Research Article Open Access
Mercury Accumulation and the Mercury-PCB-Sex Interaction in Summer Flounder
Madenjian CP1*, Jensen OP2, Krabbenhoft DP3, DeWild JF3, Ogorek JM3 and Vastano AR21US Geological Survey, Great Lakes Science Center, Ann Arbor, Michigan, USA
2Department of Marine and Coastal Sciences, Rutgers University, New Brunswick, New Jersey, USA
3US Geological Survey, Wisconsin Water Science Center, Middleton, Wisconsin, USA
- *Corresponding Author:
- Madenjian CP
US Geological Survey, Great Lakes Science Center
Ann Arbor, Michigan, USA
Tel: +7342147259
Fax: +7349948780; E-mail: cmadenjian@usgs.gov
Received Date: January 29, 2016; Accepted Date: March 21, 2016; Published Date: March 28, 2016
Citation: Madenjian CP, Jensen OP, Krabbenhoft DP, DeWild JF, Ogorek JM, et al. (2016) Mercury Accumulation and the Mercury-PCB-Sex Interaction in Summer Flounder. J Marine Sci Res Dev 6:188. doi: 10.4172/2155-9910.1000188
Copyright: © 2016 Madenjian CP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
Patterns in the relative differences in contaminant concentrations between the sexes of mature fish may reveal important behavioral and physiological differences between the sexes. We determined whole-fish total mercury (Hg) concentrations in 23 female summer flounder (Paralichthys dentatus) and 27 male summer flounder from New Jersey coastal waters. To estimate the change in Hg concentration due to release of eggs at spawning, Hg concentration in the somatic tissue and ovaries of 5 of the 23 female summer flounder were also determined. To ascertain whether most of the Hg in the summer flounder was methylmercury (MeHg), whole-fish MeHg concentrations were determined in all 50 summer flounder. Whole-fish Hg concentrations averaged 113 ng/g for females and 111 ng/g for males. Thus, females were 2% higher in Hg concentration than males, on average, but the difference was not statistically significant. Based on Hg determinations in the somatic tissue and ovaries, we predicted that Hg concentration of females would increase by 3.7%, on average, immediately after spawning due to release of eggs. On average, 92% of the Hg in the summer flounder was MeHg. To determine whether the effect of sex on Hg concentration was significantly different from the effect of sex on polychlorinated biphenyl (PCB) concentration, we paired our Hg determinations with PCB determinations from a previous study, and applied regression analysis. Sex significantly interacted with contaminant type (Hg or PCBs), as males were 43% higher in PCB concentration than females, whereas females were 2% higher in Hg concentration than males. Males eliminating Hg from their bodies at a faster rate than females was a likely explanation for this discrepancy between the two contaminant types. Overall, the Hg and PCB concentrations in the summer flounder were relatively low, and therefore our findings also had implications for continued operation of the summer flounder fishery.
Keywords
Androgens; Fish consumption advisories; Hg-elimination rates; Methylmercury; PCBs; Sex differences; Summer flounder
Introduction
Three species of freshwater teleost fishes (lake trout (Salvelinus namaycush), burbot (Lota lota), and lake whitefish (Coregonus clupeaformis)) share the characteristic of the ratio of whole-fish polychlorinated biphenyl (PCB) concentration in males to wholefish PCB concentration in females substantially exceeding the ratio of whole-fish total mercury (Hg) concentration in males to whole-fish Hg concentration in females [1]. This apparent pattern has been attributed to males eliminating Hg from their bodies at a faster rate than females, whereas long-term elimination of PCBs by fish is negligible for both sexes [1-4]. The faster rate of Hg elimination in males has been linked to certain androgens [2]. For these three fish species, mature males were between 20% and 35% higher in whole-fish PCB concentration than similarly aged mature females, and this difference appeared to be primarily due to a greater rate of energy expenditure in mature males stemming from higher activity and a higher resting metabolic rate (or standard metabolic rate [SMR]) [5-7]. A consequence of this greater rate of energy expenditure is a higher rate of food consumption, which, in turn, results in a higher rate of PCB accumulation. In contrast to the relative differences in PCB concentrations between the sexes, male lake trout were only 8% higher in Hg concentration than female lake trout, and females were actually higher than males in Hg concentration for both burbot and lake whitefish.
Documentation of a substantially greater ratio of PCB concentration in males to PCB concentration in females compared with the ratio of Hg concentration in males to Hg concentration in females in a species of a marine teleost fish would mark an important milestone in assessing the pervasiveness of this characteristic throughout all teleost fish populations. To date, this characteristic has been documented in populations of freshwater teleost fishes only [1]. Marine fish differ from freshwater fish in their processes for osmoregulation, which is the maintenance of water and salt balance within the bodies of fish [8]. Perhaps these physiological differences between freshwater and marine fishes exert an influence on the difference between the two abovementioned ratios, which would then suggest that factors other than the sex-related difference in Hg-elimination rates may be contributing to the relative difference in Hg concentrations between the sexes. On the other hand, if this apparent relationship between the two abovementioned ratios holds true for marine teleost fishes, then evidence to support the contention that males eliminate Hg at a faster rate than females is further strengthened. Likewise, the implication that certain androgens are linked to enhanced Hg-elimination rate in males is further corroborated.
Of the flatfish fisheries operating along the U. S. Atlantic coast, the summer flounder or fluke (Paralichthys dentatus) fishery is most important [9,10]. Both the summer flounder population and the commercial fishery extend from Massachusetts to North Carolina. The summer flounder recreational fishery is most concentrated in New York and New Jersey waters of the Atlantic Ocean [9-11]. Although the spawning season for summer flounder occurs during September through March, peak spawning typically occurs during October and November. Summer flounder is one of the important Predators inhabiting the western Atlantic coastal ecosystem, and the diet of adults primarily consists of fish and squid [12]. Female summer flounder grow considerably faster than male summer flounder [11]. To the best of our knowledge, the difference in whole-fish Hg concentrations between the sexes of summer flounder has yet to be quantified. Adult male summer flounder exceeded adult female summer flounder in whole-fish PCB concentration by 43%, and this difference has been attributed to a higher energy expenditure rate in males and the growth dilution effect [13].
