Review Article Open Access
Mechanisms of Neurotrophic Activities via Low-molecular-weight Compounds: Post-transcriptional Regulation in PC12 Cells and Neurons
| Hiroki Maruoka1,2 and Koji Shimoke1* | |
| 1Department of Life Science and Biotechnology, Faculty of Chemistry, Materials, and Bioengineering and Strategic Research Base, Kansai University, Japan | |
| 2Technology Research Laboratory, KURABO, Japan | |
| Corresponding Author : | Dr. Koji Shimoke Department of Life Science and Biotechnology Faculty of Chemistry, Materials and Bioengineering and Strategic Research Base Kansai University, 3-3-35, Yamate-cho Suita, Osaka 564-8680, Japan Tel: +81-6-6368-0853 Fax: +81-6-6330-3770 E-mail: shimoke@kansai-u.ac.jp |
| Received January 10, 2013; Accepted February 20, 2013; Published February 27, 2013 | |
| Citation: Maruoka H, Shimoke K (2013) Mechanisms of Neurotrophic Activities via Low-molecular-weight Compounds: Post-transcriptional Regulation in PC12 Cells and Neurons. Clin Pharmacol Biopharm S1:003. doi:10.4172/2167-065X.S1-003 | |
| Copyright: © 2013 Maruoka H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Recently, it was reported that some low-molecular-weight compounds mimic neurotrophic activities including neurite outgrowth and neuroprotection. Carnosic acid (CA) promotes neurite outgrowth through the activation of Nrf2 in a model of neuron PC12 cells. CA also protects neurons from oxidative stress via the keap/Nrf2 transcriptional pathway. Luteolin induces neurite outgrowth via MAPK, PKC, and cAMP/PKA signaling pathways. In addition, luteolin protects PC12 cells from serum withdrawal-induced oxidative stress. Forskolin-induced neurite outgrowth is mediated by the activation of the PKA signaling pathway, and this PKAmediated neurite outgrowth is achieved by the expression of Nur77 in PC12 cells. In addition, a low concentration of forskolin is closely related to the cAMP-induced protective function against L-DOPA-induced cytotoxicity. The post-transcriptional regulation of gene expression including microRNAs and the acetylation of non-histone protein plays critical roles in neurotrophic activities. Recently, it was revealed that miR-132 modulates luteolin-induced neurite outgrowth via cAMP/PKA- and MAPK-dependent CREB signaling pathways in PC12 cells. Moreover, it has been reported that acetylated Nrf2 binds to the transcriptional activator, CBP/p300 directly, and that Nur77 is acetylated in vivo and in vitro by CBP/p300. The modulation of miR-132 and acetylation of Nrf2 and Nur77 by CBP/p300 may constitute a similar novel regulatory mechanism for low-molecular-weight compounds with neurotrophic activities.
| Keywords |
| Nerve injury; Carnosic acid; Luteolin; Forskolin; miR-132; PC12 cell |
| Introduction |
| Nerve injury including traumatic brain injury (TBI) is a major public health concern in industrialized countries. It has been estimated that 1.4 million people sustain a TBI annually and 5 million people are disabled in the United States. Young children, adolescents, and the elderly, predominantly male, exhibit the highest rates of TBI [1]. The treatment of nerve injury subjects and improving their outcomes has still not been clarified [2]. |
| Nerve injury results in the formation of contusions, neuronal apoptosis, and axonal tract damage. Promoting neurite outgrowth and protecting neurons from apoptosis are important factors in the treatment of nerve injury [3-6]. It has been shown that the neurotrophic factors NGF, BDNF, NT-3, and NT-4/5 have neuroprotective and neuronal differentiation abilities [7], and are attracting attention as medicines for TBI [8]. Previous studies have demonstrated that NGF promoted electrophysiological, and histomorphological parameters and enhanced axonal regeneration following nerve injury in vivo [9-11]. The specific receptors of the neurotrophic factors NGF, BDNF plus NT-4/5, and NT-3 are TrkA, TrkB, and TrkC, respectively. These Trk family members are membrane-spanning receptors on the cell membrane of neurons [12]. |
| Intracellular signaling pathways contain several protein phosphorylation cascades and at least three signaling pathways have been identified downstream of Trk receptors: the Ras/mitogenactivated protein kinase (MAPK) pathway, phosphatidylinositol 3-kinase (PI3-K)/Akt pathway, and PLC-gamma pathway [13]; however, the delivery of exogenous neurotrophic factors is the greatest obstacle for their therapeutic application since neurotrophic factors are large polypeptide molecules that do not penetrate the bloodbrain barrier (BBB) and are easily metabolized by peptidases when administered peripherally. |
