have no effect on lipolysis [40] while the majority of studies use the rodent model to find a favorable effect [44, 45, 40]. It's unclear how much endogenous gonadal hormones influence systemic lipid and lipoprotein variations during the menstrual cycle. In the postovulatory period, however, lipids and lipoprotein patterns appear to have an estrogenic effect. The regular lipid variations observed during the menstrual cycle are comparable in magnitude to those caused by specific pharmaceutical preparations in particular variables. When studying lipid metabolism in fertile women, it's crucial to know at what stage of the menstrual cycle blood was obtained. The discovery that salivary estrogen level relates strongly with serum values in stimulated cycles contradicts a previous study [46] and supports the findings of others who have identified strong relationships, [47] in stimulated cycles [48,49]. Salivary estrogen levels are unusually high during the first half of normal ovulatory cycles; yet, the assay's sensitivity and precision allowed for successful distinction of periods of active estrogen production. The follicular phase was defined as the time before the preovulatory increase in estrogen began; the observed peak was defined as the highest value achieved each cycle in the preovulatory surge; and the postovulatory phase was defined as the time from a day after ovulation to the end of the cycle. Whenever averages are determined relative to ovulation time, the mean value is 138.2817.58 pg/ml; however, there is variation in peak estrogen timing relative to ovulation surge. A salivary estrogen concentration is measured during the trial to mimic plasma free-steroid content [46, 50]. Because of the high sensitivity attained by utilizing an I125-labeled radioligand, this simple, direct RIA used for salivary progesterone only required 500 l saliva for the assay, which most people can collect in less than 2 minutes. As a result, patients are more willing to accept samples. Progesterone levels in saliva during the preovulatory and postovulatory phases match the figures.[47] Progesterone measurement, on the other hand, is utilized to determine the incidence and merit of ovulation, with figures of 2.71±1.00 ng/ml; in contrast to the ovulatory stage, the postovulatory stage has a wider range throughout the menstrual cycle, with a mean figure of 5.40±1.31 ng/ml. Measurements collected while in the preovulatory stage are of limited clinical significance for this purpose. As indicated by the good correlation between these quantities, the levels of progesterone in saliva while in the postovulatory period is remarkably similar to that in plasma. As a result, salivary progesterone may be utilized to detect ovulation instead of plasma progesterone. Because salivary progesterone grows only 4 to 10-fold during the postovulatory phase, and plasma progesterone increases up to 100- fold, a single midluteal phase salivary progesterone measurement is less sensitive than a single midluteal phase plasma value. Because saliva collection is simple and painless, salivary progesterone measurement can be made more sensitive by analyzing multiple samples taken during the postovulatory phase. Saliva as a sample has various advantages over plasma, including the ability to collect multiple samples, which can be used to detect ovulation and, more importantly, the correct postovulatory period in a monthly cycle. Progesterone and estrogen and glucuronide conjugate metabolites in saliva closely resemble the profiles of their parent hormones in circulation [51]. Due to the noninvasive nature of saliva collection, estimations of these glucuronide conjugates in saliva outperform those of their respective parent hormones in blood, allowing for more frequent sample and patient monitoring over time. The link backs up the idea that estrogen production by the ovaries may be accurately tracked using saliva, urine, and blood samples. Since increased estrogen emission can be seen as early as three days before ovulation, it may be useful in predicting ovulation. Preovulatory estrogen secretion is accurately mirrored by an increase in estrogen emission in the late follicular phase, with an increase observable on the same day as or one day before the serum LH surge [52]. Recent research has shown that employing direct RIA to quantify ovarian steroid hormone metabolites in saliva can be utilized to study ovarian follicular maturation, as well as peri-implantation events, as an alternative to collecting blood samples daily for hormone testing. During the menstrual cycle, there is an increase in electrolytes such as sodium, potassium, and phosphate, lipids such as phospholipids and HDL-cholesterol, and hormones such as estrogen and progesterone conjugates such as E-1-G and pd-3-G activity, which is especially noticeable from the preovulatory to postovulatory phase, when ovarian estrogen production is at its peak. In addition, the current study reveals a clear relationship between hormonal results and the physiologically protective role of numerous reproductive functions. As a result, the presence of salivary biochemical such electrolytes, lipids, and hormone conjugates in saliva during ovulation could lead to the development of an ovulation biomarker.
Conclusion
To find out the exact time of ovulation is important for women who need to get conceived or undergo Invitro fertilization. The observation of ovulation has long been practiced by women pursuing or avoiding pregnancy. The fertility window begins approximately 3–5 days (sperm lifespan) before ovulation and continues to a point approximately 1–2 days (oocyte lifespan) after ovulation. Time of fertility window is used to detect simply identifying or detecting ovulation, is vital for encouraging or discouraging contraception. Fluctuation of electrolytes like sodium, potassium during phases of menstrual cycle is increased and falls significantly (<0.01) during ovulatory phase, lower in postovulatory phases of cycle. LH surge was found to be extremely up-and-down in the relationship, amplitude and period of cyclic changes during menstruation period. The estrogen and progesterone conjugates like E-1-G and pd-3-G was found to be increased significantly at (<0.01) in the phases of ovulation and post ovulation. The assisted reproductive technologist or women who wish to know if menstrual cycle is normal or to evaluate ovarian function, a test that retrospectively confirms ovulation needs to be adequate, but in order to avoid invitro fertilization for retrospective protocol, time of ovulation and fertility window should be define evidently. Thus, the presence of salivary biochemical like electrolytes, lipids and hormone conjugates expressed in saliva during ovulation makes the possibility of developing biomarkers for the detection of ovulation.