The overall goal of our study was to characterize Hg accumulation in mature summer flounder from the coastal waters of New Jersey. Specific objectives included: (1) quantify the difference in whole-fish Hg concentrations between the sexes of summer flounder caught from a spawning aggregation off the New Jersey coast, (2) quantify the difference between somatic tissue Hg concentration and ovary Hg concentration in female summer flounder, (3) estimate the change in whole-fish Hg concentration of female summer flounder associated with release of eggs at spawning, (4) determine whether the bulk of Hg in summer flounder is MeHg, (5) determine whether the effect of sex on Hg concentration was significantly different from the effect of sex on PCB concentration in summer flounder, and (6) discuss the implications of our findings with regard to the continued operation of the summer flounder fishery. Of special interest was whether the ratio of Hg concentration in males to Hg concentration in females was less than the ratio of PCB concentration in males to PCB concentration in females for summer flounder.
Methods
Field methods
The same 50 adult summer flounder used by Madenjian et al. [13] in their study on the difference in PCB concentrations between the sexes were used in our study. In brief, these fish were captured via trawling by a commercial fishing boat operating in coastal waters about 30 km east of Barnegat Light, New Jersey on 12 November 2013. We purchased 85 summer flounder from Viking Village, the commercial fish producer at Barnegat Light, New Jersey, and then transported the fish on ice in coolers to the Rutgers University Marine Field Station (RUMFS) in Tuckerton, New Jersey for further processing. Total length (TL) of each summer flounder was measured to the nearest mm, and each summer flounder was weighed to the nearest g. Sex and maturity of each summer flounder was determined by visual inspection of the gonads. We bagged each fish individually, placed a cardboard tag with a unique identification number in the bag, and then kept the fish frozen at -20°C until further processing. Refer to Madenjian et al. [13] for more details.
Hg, MeHg, and age determinations
To determine Hg concentration in somatic tissue and ovaries, five female summer flounder were randomly selected and partially thawed. Otoliths were then removed for aging purposes, ovaries were removed and weighed to the nearest g, and the remaining somatic tissue was also weighed to the nearest g. We homogenized ovaries and somatic tissue separately in appropriately sized blenders. About 100 g of each somatic tissue homogenate was placed in a contaminant-free glass jar, sealed with a lid, and then stored at -20°C. All of the available homogenate of each pair of ovaries (between 50 and 85 g) was placed in a contaminantfree glass jar, sealed with a lid, and then stored at -20°C. For the 18 remaining female summer flounder, we partially thawed each fish and removed the otoliths for aging. Then, each whole fish was homogenized using appropriately sized blenders, and approximately 100 g of the homogenate was then placed in a contaminant-free glass jar, sealed with a lid, and stored at -20°C. Of the remaining 62 males, 27 were randomly selected and then processed in the same manner that the 18 females used for whole-fish Hg determinations were processed. Frozen homogenates were shipped to the US Geological Survey (USGS) Mercury Research Laboratory in Middleton, WI for Hg and MeHg determinations. All samples were lyophilized prior to mercury analysis. At the National Oceanic and Atmospheric Administration (NOAA) Northeast Fisheries Science Center at Woods Hole, MA, the summer flounder otoliths were aged via thin sectioning and enumeration of annuli, under supervision of NOAA fishery scientists. Otoliths for one of the summer flounder were damaged during their removal, and therefore an age was not assigned to this fish. Hg concentrations in fish homogenates were measured using a Nippon MA-2000 direct combustion analyzer (Tokyo, Japan), following USEPA Method 7473 [14]. Details are provided by Madenjian et al. [1,4]. An aliquot of fish homogenate is combusted at 850°C, resulting in the reduction of all species of mercury to gaseous elemental mercury, which is detected in the sample stream by atomic absorption. We performed quality assurance/ quality control (QA/QC) protocols throughout the Hg determination process, including regular checks of instrument calibration, analysis of analytical blanks, sample replicates, standard reference materials (SRMs), and laboratory practices to prevent sample contamination. Calibration of the instrument is checked prior to each run, and is based on a 7-point polynomial calibration curve (R2>0.995). Triplicate analyses were performed once every 10 samples and the relative standard deviation (RSD) for the triplicate analyses ranged from 0.8 to 16.5% (mean of 5.1%). International Atomic Energy Agency Reference Material 407 (IAEA 407) was used as the SRM, and at a minimum an SRM analysis was included in every set of 10 samples. In total, 17 SRM determinations were made, and the average SRM recovery was 105.3%, which is well within the range of acceptance (75-125% recovery) for the USGS Mercury Research Laboratory. We estimated a detection limit for Hg of 7 ng/g (dry weight basis), based on multiple analyses of a fish homogenate (IAEA 407) and following USEPA protocol [15]. All summer flounder Hg concentrations were reported on a wet weight basis. MeHg concentrations in the fish homogenates were determined with a Brooks Rand MERX automated methylmercury analyzer (Brooks Rand Instruments, Seattle, Washington, USA), following an adaptation of USEPA method 1630 [16]. Samples were extracted in 4.5 M nitric acid for 8 hours at 50°C rather than using the distillation procedure outlined in USEPA method 1630. The aliquot was treated with sodium tetramethylborate, with the resulting ethylated mercury purged onto Tenex traps, thermally desorbed, mass-separated with a gas chromatograph column, and detected by cold vapor atomic fluorescence. Refer to Madenjian et al. [1,4] for more details. In fish, MeHg is typically the predominant form of mercury [17], but inorganic mercury can make a substantial contribution to Hg found in fish in some cases [18]. QA/QC protocols were followed throughout the MeHg determination process. The instrument was calibrated daily with a 6-point calibration curve (R2>0.995). Triplicate analyses were performed once every 10 samples and RSD for the triplicate analyses ranged from 0.9 to 10.5% (mean of 5.6%). IAEA 407 was used as the SRM, and a total of 11 SRM determinations were made. Mean SRM recovery was 110.2%, which is well within the range of acceptance (75-125%) for the USGS Mercury Research Laboratory. We estimated a detection limit for MeHg of 2 ng/g (dry weight basis), based on multiple analyses of a fish homogenate (IAEA 407) and following USEPA protocol [15]. All summer flounder MeHg concentrations were expressed on a wet weight basis.
