Mesenchymal Stromal Cell Uses for Acute Kidney Injury-Current Available Data and Future Perspectives: A Mini Review
Received: 21-Feb-2020 / Accepted Date: 04-Mar-2020 / Published Date: 11-Mar-2020 DOI: 10.4172/2376-127X.1000425
Abstract
There is growing evidence about the potential use of mesenchymal stromal cells (MSCs) for different tissue injuries. Initially, the intended physiological use of MSCs was due to their ability to differentiate and replace damaged cells. However, MSCs have been found to have multiple effects, including being able to significantly modulate immunological responses. MSCs are currently being tested for neurodegenerative diseases, graft versus host disease, kidney injury and other chronic unremitting tissue damage. Using MSCs in acute tissue damage is only now being studied. Acute kidney injury (AKI) is a common cause of morbidity and mortality. After the primary insult, overactivation of the immune system culminates in additional secondary potentially long-standing kidney damage. MSCs have the potential to ameliorate the secondary damage and recent studies have shed important light on their mechanisms of action. This article summarizes the basics of MSCs therapy, the newly discovered mechanisms of action and their potential application in the setting of AKI.
Keywords: Acuterenalfailure , Mesenchymal stemcells , MSC , Immuneresponse , AKI
Keywords
Acute renal failure; AKI; Mesenchymal stem cells; MSC; Immune response
Introduction
Acute Kidney Injury (AKI) is a syndrome of rapid deterioration of renal functions over a period of hours or days [1]. AKI is a common cause of morbidity and mortality, complicating 20% of hospitalized patients, half of them needing renal replacement therapy [2]. This severe form of AKI is related to a 50% increase in mortality among other devastating long-term consequences, including end stage renal disease (ESRD) and dialysis dependence [2,3].
The etiologies of AKI are varied, with pre-renal AKI and acute tubular necrosis being the most common [4,5]. AKI’s mechanism is often related to ischemia and reperfusion injury (IRI) [6]. In the last decade, cumulative evidence has shown the significant role that over activated immune responses play in the development of AKI [7]. This understanding paved the way to new therapeutic strategies for this relatively common and life-threatening acute kidney condition. Unfortunately, despite the progress in our understanding of AKI biology, treatment options for AKI in the daily clinical setting are still limited [1,3,4]. While dialysis can be relatively effective in handling the hazardous electrolytes and volume complications as a supportive therapy, there is need for a treatment that can eliminate the pathological cascade that may culminate in irreversible loss of renal tissue [1,4].
The Immune Response to Acute Kidney Injury
The immune system plays a crucial role in the mechanisms of AKI with both the innate and adaptive branches of the immune system involved [8]. Regarding the innate immune system, cytokines serve as major mediators while both increased production of cytokines and reduced clearance are reported during AKI [9] Interleukins (IL)-6, IL-8 and tumor necrosis factor (TNF)-α are usually elevated and are related to endothelial dysfunction and tubular injuy [10]. Conversely, IL-10 has an ameliorating effect by promoting immune tolerance [11].
The complement system, a part of the innate immune system, also has an important role in the pathogenesis of renal injury, and is involved in glomerular, tubulointerstitial and vascular kidney injuries [12]. The final common pathway of the complement system is the membrane attack complex that induces direct cellular damage, and causes activation and migration of neutrophils which further amplify the injury [13]. Suppressing the complement system in AKI has shown promising results in pre-clinical studies [14].
The cellular response to AKI includes both pro-inflammatory and anti-inflammatory characteristics. Dendritic cells, monocytes/ macrophages, neutrophils, T lymphocytes, and B lymphocytes are all involved in AKI, and can be detected even as early as one hour after the acute insult [15]. The involvement of these cells can directly and indirectly induce apoptosis of the renal tubular cells [15]. In contrast, M2 macrophages and regulatory T cells are essential for suppression of the overactivated inflammatory response and for the regeneration of damage renal tissue and are detected while recovering from the acute insult [8].