| In this review, we present recent topics regarding low-molecular weight compounds with neurotrophic activity in PC12 cells and neurons. |
| Natural Products and Neurotrophic Activities |
| Natural products may harmonize very well for the treatment of neuronal injury [14-19]. Recently, many compounds from natural sources were demonstrated to possess neurotrophic and neuroprotective abilities [20]. Current research has also confirmed the role of natural products in enhancing the neurite outgrowth activity of NGF in various experimental models [21]. |
| Carnosic acid (CA) is a phenolic diterpene found in the dietary herb rosemary (Rosmarinus officinalis L.) (Figure 1) that exerts antioxidant properties by acting as a radical scavenger [22,23]. Previously, it was demonstrated that CA functions as a peroxisome proliferator-activated receptor g (PPARg) agonist or 5-lipoxygenase inhibitor in mammalian cells [24,25]. However, the role of CA in nerve cells is still unknown. |
| Recently, it was reported that CA stimulates NGF gene expression through an NF-E2-related factor 2 (Nrf2)-dependent pathway and induces NGF production in astroglial cells [26,27]. Nrf2 is a CNCbZip transcription factor that plays a key role in redox regulation and drug metabolism [28,29]. Previous reports have revealed that Nrf2 is activated by Reactive Oxygen Species (ROS) and exogenous and endogenous electrophiles, such as sulphoraphane and 6-methylsulfinylhexyl isothiocyanate. Moreover, CA can cross the BBB and attenuate middle cerebral artery occlusion (MCAO)-induced neuronal cell death by upregulating the expression of antioxidative Nrf2 target genes, such as HO-1 [30]. |
| In PC12 cells, it has been shown that CA promotes neurite outgrowth and CA-activated Nrf2-induced p62/ZIP expression is essential for the neuronal differentiation of PC12 cells. Furthermore, it has been reported that CA-activated MAPK 1/2 and PI3-K, independent of Nrf2 activation and the activation of these kinases leads to the enhancement of Nrf2 accumulation in CA-mediated neuronal differentiation [26]. In this way, it is thought that Nrf2 contributes to CA-induced neuronal differentiation via the induction of p62/ZIP expression. |
| In addition to the effect on neurite outgrowth, CA exhibited neuroprotective activity against glutamate/ oxidative stress and cerebral ischemia both in vitro and in vivo. Previous reports revealed that CA activates the keap/Nrf2 transcriptional pathway by binding to specific keap1 cysteine residues, thereby protecting neurons from oxidative stress and excitotoxicity [30-32]. |
| From these findings, it is thought that CA may be a treatment for nerve injury. However, the detailed molecular mechanism by which CA enhances NGF production and the roles of neurotrophic activities in neurons remain unknown. A detailed analysis is expected in the future. |
| Luteolin (3’,4’,5,7-tetrahydroxyflavone), which is a natural flavonoid that exists in several types of vegetables, fruits, and medicinal herbs also exhibits neurotrophic activity (Figure 2). Luteolin is an ingredient of rosemary, similar to CA. In the mammalian Central Nervous System (CNS), it has been shown that luteolin can permeate through the blood-brain barrier (BBB), show anti-amnesic effects against the toxicity of amyloid in mice, and attenuate scopolamineinduced amnesia in rats [33,34]. |
| In neurite outgrowth, Lin et al. [35] suggested that luteolin promotes neurite outgrowth through the activation of MAPK, PKC and cAMP/PKA signaling pathways in PC12 cells. They also reported that this neurite outgrowth induced TrkA- and EGFR-independent signaling pathways [35,36]. |
| For neuroprotective activity, luteolin has been found to possess anti-inflammatory and neuroprotective activities in microglia [37] and attenuate the neurotoxicity induced by peroxide [38], the neurotoxic agent N-methyl-4-phenyl-pyridinium (MPP+) [39], and amyloid beta protein [40]. Furthermore, luteolin protects PC12 cells from serum withdrawal-induced oxidative stress through Nrf2- mediated transcriptional activation of HO-1 [35]. These findings demonstrate the possibility that luteolin as well as CA may be useful in the treatment of nerve injury. |