Data analyses
The mass balance approach described by Niimi [19] was used to calculate whole-fish Hg and whole-fish MeHg in each of the five females selected for mercury determinations of ovaries and somatic tissue. The body burden of mercury (either Hg or MeHg) in a portion of the fish is the weight of mercury contained in that portion of the fish. Body burdens in the ovaries and somatic tissue were calculated by multiplying the mercury concentration in the tissue by the weight of the tissue. Then, these body burdens were summed to yield the whole-fish body burden, and this sum was then divided by the sum of the weights for the ovaries and somatic tissue to yield the whole-fish mercury concentration. In addition, the expected percent change in mercury concentration due to release of eggs at spawning was estimated for each of the five females by calculating the ratio of mercury concentration in the somatic tissue to the estimated whole-fish mercury concentration, using the procedure described by Niimi [19]. For each of the five females, gonadosomatic index (GSI) was calculated by dividing the weight of the ovaries by the total weight of the fish and then multiplying by 100.
We considered four analyses of covariance (ANCOVA) models to test whether sex had a significant effect on Hg concentration. Likewise, we considered four ANCOVA models to test whether sex had a significant effect on MeHg concentration. For each ANCOVA, mercury (either Hg or MeHg) concentration was the dependent variable, sex was the main effect, and TL, weight, Fulton’s condition K (equal to weight â?? 105 â?? TL-3), or age was the covariate. We found that the covariate had an insignificant effect on mercury concentration in all eight ANCOVA models. Thus, a two-sample t test was used to determine whether sex had a significant effect on Hg concentration and whether sex had a significant effect on MeHg concentration. Assumptions of normality and homogeneity of variances were met for the two-sample t test applied to Hg concentration data (Shapiro-Wilk statistic: W=0.97; P=0.1512; Folded F statistic: F=1.25; df=22, 26; P=0.5792). For the two-sample t test applied to MeHg data, the homogeneity of variances assumption was met (Folded F statistic: F=1.05; df=22, 26; P=0.9015), but the normality assumption was violated (Shapiro-Wilk statistic: W=0.93; P=0.0071). However, given the robustness of the two-sample t test to departures from the normality assumption [20], its application to the MeHg data was still appropriate. Data for 23 females and 27 males were included in the statistical analyses. The ratio of mean Hg concentration of males to mean Hg concentration of females and the ratio of mean MeHg concentration of males to mean MeHg concentration of females were then calculated. We set α=0.05 for all of our statistical testing.
To determine whether the bulk of the Hg in the summer flounder was MeHg, the fraction of Hg as MeHg was calculated by the ratio of MeHg concentration to Hg concentration for each of the 50 summer flounder. The mean of these ratios was then calculated using data for all 50 summer flounder. In addition, we calculated mean ratios by sex.
To determine whether contaminant type (Hg or PCBs) significantly interacted with sex, we tested whether the regression line of PCB concentration as a linear function of Hg concentration for females was significantly different from the regression line of PCB concentration as a linear function of Hg concentration for males. To perform this analysis, Hg concentration was paired with PCB concentration for each of the 50 summer flounder, using PCB concentration data from Madenjian et al. [13]. A simple linear regression analysis of PCB concentration as a function of Hg concentration was performed for both females and males. We used an F test for equality of regression lines to determine whether the two regression lines were significantly different from one another.
Results
Differences in whole-fish Hg and MeHg concentrations between the sexes
Female summer flounder were substantially larger than male summer flounder, even though mean age varied little between the sexes (Table 1). Females ranged from 2 to 6 years in age, while age of males ranged from 3 to 8 years.
Mean age for males (4.2 years) was just slightly higher than mean age for females (3.7 years). Females averaged 497 mm in TL, while males averaged 433 mm in TL. Females averaged 1264 g in weight, while males averaged 863 g in weight (Table 1). Gonad condition of all 50 summer flounder was ripe or nearly ripe.
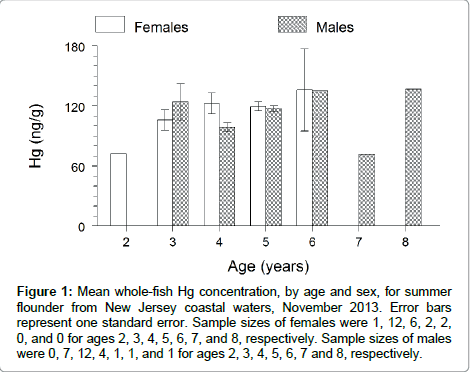
Hg concentration did not significantly vary between the sexes of summer flounder (t test: t=0.25; df=48; P=0.8056). Mean whole-fish Hg concentration averaged 113 ng/g for females and 111 ng/g for males (Table 1). Ratio of mean whole-fish Hg concentration in males to mean whole-fish Hg concentration in females was equal to 0.98. Thus, females exceeded males in Hg concentration by 2%. Hg concentration trended neither upward nor downward with increasing age of summer flounder (Figure 1).