References
- Dam JG, Loenen AC (1978) De aanwezigheid van geneesmiddelen in speeksel. Pharmaceutisch Weekblad. 113: 65-74.
- Vining RF, McGinley RA (1985) Hormones in saliva. Crit Rev Clin Lab Sci. 23: 95-146.
- Dadlani AG, Chandwani S, Desai CA, Pandya KD (1982) Serum electrolytes during various phases of menstrual cycle. Indian J Physiol Pharmacol 26: 302-306.
- Silberberg M, Silberberg R (1956) In Biochemistry and physiology of bone. Ed.Bourne GH Academic press. 632-644.
- Wernly M, Davis ME (1965) Eur J Obstetrics Ed. Greenhill JP W.B Saunders Co. London: 244.
- Southam AL, Gonzaga FP (1965) Systemic changes during the menstrual cycle. Am J Obstet Gynecol. 91:142-165.
- Solomon SF, Kurer MS, Calloway DM (1982) Menstrual cycle and basal metabolic rate in women. Am J Clin Nutr 36:611-616.
- Landau RL, ugibihl K (1958) Inhibition of the sodium-retaining influence of aldosterone by progesterone. J Clin Endocrinol Metab 18: 1237-1245.
- Korda AR, Horvath JS (1979) Renal physiology. In human reproductive physiology RP Shearman Ed Blackwell Scientific Publications London.
- Vining RF, McGinley RA, Symons RG (1983) Hormones in saliva: mode of entry and consequent implications for clinical interpretation. Clin Chem 29: 1752-1756.
- Read GF (1993) Status report on measurement of salivary estrogens and androgens. Ann NY Acad Sci 694: 146-160.
- Hofman L (2001) Human saliva as a diagnostic specimen. J Nutr 131: 1621-1625.
- Lipson SF, Ellison PT (1996) Comparison of salivary steroid profiles in naturally occurring conception and non-conception cycles. Hum Reprod 11:2090-2096.
- Navazesh M (1993) Methods for collecting saliva. Ann N Y Acad Sci 20: 72-77.
- Bosch JA, Brand HS, Ligtenberg TJ, Bermond B, Hoogstraten J, et al. (1996) Psychologicalstress as a determinant of protein levels and salivary-induced aggregation of Streptococcus gordonii in human whole saliva. Psychosom Med 58: 374-382.
- Baron DN, Bell JL (1959) Compleximetric determination of calcium in pathological and physiological specimens. J Clin Pathol 12: 143-148.
- Neill DW, Neely RA (1956) The estimation of magnesium in serum using titan yellow. J Clin Pathol 9:162-163.
- Trinder P (1951) A rapid method for the determination of sodium in serum. Analyst 76: 596.
- Jacobs RD, Hoffman WS (1931) Quantitative Estimation of Urinary Potassium. J Biol Chem 93: 685.
- Ben-Aryeh H, Filman S, Gutman D, Szargel R, Paldi E, et al. (1976) Salivary phosphate as an indicator of ovulation. Am J Obstet and Gynecol 125: 871.
- Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497-509.
- Walton KW, Scott PJ, Dykes PW, Davies JW (1965) The significance of alterations in serum lipids in thyroid dysfunction. II. Alterations of the metabolism and turnover of 131-I-low-density lipoproteins in hypothyroidism and thyrotoxicosis. Clin Sci 29: 217-238.
- Pitkin RM, Reynolds WA, Williams GA, Hargis GK (1978) Calcium-regulating hormones during the menstrual cycle. J Clin Endocrinol Metab. 47: 626-632.
- DeLuca HF (1976) Metabolism of vitamin D: current status. Am J Clin Nutr 29: 258-270.
- Mikkelsen WM (1965) The possible association of hyperuricemia and/or gout with diabetes mellitus. Arthritis Rheum 8: 853-864.
- Patricia AD (1987) Magnesium and zinc status during menstrual cycle. Am J Obstet and Gynecol 157: 964-968.
- Goldsmith NF, Johnston JO (1975) Bone mineral: effects of oral contraceptives, pregnancy, and lactation. J Bone Joint Surg 657-668.
- Voda AM, Wilde K, Gill BP (1980) Serum phosphorus during the menstrual cycle. Contraception. 22: 63-69.
- Frank HA, Carr MH (1957) Normal serum electrolytes with a note on seasonal and menstrual variation. J Lab and Clin Med 42: 246-252.
