Research Article Open Access
Metabolic and Exercise Performance Responses to Two Different Oral Doses of Calcium Lactate
Morris DM1,2* Beloni RK1, Wofford H1 and Roslanova E2
1Department of Health and Exercise Science, Appalachian State University, Boone, NC, USA
2Department of Kinesiology, University of Texas – Permian Basin, Odessa, TX, USA
- *Corresponding Author:
- David Morris
Department of Kinesiology
University of Texas of the Permian Basin
Mesa Building, Rm. 3152 4901
East University Blvd. Odessa, TX, USA, 79762-0001
Tel: 432-552-2332
E-mail: morris_da@utpb.edu
Received Date: December 19, 2016; Accepted Date: December 27, 2016; Published Date: December 30, 2016
Citation: Morris DM, Beloni RK, Wofford H, Roslanova E (2016) Metabolic and Exercise Performance Responses to Two Different Oral Doses of Calcium Lactate. Sports Nutr Ther 1: 117. doi: 10.4172/2473-6449.1000117
Copyright: © 2016 Morris DM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Nutrition Science Research
Abstract
We investigated the effects of two different oral doses of lactate on blood lactate, bicarbonate (HCO3-) and pH levels and performance of high-intensity exercise in endurance-trained, competitive cyclists. Twelve subjects consumed 120 (L120) or 300 mg lactate/kg bm (L300) or placebo (PL) 80 min prior to performing an exercise performance test consisting of five high-intensity efforts followed by a timed, high-intensity effort to exhaustion. Seventy minutes following lactate ingestion, blood HCO3- increased in L120 by 10% (p=0.03) and in L300 by 9% (p=0.02) with no significant difference observed between lactate treatments (p=0.74). No significant change in blood HCO3- was seen following consumption of PL (- 0.1%, p=0.98). Blood lactate increased by 27% in L300 (p=0.02) with no significant changes in response to PL or L120. No changes in pH were observed due to treatment. Time to exhaustion in the performance test was increased by 14% in L120 (p=0.004) and 26% in L300 (p=0.001) when compared to PL. There was no significant difference in time to exhaustion between the lactate treatments (p=0.09). Consumption of 120 or 300 mg lactate/kg bm by endurance-trained subjects increased blood HCO3- and improved exercise performance during high-intensity exercise bouts of approximately 150–180 s. However, consuming 300 mg lactate/kg bm provided no clear ergogenic effect compared to consuming 120 mg lactate/kg bm.
Keywords
Buffering; Bicarbonate; Performance
Abbreviations
PETE: Progressive Exercise Test to Exhaustion; MPO: Maximum Power Output during PETE; IPT: Interval Performance Test; L120: Experimental trial using 120 mg lactate/kg body mass; L300: Experimental trial using 300 mg lactate/kg body mass; PL: Experimental trial using Placebo; TTE: Time to Exhaustion; Pre-Con: Time period immediately prior to lactate consumption; Post- Con: Time period 70 min following the consumption of lactate
Introduction
Prolonged, high-intensity exercise can result in the release and accumulation of hydrogen ions (H+), which causes acidosis and contributes to muscular fatigue [1-4]. Numerous mechanisms can buffer H+, including bicarbonate (HCO3-) found in the blood. Metaanalysis of studies of sodium bicarbonate (NaHCO3) consumption revealed that oral consumption of NaHCO3 can increase blood HCO3 and pH levels and improve exercise performance [5]. Furthermore, the ergogenic effect of NaHCO3 appears to be dose related with consumption levels of 300 mg/kg bm providing greater and more consistent effects than lower doses [5].
Blood HCO3- levels can also be enhanced by oral lactate consumption [6-8]. When lactate is converted into glucose, 2 C3H5O3-+ 2 H+ → C6H12O6, or is oxidized, C3H5O3- + 3 O2 + H+ → 3 CO2 + 3 H2O, H+ are consumed which fortifies blood HCO3- by reducing HCO3- degradation. Three previous investigations have assessed metabolic and ergogenic effects of oral lactate consumption. Morris et al. [7] observed a significant improvement in high-intensity cycling exercise to exhaustion following the consumption of 120 mg lactate/kg bm in trained, competitive cyclists. Van Monfoort et al. [8] saw a four percent increase in high-intensity running to exhaustion in trained competitive runners following consumption of 318 mg lactate/kg bm; however, these authors did not disclose whether this improvement was statistically significant. De Salles Painelli et al. [6] compared the consumption of 122 and 244 mg lactate/kg bm in untrained subjects and observed significant increases in blood HCO3 - following lactate ingestion compared to placebo; however, no differences were noted between the doses. Furthermore, De Salles Painelli et al. [6] found no ergogenic effect of lactate consumption on high-intensity armcranking ergometry.