Figure 1: Mean whole-fish Hg concentration, by age and sex, for summer flounder from New Jersey coastal waters, November 2013. Error bars represent one standard error. Sample sizes of females were 1, 12, 6, 2, 2, 0, and 0 for ages 2, 3, 4, 5, 6, 7, and 8, respectively. Sample sizes of males were 0, 7, 12, 4, 1, 1, and 1 for ages 2, 3, 4, 5, 6, 7 and 8, respectively.
MeHg concentration did not significantly vary between the sexes of summer flounder (t test: t=0.43; df=48; P=0.6656). Mean wholefish MeHg concentration averaged 104 ng/g for females and 101 ng/g for males (Table 1). Ratio of mean whole-fish MeHg concentration in males to mean whole-fish Hg concentration in females was equal to 0.96. Thus, females exceeded males in MeHg concentration by 4%.
| Characteristic | Females | Males | ||
| n | Mean | n | Mean | |
| Total length (mm) | 23 | 497 (7) | 27 | 433 (7) |
| Weight (g) | 23 | 1264 (53) | 27 | 863 (48) |
| Age (years) | 23 | 3.7 (0.2) | 26 | 4.2 (0.2) |
| Hg (ng/g) | 23 | 113 (7) | 27 | 111 (6) |
| MeHg (ng/g) | 23 | 104 (6) | 27 | 101 (6) |
Table 1: Mean values for total length, weight, age, Hg concentration, and MeHg concentration, by sex, of the summer flounder from New Jersey coastal waters, November 2013, used in the study. Standard error of the mean enclosed within parentheses. n = number of fish.
Hg and MeHg concentrations in ovaries and somatic tissue
Mean Hg concentration in somatic tissue was about five times higher than mean Hg concentration in the ovaries (Table 2). On average, whole-fish Hg concentration of the females was expected to increase by 3.7% immediately after spawning due to release of eggs. Similarly, mean MeHg concentration in somatic tissue exceeded mean Hg concentration in the ovaries by roughly a factor of five (Table 2). On average, whole-fish MeHg concentration of the females was expected to increase by 3.6% immediately after spawning due to release of eggs. Mean GSI for the females was 4.3% (Table 2).
| Characteristic | Mean |
| Hg concentration in ovaries (ng/g) | 21 (1) |
| Hg concentration in somatic tissue (ng/g) | 119 (11) |
| Expected percent change in whole-fish Hg concentration immediately after spawning due to release of eggs (%) | +3.7 (0.3) |
| MeHg concentration in ovaries (ng/g) | 21 (2) |
| MeHg concentration in somatic tissue (ng/g) | 109 (11) |
| Expected percent change in whole-fish MeHg concentration immediately after spawning due to release of eggs (%) | +3.6 (0.3) |
| GSI (%) | 4.3 (0.4) |
Table 2: Mean values for Hg concentration in ovaries, Hg concentration in somatic tissue, expected percent change in whole-fish Hg concentration immediately after spawning due to release of eggs, MeHg concentration in ovaries, MeHg concentration in somatic tissue, expected percent change in whole-fish MeHg concentration immediately after spawning due to release of eggs, and gonadosomatic index (GSI) of the five female summer flounder from New Jersey coastal waters, November 2013, used in the study. Standard error of mean enclosed within parentheses.
Proportion of Hg represented by MeHg
Mean ratio of whole-fish MeHg concentration to whole-fish Hg concentration (standard error of mean in parentheses) was 0.92 (0.02), based on data for all 50 summer flounder. Thus, on average, MeHg accounted for 92% of the Hg found in the summer flounder. By sex, ratio of whole-fish MeHg concentration to whole-fish Hg concentration averaged 0.93 (0.02) for females and 0.92 (0.03) for males. Thus, ratio of MeHg concentration to Hg concentration varied little between the sexes.
Sex-contaminant type (Hg or PCBs) interaction
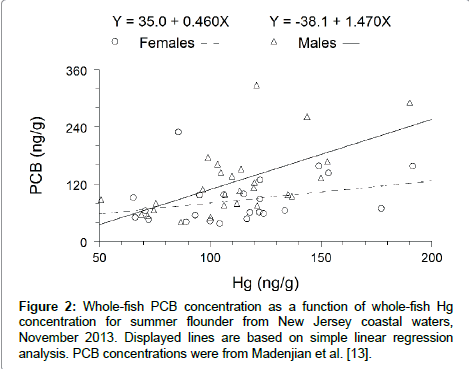
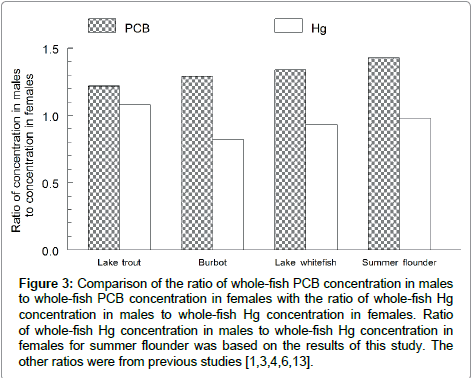
Sex significantly interacted with contaminant type (Hg or PCBs) (F=5.57; df=2, 46; P=0.0068) (Figure 2). Thus, the effect of sex on Hg concentration was significantly different from the effect of sex on PCB concentration. Males were 43% higher in PCB concentration, but females were 2% higher in Hg concentration. The ratio of Hg concentration in males to Hg concentration in females was lower than the ratio of PCB concentration in males to PCB concentration in females in summer flounder (Figure 3). A similar pattern has been observed in lake trout, burbot and lake whitefish.