- Heaney RP (1993) Thinking straight about calcium N Engl J Med 328: 503-505.
- Puskulian L (1972) Salivary electrolyte changes during the normal menstrual cycle. J Dent Res 51: 1212-1216.
- Jones DY, Judd JT, Taylor PR, Campbell WS, Nair PP, et al. (1988) Menstrual cycle effect on plasma lipids.Metabolism 37: 1-2.
- Das TK, Jana H (1991) Basal oxygen consumption during different phases of menstrual cycle. Indian J Med Res 94: 16-19.
- Lyons PM, Truswell AS, Mira M, Vizzard J, Abraham SF, et al. (1989) Reduction of food intake in the ovulatory phase of the menstrual cycle. Am J Clin Nutr 49: 1164-1168.
- Haines CJ, Cheung LP, Lam CW (1997) Changes in atherogenic lipids and lipoproteins during natural and hyper stimulated cycles in healthy women. Fertil Steril 68: 231-235.
- Mattsson LA, Silfverstolpe G, Samsioe G (1984) Lipid composition of serum lipoproteins in relation to gonadal hormones during the normal menstrual cycle. Eur J Obstet Gynecol Reprod Biol 17: 327-335.
- Rebuffe-Scrive M, Lonnroth P, Marin P, Wesslau C, Bjorntorp P, et al. (1987) Regional adipose tissue metabolism in men and postmenopausal women. Int J Obes 11: 347-355.
- Barnett JB, Woods MN, Lamon-Fava S, Schaefer EJ, McNamara JR, et al. (2004) Plasma lipid and lipoprotein levels during the follicular and luteal phases of the menstrual cycle. J Clin Endocrinol Metab 89: 776-782.
- Venners SA, Liu X, Perry MJ, Korrick SA, Li Z, et al. (2006) Urinary estrogen and progesterone metabolite concentrations in menstrual cycles of fertile women with non-conception early pregnancy loss or clinical pregnancy. Hum Reprod 21: 2272-2280.
- Rebuffe-Scrive M, Enk L, Crona N, Lonnroth P, Abrahamsson L, et al. (1985) Fat cell metabolism in different regions in women. Effect of menstrual cycle, pregnancy, and lactation. J Clin Invest 75: 1973-1976.
- Yen SS, Vela P, Rankin J, Littell AS (1970) Hormonal relationships during the menstrual cycle. JAMA 2: 1513-1517.
- Yki-Jarvinen H (1984) Insulin sensitivity during the menstrual cycle. J Clin Endocrinol Metab 59: 350-353.
- Sufi SB, Donaldson A, Gandy SC, Jeffcoate SL, Chearskul S, et al. (1985) Multicenter evaluation of assays for estradiol and progesterone in saliva. Clin Chem 31: 101-103.
- Johnson AJ, James DO, Baumber JS, Schneider E (1970) Effect of estrogen and progesterone on electrolyte balance in normal dog. Am J Physiol 219: 1691-1697.
- Kim HJ, Kalkhoff RK (1979) Changes in lipoprotein composition during the menstrual cycle. Metabolism 28: 663-668.
- Choe JK, Khan-Dawood FS, Dawood MY (1983) Progesterone and estradiol in the saliva and plasma during the menstrual cycle. Am J Obstet Gynecol 1: 557-562.
- Davis RH, Balin H (1973) Saliva glucose: a useful criterion for determining the time of fertility in women. Am J Obstet Gynecol 15: 287- 288.
- Brannstrom M, Mayrhofer G, Robertson SA (1993) Localization of leukocyte subsets in the rat ovary during th periovulatory period. Biol Reprod 48: 277-286.
- Brown WV, Levy RI, Fredrickson DS (1970) Further characterization of apolipoproteins from the human plasma very low density lipoproteins. J Biol Chem 25: 6588-6594.
- Riad-Fahmy D, Read GF, Walker RF, Griffiths K (1982) Steroids in saliva for assessing endocrine function. Endocr Rev Fall 3: 367-395.
- Stanczyk FZ, Miyakawa I , Goebelsmann U (1980) Direct radioimmunoassay of urinary estrogen and pregnanediol glucuronides during the menstrual cycle. Am J Obstet Gynecol 15: 43-50.
- Munro CJ, Stabenfeldt GH, Cragun JR, Addiego LA, Overstreet JW, et al. (1991) Relationship of serum estradiol and progesterone concentrations to the excretion profiles of their major urinary metabolites as measured by enzyme immunoassay and radioimmunoassay. Clin Chem 37: 838–844.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Alagendran S, Ponraj M, Rajasekaran M, Saarvedra GF, Chiyomba L (2022) Metabolomic Profiling of Electrolytes, Lipid and Steroids in Saliva during Menstrual Cycle. Int J Res Dev Pharm L Sci, 8: 138.
Copyright: © 2022 Alagendran S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1898
- [From(publication date): 0-2022 - Dec 22, 2024]
- Breakdown by view type
- HTML page views: 1691
- PDF downloads: 207