The relation between the different arms of the immune system can either escalate or downgrade the final injury [10,13]. To veer the cells and factors towards a less devasting rout, new treatments are being investigated including the use of stem-cell therapy.
Mesenchymal Stromal Cells
Mesenchymal stromal cells (MSCs) are fibroblast-like multipotent cells that can differentiate into mesodermal-line cells including adipocytes, chondroblasts, osteoblasts and renal tubular cells [16,17].
These cells exhibit self-renewal properties, with a potential to replace damaged cells [18].
MSCs are defined by three main characteristics:
Plastic-adherent when maintained in standard culture conditions;
Expression of CD105, CD73 and CD90, with no expression of other CDs that are not mesenchyme related (including CD45, CD34, CD14 or CD11b, CD79-α or CD19 and HLA-DR) surface molecules.
The ability to differentiate into a mature mesoderm related cell-line in vitro .19 Unlike embryonic stem cells, MSCs can be found in many organs even in adults [17,19,20].
In the past two decades, MSCs from different origins are being used in different clinical trial settings [21]. For example, bone-marrow derived MSCs are used in children to treat graft-versus-host disease autologous marrow MSCs for heart disease [20] and both bonemarrow and adipose-derived MSCs are used in Crohn ’ s-related enterocutaneous fistular disease [22]. In the neurodegenerative field, MSCs are being studied in amyotrophic lateral sclerosis, multiple system atrophy, Parkinson’s disease, Alzheimer’s disease and multiple sclerosis. While animal studies have been promising, clinical studies have demonstrated conflicting results [23,24]. The encouraging results obtained in the field of degenerative diseases can be related, among others, to the effect that MSCs have on the immune factors in these diseases setting [23,24].
The Biology of Mesenchymal Stromal Cells
MSCs can affect and be affected by other cells through different immune mediators. Cytokines, chemokines, and transcription factors can influence the differentiation of MSCs. Expression in MSCs of specific transcription factors, including Runx2, Sox9, PPARγ, MyoD, GATA4, and GATA6, may promote their differentiation into specific cell lineage [17].
The primary rational for using MSCs to rejuvenate damaged tissue was initially related to their ability to differentiate into the damaged tissue related cells. Following ischemic-reperfusion injury (IRI), MSCs migrate to the injured site and alleviate the damage [18]. Intriguingly, studies have demonstrated that MSCs have beneficial effects even at very early stages after their migration, before any differentiation and proliferation can be expected [25]. This observation has led to the understanding that early beneficial effects are related to their paracrine activity of the surrounding tissue [26,27].
Recent studies have demonstrated that MSCs can induce both local and remote anti-inflammatory effects [28]. The immunomodulatory effect of MSCs is broad and covers much of the innate and adaptive immune systems [16]. For example, MSCs can secrete factors such as insulin-like growth factor 1, vascular endothelial growth factor, angiopoietin 1, keratinocyte growth factor, and macrophage inflammatory protein 1α. These broad signaling factors are capable of promoting cell proliferation, angiogenesis, and wound healing [27].
MSCs can present both pro-inflammatory and anti-inflammatory profiles. These different phenotypes are related to their ability to sense the environment and respond to changes in the tissue. The effect is induced by activation of different macrophage populations.16 Macrophages are divided to two main groups, M1 and M2 macrophages. M1 macrophages are considered pro-inflammatory cells and secrete pro-inflammatory cytokines including IL-1, IL-6, TNF-α, and interferon-γ. M2 macrophages are anti-inflammatory cells that secret anti-inflammatory cytokines such as IL-10 and transforming growth factor (TGF)-β1 [16,29,30]. Thus, MSCs can induce differentiation of monocytes to one of the macrophage phenotype groups according to the inflammatory status of the damaged tissue [16].