Figure 3: Comparison of the ratio of whole-fish PCB concentration in males to whole-fish PCB concentration in females with the ratio of whole-fish Hg concentration in males to whole-fish Hg concentration in females. Ratio of whole-fish Hg concentration in males to whole-fish Hg concentration in females for summer flounder was based on the results of this study. The other ratios were from previous studies [1,3,4,6,13].
Discussion
The most likely explanation for the ratio of Hg concentration in males to Hg concentration in females being substantially lower than the ratio of PCB concentration in males to PCB concentration in females in summer flounder was that males eliminated Hg from their bodies at a considerably faster rate than females. In contrast, long-term elimination of PCBs by fish has been shown to be negligible for both sexes [21]. Faster Hg-elimination rates in males compared with females have been reported for northern pike (Esox lucius) and laboratory mice (Mus musculus). Based on the tracking of isotopically enriched Hg body burdens in six (three males and three females) northern pike over a 3-year period, Hg-elimination rate in mature males was estimated to be more than twice as fast as Hg-elimination rate in mature females [2]. As juveniles, rates of Hg excretion in urine did not vary between the sexes of laboratory mice [22]. However, as the mice approached maturity, males eliminated Hg in their urine at a rate three to four times higher than that of females. As adults, males continued to eliminate Hg in their urine at a substantially faster rate than females. Enhancement of Hg-elimination rate in male laboratory mice has been attributed to testosterone [22-25]. Madenjian et al. [1,2,4] hypothesized that certain androgens, such as testosterone and 11-ketotestosterone, enhance Hg-elimination in male teleost fishes as well. The pattern of the ratio of PCB concentration in males to PCB concentration in females exceeding the ratio of Hg concentration in males to Hg concentration in females persists. This phenomenon has now been documented in summer flounder, a marine fish species, as well as in three freshwater fishes, namely lake trout, burbot, and lake whitefish. Because testosterone and 11-ketotestosterone are found in all male teleost fishes [26], we may expect this pattern to hold true for all species of teleost fishes.
The magnitude of the difference between the ratio of PCB concentration in males to PCB concentration in females and the ratio of Hg concentration in males to Hg concentration in females was not solely dependent on the relative size of the testes (GSI). Because androgens have been linked to enhanced Hg-elimination rates, and because androgen concentration in the blood is positively related to GSI of males, Madenjian et al. [4] proposed that the difference between these two ratios should increase with increasing GSI. Of the four species examined to date, burbot exhibited the highest GSI of males, as GSI of males averaged 10.8%. Mean GSIs of males of the other three species were 3.1% for lake trout, 2.1% for lake whitefish, and 1.7% for summer flounder [1,4,13]. The largest difference between these two ratios was for burbot, thereby supporting the hypothesis by Madenjian et al. [4]. However, the differences between the two ratios for lake whitefish and summer flounder were substantially greater than that for lake trout, yet GSIs for lake whitefish and summer flounder were slightly lower than that for lake trout. Clearly, factors other than GSI also contributed to the difference between the two ratios. We must point out that androgen concentration in the blood is not solely dependent on GSI [4]. That is, androgen concentration can be relatively high despite a relatively low GSI. Based on results from experiments on laboratory mice [23-25], concentrations of certain androgens were important determinants of Hg-elimination rate. But, perhaps factors other than androgen concentration also influenced Hg-elimination rate. Research on various strains of laboratory mice has suggested that Hg-elimination rate may be influenced by glutathione content in the liver and kidneys, and that glutathione content in the liver and kidneys varies substantially between strains of laboratory mice [24]. Of course, differences in androgen concentration between strains may be driving these differences in glutathione content in the liver and kidneys between strains. Interestingly, Kostyniak [27] showed that Hg-elimination rate of males of the CFW strain of laboratory mice was five times higher than that of males of the CBA/J strain of laboratory mice. Moreover, estimates of Hg-elimination rates from laboratory experiments indicated that lake whitefish were capable of eliminating Hg from their bodies at a rate nearly three times faster than that for lake trout [1]. These estimates were derived from mixed-sex fish populations held in laboratory tanks. In addition, Hg-elimination rate in fish has been shown to increase with increasing water temperature [28]. In sum, the ratio of PCB concentration in males to PCB concentration in females minus the ratio of Hg concentration in males to Hg concentration in females was positive for all four fish species, suggesting that males eliminated Hg at a faster rate than females in all four species. The magnitude of the difference between the two ratios may be positively related to androgen concentration, but other factors may also be involved. Certainly, Hg-elimination rate can vary considerably from one species to another.
Expected mean percent increase in Hg concentration of females due to release of eggs at spawning was lower in summer flounder than in other fish species that have been studied. We estimated that Hg concentration of summer flounder females would increase by 3.7%, on average, immediately after spawning due to release of eggs. In lake whitefish from northern Lake Huron, Hg concentration of females was expected to increase by 17.9%, on average, immediately after spawning [1]. In burbot from Great Slave Lake (Northwest Territories, Canada), females were expected to increase in Hg concentration by 6.2%, on average, due to shedding of eggs at spawning [4]. Mean percent increases in Hg concentration of females due to release of eggs at spawning were estimated to be 15.3% in rainbow trout (Oncorhynchusmykiss), 16.0% in white sucker (Catostomus commersoni), 10.9% in white bass (Morone chrysops), 5.5% in smallmouth bass (Micropterus dolomieu), and 22.4% in yellow perch (Perca flavescens) [19]. The relatively modest change in Hg concentration of summer flounder females due to shedding of eggs was attributable, in part, to the relatively low GSI of female summer flounder. Female summer flounder averaged 4.3% in GSI, whereas mean GSI of females of the other species ranged from 7.4% to 22.3% [1,4,19].