| Similar to the effects of neurotrophic factors, cyclic AMP (cAMP) can also promote neurite outgrowth and neuroprotective activity either on its own or via the activation of MAPK and cyclic AMPdependent protein kinase (PKA) in nerve cells [41]. Previously, it was shown that intracellular cAMP protects against oxidative stress when used alone and in association with the neurotrophic factors, NGF and EGF in PC12 cells [42]. In addition, it has also been reported that the cAMP analogue dbcAMP promotes neurite outgrowth in human neuroblastoma SH-SY5Y cells and PC12 cells [43,44]. Furthermore, we have reported that treatment with dbcAMP leads to the expression of immediate early genes (IEGs), including c-fos and Nur77, as does treatment with NGF in PC12 cells. We also observed that the cAMPPKA- Nur77 pathway is essential for the induction of differentiation by dbcAMP in PC12 cells and their expressions are regulated via the acetylation of histone H3 [44]. It is thought that detailed studies on low-molecular-weight compounds with neurotrophic activity will be necessary for advancing this field. However, the detailed mechanisms of cAMP/PKA have not yet been fully elucidated. |
| In CNS injury models, several studies have demonstrated that the restoration of cAMP levels improves the outcome. In spinal cord injuries, the application of rolipram to inhibit the degradation of cAMP promotes axon sparing and results in locomotor improvements [45,46]. Similarly, rolipram improves neuronal survival in the hippocampus and hippocampal-dependent learning in transient global ischemia [47-49]. |
| However, it has been reported that rolipram is characterized for its emetic and other problematic effects, and the development of a cAMP activator other than rolipram is expected. |
| Forskolin, one of the natural products, is a cAMP activator that is used to raise the level of cAMP. Forskolin also plays a useful role in neurite outgrowth and neuroprotective activity. Forskolin is a labdane diterpene that is produced by the Indian Coleus plant (Figure 3). Moreover, it is also known that forskolin is a BBB permeant. |
| Previously, it was shown that the forskolin induced neurite outgrowth of PC12 cells is mediated by the activation of the PKA signaling pathway and synergistic activation of the ERK signaling pathway [43,50]. |
| On the other hand, Jin et al. [41] suggested that a low concentration of forskolin is closely related with the cAMP-induced protective function against L-DOPA-induced cytotoxicity and that a high concentration of forskolin induces the cAMP-mediated apoptotic process, which enhances L-DOPA-induced cytotoxicity in PC12 cells. |
| These findings reveal the possibility that forskolin may be a useful tool for the treatment of nerve injury. A detailed investigation is expected in the future. |
| The Post-Transcriptional Regulation of Gene Expression: MicroRNAs and the Acetylation of Non- Histone Protein |
| The post-transcriptional regulation of gene expression plays critical roles in neurotrophic activities including neurite outgrowth and neuroprotective activity. MicroRNAs (miRNAs) form part of the post-transcriptional machinery. miRNAs are a class of small, noncoding RNAs of 21-23 nucleotides that regulate gene expression at the posttranscriptional level by binding to the mRNA of protein coding genes [51]. It has been reported that miRNAs are involved in several biological processes, such as development, morphogenesis, cell proliferation, cell differentiation, and apoptosis [52]. In the mammalian CNS, several miRNAs are specifically transcribed and enriched and may play important regulatory roles in neuronal development and brain function [53-55]. A previous study revealed that miR-132, an miRNA that is enriched in mammalian brain tissue, could be induced by neurotrophic factors and that this could represent a mechanism for fine-tuning protein expression following neurotrophic action [56,57]. |
| Recently, it was reported that miR-132 modulates luteolin-induced neurite outgrowth in PC12 cells. Furthermore, it has been revealed that the cAMP/PKA- and MAPK-dependent CRE binding protein (CREB) signaling pathways are involved in the luteolin-mediated miR-132 expression and neuritogenesis of PC12 cells [36]. CREB is a transcription factor that binds to the cAMP-responsive element (CRE), a consensus sequence found in the promoter regions of many target genes. It has been reported that miR-132 is induced by CREB and is involved in the modulation of dendritic morphology, neurite outgrowth, synaptic plasticity and neuroprotection [56,58-60]. Therefore, it is thought that miR-132 also modulates a lot of CREBregulated genes in nerve cells. |