MSCs can also affect T-cell activation and differentiation toward Tregulatory cells that have anti-inflammatory properties [31]. In addition to the paracrine effect on the immune system, MSCs can transfer mitochondria into the damaged cells, enabling better energy utilization, and restoration of the adenosine triphosphate (ATP) supply, thus promoting cellular recuperation [31]. MSCs might also assist in preserving tubular mitochondria thus preserving the functionality of these cells [32]. Since oxygen metabolism and energy utilization are improved, MSCs reduce the oxidative stress and induce anti-oxidant activity [33].
Treatment with Mesenchymal Stromal Cells in Acute Kidney Injury
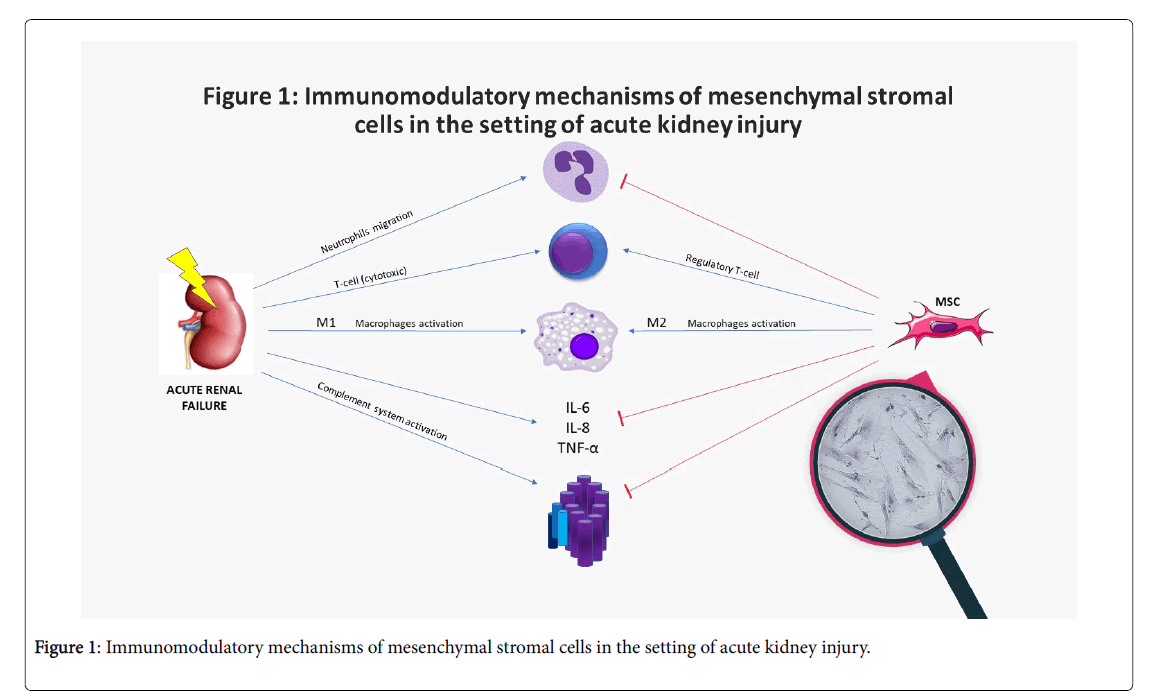
In the setting of AKI, MSCs promote protective effects on the injured kidney and ameliorate tissue damage [31,33]. The beneficial effects of MSCs are noticeable early after their injection and can be attributed the following paracrine related mechanisms (Table 1 and Figure 1) [34-49].