We found that 92%, on average, of the Hg in summer flounder from New Jersey coastal waters was MeHg. Similarly, 91% of the Hg in burbot from Great Slave Lake was MeHg [4], and 91% of the Hg in lake whitefish from northern Lake Huron was MeHg [1]. In contrast, 84% of the Hg in fillets of white perch (Morone americana) from the Hackensack River (NJ, USA) was in the inorganic mercury form, while only 16% of the Hg was MeHg [18]. Raymond and Rossmann [17] reported that between 60 and 70% of the Hg found in alewives (Alosa pseudoharengus) from Lake Michigan was MeHg, whereas all (100%) of the Hg in Lake Michigan lake trout was MeHg. Further, all (100%) of the Hg in Lake Erie burbot was determined to be MeHg [4]. We also found that the percentage of Hg represented by MeHg differed little between the sexes of summer flounder. Similarly, the percentage of Hg represented by MeHg showed a minimal amount of variation between the sexes of burbot from Great Slave Lake [4], as well as lake whitefish from northern Lake Huron [1].
Long-term elimination of Hg by fish is very slow, but measurable. Trudel and Rasmussen [28] reported half-lives of Hg in fish ranging from 130 to 1030 days, based on results taken from the literature for experiments that were greater than 90 days in duration. Using regression modeling, Hg-elimination rate was found to depend on fish weight and water temperature. However, sex of the fish was not considered as an explanatory variable in the regression analyses [28]. Results from more recent research have suggested that the half-life of Hg in fish is greater than previously reported estimates [29,30]. In other words, the rate of elimination of Hg by fish is slower than once thought. Using isotopically enriched Hg in whole-lake experiments, half-lives of Hg in yellow perch and northern pike were estimated to be 489 days and 1193 days, respectively [29,30]. In a follow-up study, half-lives of Hg in male and female northern pike were estimated to be 950 days and 2039 days, respectively; this was the first study, to the best of our knowledge, to examine the difference in Hg-elimination rates between the sexes of fish [2]. Long-term elimination of PCBs by fish is so slow that it is undetectable [21]. In four experiments designed to estimate elimination rates, PCB body burden in fish showed no further decrease after an initial phase (several days in duration), during which PCB body burden did decrease immediately after dosing of the fish.
Results from previous studies have shown that ratio of Hg concentration in males to Hg concentration in females can vary substantially from one species of fish to another [1]. As mentioned above, male lake trout were 8% greater in whole-fish Hg concentration than female lake trout [3], whereas female burbot were 22% greater in whole-fish Hg concentration than male burbot [4]. Based on muscle tissue determinations, males averaged higher Hg concentrations than females in some species of fish, whereas females averaged higher Hg concentrations than males in other species of fish [31-34]. For example, Bastos et al. [34] investigated sex-related differences in muscle Hg concentrations of 41 species of freshwater fishes from the Madeira River in the Amazon, and they reported that males exceeded females in Hg concentration in 23 species, while females exceeded males in Hg concentration in the other 18 species. A significant difference in Hg concentrations between the sexes was only detected in 4 of the 41 species. For 3 species, males averaged a significantly higher Hg concentration than females, whereas females averaged a significantly higher Hg concentration than males in the other species. Bastos et al. [34] acknowledged that low sample sizes contributed to the low proportion of significant differences detected between the sexes. As previously explained, we propose that this interspecific variation in the ratio of Hg concentration in males to Hg concentration in females is primarily driven by interspecific variation in the elimination rate of Hg by males.
Our findings have implications for continued operation of the summer flounder fishery. Thresholds of 300 and 1000 ng/g have been established by the US Environmental Protection Agency (EPA) and US Food and Drug Administration (FDA), respectively, for Hg concentration in fish eaten by citizens, and either restricted consumption or no consumption is advised when these thresholds are exceeded [35]. Mean whole-fish Hg concentration in the 50 summer flounder from our study was 112 ng/g, and the maximum whole-fish Hg concentration was 191 ng/g. Fillet Hg concentration is about 10% greater than whole-fish Hg concentration in most fishes [36]. Applying this conversion factor to our summer flounder Hg data, mean and maximum Hg concentrations in the summer flounder fillets were estimated to be 123 and 210 ng/g, respectively. Thus, all of the summer flounder fillets would be below the EPA and FDA thresholds for Hg concentration. Deshpande et al. [37,38] determined Hg concentration in the white muscle tissue of summer flounder caught along the New Jersey coast in the New York Bight Apex area during 1993, and Hg concentration in these fillets averaged 40 ng/g. The considerably lower Hg concentrations reported by Deshpande et al. [37,38] compared with Hg concentrations from our study were probably due, at least in part, to a difference in summer flounder sizes between the two studies. Summer flounder used in the Deshpande et al. [37,38] study averaged only 361 mm in TL, whereas summer flounder used in our study averaged 463 in TL. Our estimated average fillet Hg concentration of 123 ng/g was similar to the average fillet Hg concentration of 136 ng/g reported by Staudinger [39] for summer flounder caught in the continental shelf waters of the Atlantic Ocean between New Jersey and Massachusetts during 2002-2003 using bottom trawls. Average size of summer flounder used in our study was similar to summer flounder average size from the Staudinger [39] study. Determinations of fillet Hg concentrations of mixed species of flounder (Pleuronectiformes) from New Jersey supermarkets yielded a mean concentration of 50 ng/g [40]. The lower mean Hg concentration from the Burger and Gochfeld [40] study compared with our mean Hg concentration may simply have been due to other species of flounder being lower in Hg concentration than summer flounder. The FDA threshold for PCB concentration in fish eaten by people is 2000 ng/g [41]. Mean and maximum wholefish PCB concentrations of the summer flounder from our study were 107 and 327 ng/g, respectively [13]. Thus, all of the summer flounder PCB concentrations were well below the FDA guideline. Mean PCB concentration of the summer flounder used in the Deshpande et al. [37,38] study was less than the detection limit of 50 ng/g. The considerably lower PCB concentrations reported by Deshpande et al. [37,38] compared with our summer flounder PCB concentrations were attributable to two factors. First, Deshpande et al. [37,38] determined PCB concentrations in fillets, whereas we determined whole-fish PCB concentrations. Typically, whole-fish PCB concentrations are substantially higher than fillet PCB concentrations [42]. Second, the summer flounder used in the Deshpande et al. [37,38] study were considerably smaller than the summer flounder used in our study.