| The relationship between CA-induced neurite outgrowth and neuroprotective activity, and the regulation of miR-132 has not yet been clarified. Recently, it was revealed that CREB-binding protein (CBP) regulates Nrf2-induced gene transcription [61]. Therefore, it is expected that miR-132 is regulated by Nrf2 via CBP. Moreover, it has been shown that CA activates the MAPK-dependent CREB pathway in addition to Nrf2/ p62/ZIP in PC12 cells [26]. Therefore, it is expected that the MAPK-dependent CREB pathway induced by CA regulates miR-132. |
| On the other hand, it has been reported that forskolin promotes PKA and CREB phosphorylation and induces miR-132 expression in cultured primary rat neurons [57]. It is known that PKA phosphorylates CREB and that there are more than 100 CREB target genes including IEGs. Furthermore, we reported that dbcAMPinduced neurite outgrowth is regulated by the PKA-CREB-Nur77 pathway in PC12 cells [44]. However, the relationship between PKACREB- dependent neurite outgrowth and the regulation of miR- 132 has not yet been clarified. Recently, it was reported that miR- 132 regulates the differentiation of dopamine neurons by directly targeting Nurr1 expression [62]. It is known that Nur77 and Nurr1 are members of the Nur77 family, which also contains orphan nuclear transcription factors. Therefore, it is expected that miR-132 regulates neurite outgrowth by Nur77 expression, similar to Nurr1. The role of Nur77 on miR-132-mediated PC12 differentiation remains to be investigated. A detailed investigation is expected in the future. |
| The transcriptional regulation of acetylation is also one of the most important potential mechanisms by which signaling transduction cascades may control their cellular functions [63]. The control of transcription by epigenetic modifications has proven to be important for neurite outgrowth and neuroprotection activity during neuronal development in the nervous system. |
| Recent studies have shown that many non-histone proteins, particularly transcription factors, are substrates for CBP/p300, greatly expanding the possible mechanisms of CBP/p300 in transcriptional activation [64]. CBP and p300 proteins are common co-activators for a variety of transcription factors [65,66]. |
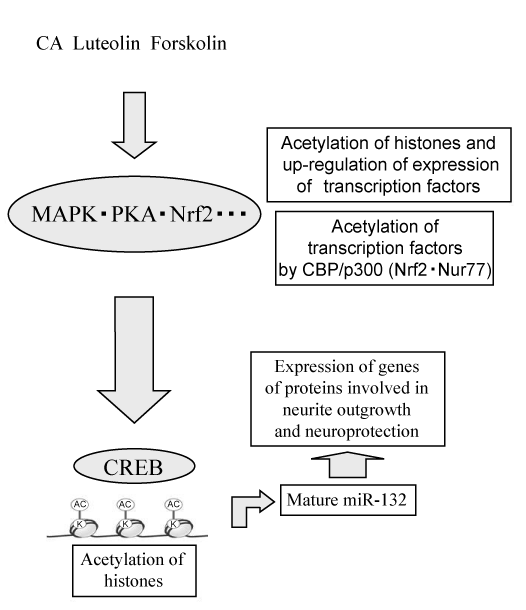
| Sun et al. revealed that CBP/ p300 directly bound to and acetylated Nrf2 in response to arsenite-induced oxidative stress [67]. Acetylation of Nrf2 by CBP/p300 showed the possibility to constitute a novel regulatory mechanism for Nrf2-dependent neurotrophic activity. Nur77 is acetylated in vivo and in vitro by CBP/p300 and has been detected using acetylation specific antibodies, including anti-Panacetyl and antiacetylated Lys antibodies [68]. We reported that Nur77 was involved in dbcAMP-induced neurite outgrowth in PC12 cells [44]. Acetylation of Nur77 by CBP/ p300 may also constitute a novel regulatory mechanism for Nur77-dependent neurotrophic activity. As shown in figure 4, we propose a similar novel regulatory mechanism by which low-molecular-weight compounds induce neurite outgrowth and neuroprotection. |
| Conclusion |
| It has become evident that low-molecular-weight-compounds including natural products may work as therapeutic agents possessing neurotrophic activities and that they may exert many effects on cell function in the central and peripheral nervous systems. However, molecular mechanisms of neurotrophic activities via low-molecularweight- compounds are largely unknown. In this review, we introduced the possibility that three low-molecular-weight compounds CA, luteolin and forskolin showed neurotrophic activities through the same mechanism in the post-translational regulation. |
| Low-molecular-weight compounds may lead to posttranscriptional regulation including miRNAs and acetylation of histones, and, thus, induce the expression of transcription factors. These transcription factors may be acetylated by CBP/p300. Both acetylated transcription factors and acetylated histones may lead to the increased expression of the genes of proteins involved in neurite outgrowth and neuroprotection (Figure 4). |
| It is expected that the detailed relationship between the neurotrophic activities of low-molecular-weight compounds and gene expression will be revealed in the future. |
| Acknowledgements |
| This work was supported, in part, by Grants-in-Aid for Scientific Research from MEXT (Ministry of Education, Culture, Sports, Science, and Technology of Japan), HAITEKU (2002-2006) and SENRYAKU (2008-2012) from MEXT, and the Kansai University Special Research Funds, 2007 and 2009. |
| References |
References
- Faul M, Xu L, Wald MM, Coronado VG (2010) Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths. National Center for Injury Prevention and Control 1-71.