| Part of the immune system | Mechanism | Reference |
|---|---|---|
| Complement system | Amelioration of Complement system activation | Zilberman-Itskovich, et al. [25] Tang et al. [50] |
| Cytokines | Down regulation of pro-inflammatory cytokines: IL-1β, IL-6, IL-17, TNF-α, INF-γ, TGF-β | Tögel et al. [51], Zilberman-Itskovich, et al. [25]. Semedo et al. [52], Semedo, et al. [53] Cao, et al. [54], Furuichi, et al. [55] Sun, et al. [56] |
| Upregulation of anti-inflammatory cytokines: IL-10, IL-4, bFGF, TGF-α, and Bcl-2 | Tögel, et al. [51]. Zilberman-Itskovich, et al. [25]. Semedo, et al. [52], Luo, et al. [57], Tsuda et al. [58], Sun, et al. [56] | |
| Macrophages | Proliferation and migration of M2 macrophages population | Zilberman-Itskovich, et al. [25], Geng, et al.[59], Sun, et al.[56]. |
| Inhibition of macrophages infiltration | Tsuda, et al. [58] | |
| T-cells | Inhibition of T-cell infiltration | Tsuda, et al. [58], Sun, et al. [56]. |
| Differentiation to T-cell regulatory cells | Kilpinen, et al. [60], Semedo, et al.[53 ], Hu, et al.[61], Sun, et al.[56] | |
| Neutrophils | Inhibition of neutrophils infiltration | Sun, et al. [56], Tian, et al. [62] |
Abbreviation: IL: Interleukin; TNF: Tumor Necrosis Factor; INF: Interferon; TGF: Transforming Growth Factor; bFGF: basic Fibroblast Growth Factor; Bcl: B-cell lymphoma; MSC: Mesenchymal Stromal Cell
Table 1: Immunomodulatory mechanisms of mesenchymal stromal cells in the setting of acute kidney injury.
Illustration of the immune mechanisms of acute kidney injury and the immunomodulatory effect of mesenchymal stromal cells. Acute kidney injury is accompanied by increase in inflammatory cytokines, complement activation and immune cell activation. Mesenchymal stromal cells inhibit cytokines release, complement system activation, and neutrophils migration, while promoting M2-anti-inflammatory macrophages and regulatory T-cells proliferation; in magnifying glass: microscopic picture of mesenchymal stromal cells- placenta origin; MSC= mesenchymal stromal cell; IL= Interleukin; TNF= tumor necrosis factor.
In magnifying glass: microscopic picture of mesenchymal stromal cells-placenta origin.
An increase of the M2 macrophage CD68/CD163 population. As discussed, these M2 macrophages have anti-inflammatory and proregenerative phenotypes [25,29].
A shift from the pro-inflammatory cytokines TNF-α, and IL-1β to the anti-inflammatory cytokine IL-10 with a favorable expression of homing adhesion molecules ICAM-1 and VCAM-1[34]
An inhibitory effect of the complement system’s overactivation and the related cellular damage generated by the membrane attack complex [25].
Exosomes-One of the most exciting discoveries in intercellular communication is exosomes. Exosomes are membrane bound extracellular vesicles that can be produced by most eukaryotic cells. Their size is about 30 to 120 nanometers (nm) in diameter (around the size of lipoproteins) and they contain various molecular constituents of their cell of origin, including proteins, mRNA and miRNA or doublestranded DNA [35,36]. Recent studies have demonstrated that administration of exosomes derived from MSCs can ameliorate the expected renal damage in the setting of AKI [35]
Epigenetic effects a shift in gene expression. Xie et al. demonstrated that overexpression of the Klotho gene, which regulates apoptosis, can reinforce the protective effect of MSCs in the setting of AKI [37] Chen et al. demonstrated that the protective effect of MSCs in the setting of AKI can be related to TNF-inducible gene 6 protein expression. This protein, in addition to its anti-inflammatory effect, can promote renal tubular epithelial cell proliferation [38].