The use of untrained subjects by De Salles Painelli et al. [6] makes it difficult to compare those results to the results of Morris et al. [7] and Van Monfoort et al. [8]. Training status affects rates of lactate oxidation and conversion to glucose [9,10] and, therefore, could affect the HCO3- and ergogenic responses to lactate consumption. The ability of untrained subjects to replicate performance during unfamiliar modes of exercise is also cause for concern. Thus, the interrelationship between lactate dose, blood HCO3-, and exercise performance in endurancetrained athletes is unclear. We sought to verify the ergogenic efficacy of oral lactate consumption and investigate dose-response relationships of two different lactate doses on blood levels of HCO3- and pH and performance of high-intensity exercise to exhaustion in trained, competitive cyclists.
Materials and Methods
Prior to subject recruitment, the procedures of this investigation were reviewed and approved by the institutional review board of Appalachian State University. Twelve competitive cyclists (11 males, 1 female) with at least one year of competitive road cycling experience were recruited and completed this study. All subjects were currently training and competing and each demonstrated his/her willingness to participate by providing written informed consent. Mean ± SD descriptive values for these subjects were 21 ± 2 years, 70.1 ± 7.3 kg, 172.4 ± 8.1 cm, with a VO2 max of 64.7 ± 4.8 mL/kg/min.
During their initial visit, subjects completed a progressive exercise test to exhaustion (PETE) to determine their maximal oxygen consumption (VO2 max) and power output at VO2 max (MPO). Subjects then performed a practice trial of an interval performance test (IPT) that was performed during subsequent visits to evaluate the ergogenic effects of the lactate supplements. After the initial visit, subjects returned on three occasions to perform the IPT following acute consumption of 120 mg lactate/kg bm (L120), 300 mg lactate/ kg bm (L300), or an equal volume of aspartame placebo (PL). All tests were performed on a Lode Excalibur Sport electronically braked cycle ergometer (Lode, Groningen, The Netherlands) placed in the hyperbolic (cadence independent) mode with the saddle and handlebar positions adjusted to the dimensions of each subject’s bicycle.
PETE and determination of MPO
Following a standardized warm-up, the PETE began at 3 W/kg bm and increased in a step-wise fashion by 0.3 W/kg bm/min until exhaustion. Expired gas was analyzed using a Parvomedics Trueone 2400 metabolic cart (Parvomedics, Sandy, UT) using 10-s rolling averages. Criteria for a successful test were volitional exhaustion of the subject combined with an RER greater than 1.15, and a maximal heart rate similar to the age-estimated maximal heart rate (220 – age). At least 30 s of the final stage had to be completed for that work rate to be used as the MPO, otherwise, MPO was taken from the final completed stage.
Interval Performance Test (IPT)
The IPT was identical to that used previously [7] and consisted of four, one-min work intervals at 100% of subjects’ MPO, each followed by a one-min active recovery period performed at 25% of MPO. Immediately following the final recovery period, subjects performed a fifth effort to exhaustion at 100% of MPO. Pedaling cadence during the work intervals was maintained at a similar rate to the cadence of the PETE. Exhaustion during the final effort of the IPT was marked by volitional exhaustion of the subject or if cadence dropped below 50 rpm for more than eight consecutive seconds. Consistent verbal encouragement was provided throughout the IPT from two members of the research team. Time to exhaustion (TTE) was measured to the nearest second on the final effort with a hand held stopwatch. Workloads and timing of the intervals for each IPT were controlled by the automatic program feature of the Lode ergometer.