Although we did not detect a significant increase in Hg concentration with increasing summer flounder TL, Staudinger [39] found that Hg concentration of summer flounder significantly increased with increasing TL. This discrepancy between the two studies could be attributable to differences in TL ranges between the two studies. Our summer flounder ranged from 381 to 560 mm in TL, while the summer flounder used by Staudinger [39] ranged from 280 to 750 mm in TL. We would have likely detected a significant increase in Hg concentration with increasing TL had we sampled from a slightly wider range of TLs. Some of the summer flounder used in the Staudinger [39] study were probably immature fish, because most summer flounder less than 290 mm TL are immature [43].
Conclusion
Mean whole-fish Hg concentration of summer flounder caught in New Jersey coastal waters was 113 ng/g for mature females and 111 ng/g for mature males, but this difference was not statistically significant. However, sex did significantly interact with contaminant type (Hg or PCBs), as males were 43% greater in PCB concentration than females, but females were 2% higher in Hg concentration. This significant interaction effect was attributed to males eliminating Hg from their bodies at a faster rate than females, whereas long-term elimination of PCBs by both sexes was negligible. The ratio of PCB concentration in males to PCB concentration in females exceeding the ratio of Hg concentration in males to Hg concentration in females has now been documented in summer flounder, a marine species, as well as in three freshwater fish species, namely lake trout, burbot, and lake whitefish. MeHg represented 92% of the Hg found in the summer flounder. Both Hg and PCB concentrations in the summer flounder were relatively low, well below the EPA and FDA guidelines, and therefore our findings had implications for continued operation of the summer flounder fishery.
Acknowledgements
Ganzorig Batsaikhan, Maria Berezin, Kaycee Coleman, Julia Criscione, Christopher Filosa, James Fiorendino, Christopher Free, Evan Kwityn, Andrew Lahr, Margaret Shaw, and Thomas Siciliano assisted with homogenization of the fish tissues. Jason Morson obtained the summer flounder and provided further assistance throughout this study. Blanche Jackson and Eric Robillard contributed their expertise in aging summer flounder otoliths. James Hurley reviewed the manuscript and made helpful suggestions for its improvement. Use of trade, product, or firm names does not imply endorsement by the United States Government. This article is Contribution 2029 of the US Geological Survey Great Lakes Science Center.
References
- Madenjian CP, Ebener MP, Krabbenhoft DP (2016) Mercury accumulation, and the mercury-PCB-sex interaction, in Lake Whitefish (Coregonusclupeaformis). Environments 3: 7.
- Madenjian CP, Blanchfield PJ, Hrenchuk LE, Van Walleghem JLA (2014) Mercury elimination rates for adult northern pike Esoxlucius: evidence for a sex effect. Bull EnvironContamToxicol 93: 144-148.
- Madenjian CP, Keir MJ, Whittle DM (2011) Sexual difference in mercury concentrations of lake trout (Salvelinusnamaycush) from Lake Ontario. Chemosphere 83:903-908.
- Madenjian CP, Stapanian MA, Cott PA, Krabbenhoft DP, Edwards WH, et al. (2015) Females exceed males in mercury concentrations of burbotLotalota. Arch Environ ContamToxicol 68: 678-688.
- Madenjian CP (2011) Sex effect on polychlorinated biphenyl concentrations in fish: a synthesis. Fish and Fisheries 12: 451-460.
- Madenjian CP, Stapanian MA, Cott PA, Rediske RR, O’Keefe JP (2014) Polychlorinated biphenyl concentrations of burbotLotalota from Great Slave Lake are very low but vary by sex. Arch EnvironContamToxicol 66: 529-537.
- Madenjian CP, Ebener MP, Sepúlveda MS (2015) PCB concentrations of Lake Whitefish (Coregonusclupeaformis) vary by sex. Journal of Great Lakes Research 41: 1185-1190.
- Marshall WS, Grosell M (2006) Ion transport, osmoregulation and acid-base balance. In: Evans DH, Claiborne JB (eds.) The physiology of fishes. Taylor & Francis CRC Press, Boca Raton, FL, USA.
- Terceiro M (2002) The summer flounder chronicles: Science, politics, and litigation 1975-2000. Reviews in Fish Biology and Fisheries 11: 125-168.
- Terceiro M (2011) The summer flounder chronicles II: new science, new controversy 2001-2010. Reviews in Fish Biology and Fisheries 21: 681-712.
- Morson JM, Bochenek EA, Powell EN, Gius JE (2012) Sex at length of summer flounder landed in the New Jersey recreational party boat fishery.North American Journal of Fisheries Management 32: 1201-1210.
- Wuenschel MJ, Able KW, Vasslides JM, Byrne DM (2013) Habitat and diet overlap of 4 piscivorous fishes: variation on the inner continental shelf off New Jersey. Fish Bull 111: 352-369.
- Madenjian CP, Jensen OP, Rediske RR, O’Keefe JP, Vastano AR, et al. (2016) Differences in energy expenditures and growth dilution explain higher PCB concentrations in male summer flounder. PLoS ONE 11: e0147223.
- United States Environmental Protection Agency (2007) Method 7473: mercury in solids and solutions by thermal decomposition, amalgamation, and atomic absorption spectrophotometry. Office of Water, Washington, DC, USA.