While there is growing body knowledge in pre-clinical studies, the available clinical data on MSCs in AKI is still scarce. A recent study using MSCs in post-cardiac surgery patients did not show beneficial effects regarding post-surgery AKI [39]. This unfortunate result can be attributed to time of MSCs administration. The optimal results are obtained if the administration of MSCs is closest to the initiation of IRI [34]. The detection of AKI, based on commonly used blood markers in humans (serum creatinine and urea) is usually late, after AKI and tubular necrosis is well established [6] In this scenario, while the damage is already well established, the potential immunological benefits of MSCs are probably negligible. In addition, the MSCs themselves can be injured by an overactivated complement system [40]
In addition to AKI, there is growing evidence of MSCs benefits in the setting of chronic kidney disease (CKD). Even though the clinical studies done so far included relatively a small number of patients, the evidence looks promising with regards to the ability of MSCs to prevent the expected kidney function deterioration over time [41-43]. In patients suffering from chronic diabetic nephropathy, allogeneic transplantation of MSCs demonstrated improvement of renal functions compared to placebo [43]. The effect might be attributed to the paracrine secretion of the vascular endothelial growth factor and insulin growth factor-1 by MSCs [42].
One of the relevant clinical settings where MSCs have the potential to have beneficial effects is in post-renal transplantation patients. In the immediate post-transplantation period, IRI is one of the main reasons for AKI [44]. Thanks to the above discussed immunomodulating effects of MSCs, there are promising results in pre-clinical trials, and clinical studies are currently ongoing [45].
Current Available Safety Data on MSCs
Several safety concerns are related to the use of MSCs in the clinical setting. The first concern is related to the administration technique. When the cells are administered intravenously (IV), most of the cells can be found within the lungs [25,46]. If the lung capillaries are blocked with these cells, ventilation and respiratory difficulties are expected. Therefore, higher dosage with high concentration of MSCs should be avoided. The second concern is related to exposing the immune system to foreign cells, when administering cells from a donor. Luckily, MSCs do not stimulate a profound immune response, since they only express the human leucocyte antigen (HLA)-DR but lack other HLA typings [26]. In a CKD trial, none of the patients developed persistent donor specific anti-HLA antibodies [43].
In particular, fetal MSCs have very low immunogenicity by nature, and those can be used to overcome this potential barrier [33].The last concern is related to the proliferation and differentiation of pluripotent cells injected to a living body, with the potential of transforming into malignant cells. This concern is probably irrelevant, since stromal cells need a special environment and signaling factors to act as stem cells and differentiate, and usually do not survive after administration [17,46]. In any case, to address this scenario, more research with longterm follow-up is needed.
Even though clinical trials with long-term follow-up are still lacking, some preclinical trials have addressed the safety issues. Till now, no serious adverse effects were reported in either preclinical [47,48] and clinical studies [18,39,43,49].
Conclusion
The ongoing cumulative data on the beneficial physiological effects of MSCs open new treatment opportunities for diseases that are currently being managed with only supportively therapy. While other types of stem cells, such as hematopoietic stem cells, are used in the clinical practice, the clinical data on MSCs is still scarce. In the setting of AKI, MSCs by way of their paracrine effects may modulate the hazardous results of an overactivated inflammatory response. MSCs hold the hope for future novel therapies, and a better understanding of the immune-biological effects of these cells will enable development of new treatment strategies.
Acknowledgments
We thank Dr. Mechael Kanovsky for his help in professionally editing the manuscript.
Author Declaration
All authors are in agreement with the content of the manuscript. Each author’s contributed to the paper significantly.
References
- Kellum JA, Lameire N, Group KAGW (2013) Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit Care 17: 1-15.
- Levey AS, James MT (2017) Acute kidney injury. Ann Intern Med 167: 66-80.
- Chawla LS, Kimmel PL (2012) acute kidney injury and chronic kidney disease: An integrated clinical syndrome. Kidney Int 82: 516-524.
- Bellomo R, Kellum JA, Ronco C ( 2012) Acute kidney injury. Lancet 380: 756-766.
- Liano F, Pascual J (1996) Epidemiology of acute renal failure: A prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 50: 811-818.