Protocol
Subjects performed the IPT on four occasions. The first IPT was used only to familiarize the subjects and the results were not used in the data analyses. Subjects then returned to the laboratory on three occasions to perform the IPT after consuming their experimental treatment. Treatments were applied in a randomized, double-blind, crossover fashion and were consumed over a short (<5 min) time period with 30 mL water/g lactate consumed during L300. Calcium lactate, from a factory sealed container (Puracal, Purac America, Lincolnshire, IL), was weighed to the nearest milligram, adjusted for calcium content, and placed in clear gelatin capsules. The number of capsules was consistent among the treatments. During L120, subjects received a combination of calcium lactate and placebo to keep the number of capsules consistent between L120 and the other two treatments. Placebo capsules were visibly indistinguishable from the lactate capsules. Capsules from each treatment were sprinkled with small amounts of aspartame to disguise them from any residual aspartame that may have remained on the gelatin capsules of the placebo. At least 48 h separated each IPT and all were completed within a 14-day period. The number of days between each IPT and the time of day for each visit was consistent for each subject. Subjects were required to refrain from vigorous exercise on the day before each trial (no more than 30 min of total activity at a heart rate of ≤ 65% of maximal heart rate) and to maintain similar training schedules and dietary regimens for 48 h prior to each IPT. Subjects were required to consume a carbohydrate rich meal the evening before the day of each laboratory visit. Training and dietary records were kept by each subject for the duration of his/her involvement in the investigation and were inspected upon arrival for each IPT.
Subjects arrived at the laboratory three-hours post-prandial. If dietary and training requirements had been followed, a 22 g catheter was placed in a prominent antecubital vein. Subjects then rested quietly in a seated position for 10 min. After this rest but before consumption of the experimental treatment, a 1 mL blood sample was drawn and discarded followed by a 1 mL sample that was immediately analyzed for blood lactate, pH, and HCO3- using an Abbott iStat1 analyzer with a CG-4+cartridge (Abbott Point of Care, Princeton, NJ) calibrated to manufacturer’s specifications. This device has been previously validated for accuracy and consistency in measuring blood HCO3-, lactate, and pH [11]. Subjects were then asked to rate their level of perceived illness (RPI) and level of perceived stomach ache (RPSA) on a 10-point Like scale with 0 being no illness or stomach ache and 10 being vomiting and doubled-over for the RPI and RPSA, respectively. Following collection of the pre-consumption (Pre-Con) blood, RPI, and RPSA data, subjects consumed their experimental supplement. Subjects then rested, consuming no food or water, for 70 min. Following the 70- min rest period, a second blood sample was obtained and analyzed as previously described and RPI and RPSA were measured (Post-Con). Subjects then mounted the cycle ergometer and began a standardized 10-min warm-up for the IPT. Immediately following the warm-up, the subject pedaled at a work rate of 25% of MPO for one minute before beginning the first work interval of the IPT.
Statistical analyses
Two-way repeated measures ANOVAs were performed to detect differences in blood bicarbonate, lactate, and pH resulting from changes in time and treatments. A one-way repeated measures ANOVA was used to detect differences between treatments for TTE. Post hoc tests using Sidak correction procedures were used where appropriate. A Pearson correlation was performed to measure the correlation between changes in blood bicarbonate (Pre-Con vs. Post-Con) and changes in TTE. All results are reported as M ± SD. Level of significance was set a priori at P ≤ 0.05.
Results
Blood measures
No significant differences in Pre-Con HCO3- levels were observed among treatments. Mean Post-Con HCO3- did not change following placebo consumption (range: -0.7 - + 0.8 mmol/L, p=0.98) but were significantly elevated compared to Pre-Con in L-120 by 2.5 ± 1.7 mmol/L (range: 0.7 – 6.7 mmol/L, p=0.03) and L-300 by 2.6 ± 1.2 mmol/L (range: 0.4 – 4.5 mmol/L, p=0.02). The increases in blood HCO3- of L120 and L300 were not significantly different (P=0.74). There were no significant effects of treatment on blood pH (p=0.22–0.50). Changes in blood lactate Pre-Con vs. Post-Con were not significant for PL (p=0.61) or L120 (p=0.84), but was increased significantly in L300 (p=0.002). Blood results are presented in Table 1.