- United States Environmental Protection Agency (1990) Guidelines establishing test procedures for the analysis of pollutants.Office of Water, Washington, DC, USA.
- United States Environmental Protection Agency (1998) Method 1630: methyl mercury in water by distillation, aqueous ethylation, purge and trap, and cold vapor atomic fluorescence spectrometry. Office of Water, Washington, DC, USA.
- Raymond B, Rossmann R (2009) Total and methyl mercury accumulation in 1994-1995 Lake Michigan lake trout and forage fish. Journal of Great Lakes Research 35:438-446.
- Weis P, Ashley JTF (2007) Contaminants in fish of the Hackensack Meadowlands, New Jersey: size, sex, and seasonal relationships as related to health risks. ArchEnvironContamToxicol 52:80-89.
- Niimi AJ (1983) Biological and toxicological effects of environmental contaminants in fish and their eggs.Canadian Journal of Fisheries and Aquatic Sciences 40: 306-312.
- Scheffé H (1959) The analysis of variance. John Wiley & Sons, New York, NY, USA.
- Madenjian CP, Hesselberg RJ, DeSorcie TJ, Schmidt LJ, Stedman RM, et al. (1998) Estimate of net trophic transfer efficiency of PCBs to Lake Michigan lake trout from their prey. Environ SciTechnol 32: 886-891.
- Hirayama K, Yasutake A (1986) Sex and age differences in mercury distribution and excretion in methylmercury-administered mice. JToxicolEnvironHealth 18:49-60.
- Yasutake A, Hirayama K, Inoue M (1989) Mechanism of urinary excretion of methylmercury in mice.ArchToxicol 63: 479-483.
- Tanaka T, Naganuma A, Kobayashi K, Imura N (1991) An explanation for strain and sex differences in renal uptake of methylmercury in mice. Toxicology 69:317-329.
- Tanaka T, Naganuma A, Miura N, Imura N (1992) Role of testosterone in γ-glutamyltranspeptidase-dependent renal methylmercury uptake in mice. ToxicolApplPharmacol 112: 58-63.
- Borg B (1994) Androgens in teleost fishes. Comparative Biochemistry and Physiology 109C: 219-245.
- Kostyniak PJ (1980) Differences in elimination rates of methylmercury between two genetic variant strains of mice. Toxicol Lett6:405-410.
- Trudel M, Rasmussen JB (1997) Modeling the elimination of mercury by fish. Environ SciTechnol 31:1716-1722.
- Van Walleghem JLA, Blanchfield PJ, Hintelmann H (2007) Elimination of mercury by yellow perch in the wild. Environ SciTechnol 41: 5895-5901.
- Van Walleghem JLA, Blanchfield PJ, Hrenchuk LE, Hintelmann H (2013) Mercury elimination by a top predator, Esoxlucius. Environ SciTechnol47: 4147-4154.
- Monteiro LR, Isidro EJ, Lopes HD (1991) Mercury content in relation to sex, size, age and growth in two scorpionfish (Helicolenusdactylopterus and Pontinuskuhlii) from Azorean waters. Water, Air, and Soil Pollution 56: 359-367.
- Stafford CP, Hansen B, Stanford JA (2004) Mercury in fishes and their diet items from Flathead Lake, Montana. Transactions of the American Fisheries Society 133: 349-357.
- Gewurtz SB, Bhavsar SP, Fletcher R (2011) Influence of fish size and sex on mercury/PCB concentration: Importance for fish consumption advisories. Environ Int 37: 425-434.
- Bastos WR, Dórea JG, Bernardi JVE, Manzatto AG, Mussy MH, et al. (2016) Sex-related mercury bioaccumulation in fish from the Madeira River, Amazon. Environ Res144: 73-80.
- Cai Y, Rooker JR, Gill GA, Turner JP (2007) Bioaccumulation of mercury in pelagic fishes from the northern Gulf of Mexico. Canadian Journal of Fisheries and Aquatic Sciences 64: 458-469.
- Becker DS, Bigham GN (1995) Distribution of mercury in the aquatic food web of Onondaga Lake, New York. Water, Air, and Soil Pollution 80: 563-571.
- Deshpande AD, Draxler AFJ, Zdanowicz VS, Schrock ME, Paulson AJ (2002) Contaminant levels in the muscle of four species of fish important to the recreational fishery of the New York Bight Apex. Mar Pollut Bull 44: 164-171.
- Deshpande AD, Draxler AFJ, Zdanowicz VS, Schrock ME, Paulson AJ, et al. (2000) Contaminant levels in the muscle of four species of fish important to the recreational fishery of the New York Bight Apex.NOAA Technical Memorandum NMFS-NE-157, Northeast Fisheries Science Center, Woods Hole, MA, USA.
- Staudinger MD (2011) Species and size-specific variability of mercury concentrations in four commercially important finfish and their prey from the northwest Atlantic. Mar Pollut Bull 62: 734-740.
- Burger J, Gochfeld M (2005) Heavy metals in commercial fish in New Jersey. Environ Res 99: 403-412.
- United States Food and Drug Administration (2015) Tolerances of polychlorinated biphenyls (PCBs), 21 CFR. Health and Human Services, Public Health Service, Rockville, MD, USA.
- Amrhein JF, Stow CA, Wible C (1999) Whole-fish versus filet polychlorinated-biphenyl concentrations: An analysis using classification and regression tree models. Environmental Toxicology and Chemistry 18: 1817-1823.
- Northeast Fisheries Science Center (2013) 57th Northeast Regional Stock Assessment orkshop (57th SAW) Assessment Report. US Department of Commerce, Northeast Fisheries Science Center Reference Document pp: 13-16.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 11830
- [From(publication date):
April-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 10901
- PDF downloads : 929