- John F, Marcello T, Richard J J, Floge , Marcellion T ,et al. (2019) Comprehensive Clinical Nephrology (6th edn). Alberta, Elsevier Canada : 176-1573.
- Ortiz A, Sanchez-Nino MD, Izquierdo MC, Ana B. Sanz, Maria J Soler, et al. (2015) Translational value of animal models of kidney failure. Eur J Pharmacol 759: 205-220.
- Bonavia A, Singbartl K (2018) A review of the role of immune cells in acute kidney injury. Pediatr Nephrol 33: 1629-1639.
- Singbartl K, Joannidis M(2015) Â Short-term effects of acute kidney injury. Crit Care Clin 31: 751-762.
- Singbartl K, Formeck CL, Kellum JA (2019) Kidney-Immune System Crosstalk in AKI. Semin Nephrol 39: 96-106.
- Sakai K, Nozaki Y, Murao Y, Yano T, K Niki et al. (2019) Protective effect and mechanism of IL-10 on renal ischemia-reperfusion injury. Lab Inves 99: 671-683.
- McCullough JW, Renner B, Thurman JM (2013) The role of the complement system in acute kidney injury. Semin Nephrol 33: 543-556.
- Chan RK, Ibrahim SI, Verna N, Carroll M, Hechtman HB, et al. (2003) Ischaemia-reperfusion is an event triggered by immune complexes and complement. Br J Surg 90: 1470-1478.
- Zilberman-Itskovich S, Abu-Hamad R, Stark M, Efrati S (2019) Effect of anti-C5 antibody on recuperation from ischemia/reperfusion-induced acute kidney injury. Ren Fail 41: 967-975.
- Kinsey GR, Okusa MD ( 2014) Expanding role of T cells in acute kidney injury. Curr Opin Nephrol Hypertens 23: 9-16.
- Bernardo ME, Fibbe WE (2013) Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13: 392-402.
- Almalki SG, Agrawal DK (2016) Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 92: 41-51.
- Perico N, Casiraghi F, Remuzzi G (2018) Clinical Translation of Mesenchymal Stromal Cell Therapies in Nephrology. J Am Soc Nephrol 29: 362-375.
- Dominici M, Le Blanc K, Mueller I, I Slaper-Cortenbach, FC Marini, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315-317.
- Galipeau J, Sensebe L. Mesenchymal (2018) Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 22: 824-833.
- Dulak J, Szade K, Szade A, Nowak W, Jozkowicz A (2015) Adult stem cells: Hopes and hypes of regenerative medicine. Acta Biochim Pol 62: 329-337.
- Carvello M, Lightner A, Yamamoto T, Kotze PG, Spinelli A (2019) Mesenchymal Stem Cells for Perianal Crohn's Disease. Cells 8: 1-12.
- Xie C, Liu YQ, Guan YT, Zhang GX. (2016) Induced stem cells as a novel multiple sclerosis therapy. Curr Stem Cell Res Ther 11: 313-320.
- Staff NP, Jones DT, Singer W (2019) Mesenchymal stromal cell therapies for neurodegenerative diseases. Mayo Clin Proc 94: 892-905.
- Zilberman-Itskovich S, Abu-Hamad R, Zarura R, et al.,(2019) Human mesenchymal stromal cells ameliorate complement induced inflammatory cascade and improve renal functions in a rat model of ischemia-reperfusion induced acute kidney injury. PLoS One 14: 354.
- Ullah I, Subbarao RB, Rho GJ (2015) Human mesenchymal stem cells: Current trends and future prospective. Biosci Rep 35: 1-18.
- Chen L, Tredget EE, Wu PY, Wu Y (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3 : 1-12.
- Shi Y, Wang Y, Li Q, Keli L, Jianquan H, et al (2018) Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol 14: 493-507.
- Tang PM, Nikolic-Paterson DJ, Lan HY (2019) Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol 15: 144-158.