| Time | Pre-Con | Post-Con | |||
|---|---|---|---|---|---|
| Treatment | Mean ± SD | 95% CI | Mean ± SD | 95% CI | |
| PL | |||||
| HCO3- | 29.5 ± 2.0 | 27.5 – 31.6 | 29.6 ± 2.0* | 27.5 – 31.7 | |
| pH | 7.34 ± 0.02 | 7.32 – 7.36 | 7.31 ± 0.07 | 7.25 - 7.37 | |
| Lactate | 0.90 ± 0.35 | 0.58 – 1.22 | 0.96 ± 0.36 | 0.63 – 1.29 | |
| L120 | |||||
| HCO3- | 29.0 ± 2.9 | 26.3 – 31.8 | 32.0 ± 1.6† | 30.6 – 33.5 | |
| pH | 7.36 ± 0.02 | 7.34 – 7.38 | 7.36 ± 0.02 | 7.35 – 7.37 | |
| Lactate | 0.91 ± 0.24 | 0.69 – 1.13 | 0.91 ± 0.30 | 0.63 – 1.19 | |
| L300# | |||||
| HCO3- | 29.2 ± 1.9 | 27.4 – 31.0 | 31.8 ± 1.5† | 30.3 – 33.2 | |
| pH | 7.36 ± 0.03 | 7.33 – 7.39 | 7.37 ± 0.03 | 7.34 – 7.40 | |
| Lactate | 0.77 ± 0.12 | 0.47 – 1.07 | 1.07 ± 0.13† | 0.74 – 1.4 | |
Pre-Con=Pre-consumption of treatment.
Post-Con=70 min post-consumption of treatment.
PL=Placebo
L120=120 mg lactate/kg bm
L300=300 mg lactate/kg bm
All bicarbonate and lactate values are expressed in mmol/L.
*Significantly different vs. other treatments of the same time period (p<0.05).
†Significantly different vs. Pre-Con time period for the same treatment (p<0.05).
#Due to a technical error, blood values for L300 are from 11 subjects.
Table 1: Blood Bicarbonate, pH, and Lactate Responses.
Exercise performance, RPI, and RPSI
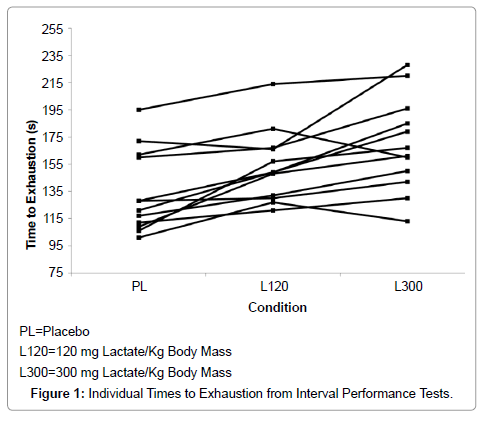
IPT TTEs were 134 ± 30 s (95% CI = 117 – 154 s) in PL and increased to 153 ± 25 s (95% CI = 137 – 169 s) in L120 (p=0.004) and to 169 ± 37 s (95% CI = 145 – 193 s) in L300 (p=0.001 vs. PL, p=0.086 vs. L120). TTE effect size and statistical power were 0.63 and 1.00, respectively. Individual performance data are presented in Figure 1. Correlation between the changes in Pre-Con vs. Post-Con blood bicarbonate and the changes in TTE was 0.46 (p=0.04). There were no reports of substantial illness or stomach ache. Mean values for RPI and RPSI were less than one for both measurement periods of each treatment and no subject reported any RPI or RPSI of greater than one.
Discussion
The novel purposes for this investigation were to compare the blood HCO3- and exercise performance responses to two different oral doses of lactate in trained cyclists. Previous works demonstrated that lactate consumption increases blood HCO3- [7] and exercise performance in trained individuals [7,8]. However, those investigations did not clearly establish optimal dose levels in trained athletes. The current investigation reaffirmed that consumption of 120 mg lactate/kg bm increases blood HCO3- and exercise performance when compared to placebo in endurance-trained athletes. When the lactate dose was increased to 300 mg/kg bm, blood HCO3- and exercise performance again significantly increased compared to placebo but was not significantly different compared to L120. Exercise performance in L300 was significantly elevated compared to PL but not compared to L120.
Previous investigations of the effects of lactate consumption have demonstrated positive responses in blood HCO3- levels [6,7] and mixed [6,7] or inconclusive [8] results with respect to exercise performance. The HCO3- responses observed in the current and previous work from our laboratory [7] exhibited similarities and differences compared to those of De Salles Painelli et al. [6]. Like De Salles Painelli et al. [6], the current results showed increases in HCO3- following lactate consumption, but no difference in response between the high and low doses. However, the magnitude of the HCO3- response to the lactate doses appeared to be greater (ten and nine percent respectively for L120 and L300) in the current investigation when compared to De Salles Painelli et al. [6] who observed increases of approximately five percent. These differences could be due to the accelerated capacity for lactate oxidation and conversion of lactate to glucose due to exercise training [9,10]. Regardless of training status, however, consumption of 120 mg lactate/kg bm appears to be sufficient to result in peak lactate-induced increases of blood HCO3- in endurance trained subjects. The inability of lactate doses above 120 mg/kg bm to further raise blood HCO3- and the significant increase in Post-Con blood lactate levels in only L300 suggests that consumption of 120 mg lactate/kg bm may provide lactate at a rate that meets the maximum rate of lactate disposal via oxidation and gluconeogenesis.