- Hamidzadeh K, Christensen SM, Dalby E, Chandrasekaran P, Mosser DM (2017) Macrophages and the Recovery from Acute and Chronic Inflammation. Annu Rev Physiol 79: 567-592.
- Erpicum P, Detry O, Weekers L, Catherine B, Chantal L, et al (2014) Mesenchymal stromal cell therapy in conditions of renal ischaemia/reperfusion. Nephrol Dial Transplant 29: 1487-1493.
- Perico L, Morigi M, Rota C, Matteo B, Caterina Me, et al., (2017) Human mesenchymal stromal cells transplanted into mice stimulate renal tubular cells and enhance mitochondrial function. Nat Commun 8: 983.
- Missoum A (2020) Recent updates on mesenchymal stem cell based therapy for acute renal failure. Curr Urol. 13 : 189-199.
- Liu X, Cai J, Jiao X, Yu X, Ding X (2017) Therapeutic potential of mesenchymal stem cells in acute kidney injury is affected by administration timing. Acta Biochim Biophys Sin (Shanghai) 49: 338-348.
- Aghajani Nargesi A, Lerman LO, Eirin A (2017) Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther 8: 273.
- Altanerova U, Jakubechova J, Repiska V, Altaner C (2017) Exosomes of human mesenchymal stem/stromal/medicinal signaling cells. Neoplasma 64: 809-815.
- Xie LB, Chen X, Chen B, Wang XD, Jiang R, et al., (2019) Protective effect of bone marrow mesenchymal stem cells modified with klotho on renal ischemia-reperfusion injury. Ren Fail 41: 175-182.
- Chen Y, Tang X, Li P, Chen Yu, Jie Liu, et al., (2019) Bone Marrow Derived Mesenchymal Stromal Cells Ameliorate Ischemia/Reperfusion Injury-Induced Acute Kidney Injury in Rats via Secreting Tumor Necrosis Factor-Inducible Gene 6 Protein. Biomed Res Int 2019: 1-12.
- Swaminathan M, Stafford-Smith M, Chertow GM, David G W, Viken Paragamian, et al. (2018) Allogeneic Mesenchymal Stem Cells for Treatment of AKI after Cardiac Surgery. J Am Soc Nephrol 29: 260-267.
- Li Y, Lin F ( 2012) Mesenchymal stem cells are injured by complement after their contact with serum. Blood. 120: 3436-3443.
- El-Ansary M, Saadi G, Abd El-Hamid SM (2012) Mesenchymal stem cells are a rescue approach for recovery of deteriorating kidney function. Nephrology (Carlton) 17: 650-657.
- Saadi G, El Ansary M, Hassaballa M, Roshdy, M El-Aziz E A, et al. (2016) vascular endothelial growth factor and insulin growth factor as an underlying paracrine action of mesenchymal stem cells transfused for the regeneration of stage II and III chronic kidney disease. Journal of the Egyptian Society of Nephrology and Transplantation 16: 3-9.
- Packham DK, Fraser IR, Kerr PG, Segal KR (2016) Allogeneic Mesenchymal Precursor Cells (MPC) in Diabetic Nephropathy: A Randomized, Placebo-controlled, Dose Escalation Study. EBioMedicine 12: 263-269.
- Chen CC, Chapman WC, Hanto DW (2015) Ischemia-reperfusion injury in kidney transplantation. Front Biosci (Elite Ed). 7: 117-134.
- Casiraghi F, Perico N, Cortinovis M, Remuzzi G (2016) Mesenchymal stromal cells in renal transplantation: opportunities and challenges. Nat Rev Nephrol 12: 241-253.
- Eggenhofer E, Benseler V, Kroemer A, FC Popp, EK Geissler, et al. (2012) Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol 3: 297.
- Park SE, Lee NK, Lee J, Changad J W, DUK L, et al. (2016) Distribution of human umbilical cord blood-derived mesenchymal stem cells in the Alzheimer's disease transgenic mouse after a single intravenous injection. Neuroreport 27: 235-241.
- Cai J, Yu X, Xu R, Y Fang, Xiaoqin Q, et al., (2014) Maximum efficacy of mesenchymal stem cells in rat model of renal ischemia-reperfusion injury: renal artery administration with optimal numbers. PLoS One 9: 1-16.
- Makhlough A, Shekarchian S, Moghadasali R, Hossein B,Nasser A, et al., (2018) Bone marrow-mesenchymal stromal cell infusion in patients with chronic kidney disease: A safety study with 18 months of follow-up. Cytotherapy 20: 660-669.
- Tang M, Zhang K, Li Y, Qian hui H, Gui-qing L, et al. (2018) Mesenchymal stem cells alleviate acute kidney injury by down-regulating C5a/C5aR pathway activation. Int Urol Nephrol 50: 1545-1553.
- Togel F, Hu Z, Weiss K, Isaac J, Lange C, et al. (2005) Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31-42.
- Semedo P, Wang PM, Andreucci TH, A P Silvaa, N O S Câmaraac, et al., (2007) Mesenchymal stem cells ameliorate tissue damages triggered by renal ischemia and reperfusion injury. Transplant Proc 39: 421-423.
- Semedo P, Palasio CG, Oliveira CD, N O S Camara,P Silva, et al. (2009) Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int Immunopharmacol 9: 677-682.
- Cao H, Qian H, Xu W,Xie Y, Yan Y, et al. (2010) Mesenchymal stem cells derived from human umbilical cord ameliorate ischemia/reperfusion-induced acute renal failure in rats. Biotechnol Lett 32: 725-732.
- Furuichi K, Shintani H, Sakai Y, Takashi W,Kaneko S, et al. (2012) Effects of adipose-derived mesenchymal cells on ischemia-reperfusion injury in kidney. Clin Exp Nephrol 16: 679-689.
- Sun P, Liu J, Li W, Wang H, Ciagan D, et al. Â (2016) Human endometrial regenerative cells attenuate renal ischemia reperfusion injury in mice. J Transl Med 14: 1-13.
- Luo J, Zhao X, Tan Z, Su Z, Meng F, Zhang M,et al. (2013) Mesenchymal-like progenitors derived from human embryonic stem cells promote recovery from acute kidney injury via paracrine actions. Cytotherapy 15(6): 649-662.
- Tsuda H, Yamahara K, Otani K, Yazawa K, Jun Y, et al. (2014) Transplantation of allogenic fetal membrane-derived mesenchymal stem cells protects against ischemia/reperfusion-induced acute kidney injury. Cell Transplant 23: 889-899.
- Geng Y, Zhang L, Fu B,Quan H,Chen X, et al. (2014) Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of M2 macrophages. Stem Cell Res Ther 5: 1-14.
- Kilpinen L, Impola U, Sankkila L,Eero M, Esko K, et al. (2013) Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury: A feature that is lost after inflammatory conditioning. J Extracell Vesicles 2: 1-16.
- Hu J, Zhang L, Wang N,S Cui,Liu X, et al., (2013) Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions. Kidney Int 84: 521-531.
- Tian H, Lu Y, Shah SP, Wang Q, Hong S, et al. (2012) 14S,21R-dihydroxy-docosahexaenoic acid treatment enhances mesenchymal stem cell amelioration of renal ischemia/reperfusion injury. Stem Cells Dev 21: 1187-1199.
Citation: Zilberman-Itskovich S (2020) Mesenchymal Stromal Cell Uses for Acute Kidney Injury-Current Available Data and Future Perspectives: A Mini Review. J Clin Exp Neuroimmunol 5: 119. DOI: 10.4172/2376-127X.1000425
Copyright: © 2020 Zilberman-Itskovich S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2148
- [From(publication date): 0-2020 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 1433
- PDF downloads: 715