No significant changes were observed in blood pH in response to lactate consumption which is consistent with previous work from our laboratory [7], but is in contrast to those of De Salles Painelli et al. [6] who observed significant increases in pH following lactate consumption. These contrasting results are perplexing and not readily explainable considering that in the current and previous [7] works from our laboratory, HCO3- response was greater than that seen by De Salles Painelli et al. [6]. The observed ergogenic effect in the current investigation without changes in blood pH is also concerning as previous investigations of the ergogenic effects of sodium bicarbonate ingestion link increases in performance with increases in pH [5]. However, blood lactate can be transported into the skeletal muscle where it can be oxidized in a process that consumes H+ (C3H5O3- + 3 O2+ H+ → 3 CO2 + 3 H2O). Thus, it is possible that oral consumption of lactate may raise muscle pH which could result in an ergogenic effect without a change in blood pH.
The performance enhancement following lactate ingestion in the current study is congruent with Morris et al. [7] but differed from De Salles Painelli et al. [6] who saw no ergogenic effect. Van Montfoort et al. [8] observed a four percent increase in high-intensity running time to exhaustion following the consumption of lactate, but it is unclear if this represented a statistically significant improvement in performance. Care must be taken in comparing the performance results from De Salles Painelli et al. [6] to the others for several reasons. First, in the current and previous [7] studies, blood HCO3- rose to a greater extent than what was reported by De Salles Painelli et al [6]. These differences could have contributed to greater H+ efflux from the muscle to the blood and resulted in an environment in the working muscle that was more favorable for muscle contraction. Secondly, in the current study and those of Morris et al. [7] and Van MontFoort et al. [8], endurancetrained athletes served as subjects, while De Salles Painelli et al. [6] used untrained individuals. Trained subjects have been shown to exhibit more consistent performances in repeated, intense exercise trials when compared to untrained individuals [12]. Third, in the current study and that of Morris et al. [7], trained cyclists were tested using cycling ergometry and in the investigation of Van MontFoort et al. [8], trained distance runners were tested using a treadmill protocol. Thus, subjects performed familiar modes of exercise and, furthermore, in all three of these investigations, the subjects performed a practice trial of the exercise performance tests prior to the experimental trials. In contrast, De Salles Painelli et al. [6] had untrained subjects perform an arm ergometry protocol and made no mention of familiarization with arm ergometry or allowing subjects to practice the exercise protocol before performing the experimental trials. Therefore, the possibility of a learning effect obscuring ergogenic effects cannot be ignored. Finally, De Salles Painelli et al. [6] used peak power during a series of Wingate tests as a performance measure. However, it was not clear if the power measurements took into consideration the acceleration of the ergometer flywheel. Acceleration of the ergometer flywheel requires substantial power input and is not measured with many Wingate analysis programs. If this aspect of performance is not measured, calculated values of peak power can be significantly underestimated [13] which could mask differences in exercise performance.
Time to exhaustion performance test has been criticized for having low levels of reliability [14]. However, most critiques of TTE tests have focused on relatively low-intensity, long duration (>1 h) tests. During long TTE tests, factors other than physical performance ability, such as boredom and discomfort of sitting on a bicycle saddle, may affect subjects’ willingness to perform and, therefore, the reliability of the test. In contrast, studies that have assessed reliability of TTE tests ranging from approximately 60–90 s have revealed relatively low (< 6%) coefficients of [15,16]. We have tested the reliability of the IPT used in the current study by having 10 subjects perform the test three times over a 6–14 day period and found an average variation of 1.5% in performance times when comparing the three rides [17]. Comparatively, in the current study, the average improvements for L120 and L300 were 14% and 26%, respectively, compared to PL.
The current investigation demonstrates that oral consumption of 120 or 300 mg lactate/kg bm increases blood HCO3- levels and improves performance of high-intensity exercise to exhaustion. Consuming 300 mg lactate/kg bm did not increase blood HCO3- levels more than consuming 120 mg lactate/kg bm when blood HCO3- is measured 70 min following ingestion. Time to exhaustion was significantly increased by 14% and 26% in following consumption of 120 and 300 mg lactate/ kg bm, respectively, compared to placebo. However, no significant difference in TTE was detected between the two lactate doses.
Acknowledgements
This work was supported by a grant from the Gatorade Sport Science Institute received by David Morris and Hannah Wofferd.
References
- Adams G, Fisher M, Meyer R (1991) Hypercapnic acidosis and increased H2PO4 concentration do not decrease force in cat skeletal muscle. Am J Physiol 260: C805-C812.
- Hultman E, Del Canale S, Sjöholm H (1985) Effects of induced metabolic acidosis on intracellular pH, buffer capacity and contraction force of human skeletal muscle. ClinSci 69: 505-510.
- Raymer G, Marsh G, Kowalchuk J, Thompson R (2004) Metabolic effects of induced alkalosis during progressive forearm exercise to fatigue. J ApplPhysiol 96: 2050-2056.
- Spriet L, Matsos C, Peters S, Heigenhauser G, Jones N (1985) Effects of acidosis on rat muscle metabolism and performance during heavy exercise. Am JPhysiol 248: C337-C347.
- Matson L, Tran Z (1993) Effects of sodium bicarbonate ingestion on anaerobic performance: a meta-analytic review. Int J Sport Nutr 3: 2-28.
- PainelliVde S, da Silva R, de Oliveira O, de Oliveira L, Benatti F, et al. (2014) The effects of two different doses of calcium lactate on blood pH, bicarbonate, and repeated high-intensity exercise performance. Int J Sport Nutr and ExercMetab 24: 286-295.
- Morris D, Shafer R, Fairbrother K, Woodall M (2011) Effects of lactate consumption on blood bicarbonate levels and performance during high-intensity exercise.Int J Sport NutrExercMetab 21: 311-317.
- Montfoort MVan, Dieren LVan, Hopkins W, Shearman J (2004) Effects of ingestion of bicarbonate, citrate, lactate, and chloride on sprint running. Med Sci Sports Exerc 36: 1239-1243.
- Emhoff C, Messonnier L, Horning M, Fattor J, Carlso T, et al. (2013) Direct and indirect lactate oxidation in trained and untrained men. J ApplPhysiol 115: 829-838.
- Podolin D, Gleeson R, Mazzeo R (1996) Hormonal regulation of hepatic gluconeogenesis: influence of age and training. American Journal of Physiology 270: R365-R372.
- Descombe B, Raeburn P, Sirotic A, Coutts A (2007) The reliability of the i-STAT clinical portable analyser. J Sci Med Sport 10: 135-140.
- Bingisser R, Kaplan V, Scherer, T, Russi E, Bloch, K (1997) Effect of training on repeatability of cardiopulmonary exercise performance in normal men and women. Med Sci Sports Exerc29: 1499-1504.
- Ballmer J, Bird S, Davison R, Doherty M, Smith P (2004) Mechanically braked Wingate powers: agreement between SRM, corrected and conventional methods of measurement. J Sport Sci 22: 661-667.
- Currell K, Jeukendrup A (2008) Validity, reliability and sensitivity of measures of sporting performance. Sports Medicine38: 297-316.
- Coggan A, Costill D (1984) Biological and technological variability of three anaerobic ergometer tests. International Journal of Sports Medicine 5: 142-145.
- Lindsay F, Hawley J, Myburgh K, Schomer H, Noakes T, et al. (1996) Improved athletic performance in highly trained cyclists after interval training. Med Sci Sports Exerc 28: 1427-1434.
- Beloni R Morris, D (2013) Reliability of a short, high-intensity exercise test to exhaustion. Proceedings of the Annual Meeting of the Southeast Chapter of the American College of Sports Medicine. P107: 69.
Relevant Topics
- Aminoacid Suppliments
- Bodybuilding Nutrition
- Clinical Sports Nutrition
- Creatine Sports Nutrition
- Diet
- Fitness Nutrition
- Food and Nutrition
- Gym Suppliments
- Herbal Suppliments
- Micronutrients
- Natural Suppliments
- Nutrition Sport Fitness
- Nutritional Health
- Protein Diet
- Protein Suppliments
- Sports Nutrition Suppliments
- Vitamin Supplement
Recommended Journals
Article Tools
Article Usage
- Total views: 3499
- [From(publication date):
December-2016 - Dec 03, 2024] - Breakdown by view type
- HTML page views : 2743
- PDF downloads : 756