- Maas AI, Stocchetti N, Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurol 7: 728-741.
- Balasubramaniyan V, Boddeke E, Bakels R, Kust B, Kooistra S, et al. (2006) Effects of histone deacetylation inhibition on neuronal differentiation of embryonic mouse neural stem cells. Neuroscience 143: 939-951.
- Saha RN, Pahan K (2006) HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell Death Differ 13: 539-550.
- Schwechter BR, Millet LE, Levin LA (2007) Histone deacetylase inhibition-mediated differentiation of RGC-5 cells and interaction with survival. Invest Ophthalmol Vis Sci 48: 2845-2857.
- Siebzehnrubl FA, Buslei R, Eyupoglu IY, Seufert S, Hahnen E, et al. (2007) Histone deacetylase inhibitors increase neuronal differentiation in adult forebrain precursor cells. Exp Brain Res 176: 672-678.
- Bothwell M (1995) Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci 18: 223-253.
- Sofroniew MV, Howe CL, Mobley WC (2001) Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 24: 1217-1281.
- Lee AC, Yu VM, Lowe JB 3rd, Brenner MJ, Hunter DA, et al. (2003) Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol 184: 295-303.
- Sun W, Sun C, Lin H, Zhao H, Wang J, et al. (2009) The effect of collagen-binding NGF-beta on the promotion of sciatic nerve regeneration in a rat sciatic nerve crush injury model. Biomaterials 30: 4649-4656.
- Chen J, Chu YF, Chen JM, Li BC (2010) Synergistic effects of NGF, CNTF and GDNF on functional recovery following sciatic nerve injury in rats. Adv Med Sci 55: 32-42.
- Kaplan DR, Miller FD (1997) Signal transduction by the neurotrophin receptors. Curr Opin Cell Biol 9: 213-221.
- Shimoke K, Fukunaga K, Matsumura Y, Kudo M, Ikeuchi T (2009) Protection from ER stress-mediated apoptosis by the neurotrophins. Curr Topics Biochem Res 11: 19-28.
- Tohda C, Kuboyama T, Komatsu K (2005) Search for natural products related to regeneration of the neuronal network. Neurosignals 14: 34-45.
- Li P, Yamakuni T, Matsunaga K, Kondo S, Ohizumi Y (2003) Nardosinone enhances nerve growth factor-induced neurite outgrowth in a mitogen-activated protein kinase- and protein kinase C-dependent manner in PC12D cells. J Pharmacol Sci 93: 122-125.
- Sagara Y, Vanhnasy J, Maher P (2004) Induction of PC12 cell differentiation by flavonoids is dependent upon extracellular signal-regulated kinase activation. J Neurochem 90: 1144-1155.
- Guo Y, Xu J, Li Y, Watanabe R, Oshima Y, et al. (2006) Iridoids and sesquiterpenoids with NGF-potentiating activity from the rhizomes and roots of Valeriana fauriei. Chem Pharm Bull (Tokyo) 54: 123-125.
- Kano Y, Horie N, Doi S, Aramaki F, Maeda H, et al. (2008) Artepillin C derived from propolis induces neurite outgrowth in PC12m3 cells via ERK and p38 MAPK pathways. Neurochem Res 33: 1795-1803.
- Shibata T, Nakahara H, Kita N, Matsubara Y, Han C, et al. (2008) A food-derived synergist of NGF signaling: identification of protein tyrosine phosphatase 1B as a key regulator of NGF receptor-initiated signal transduction. J Neurochem 107: 1248-1260.
- More SV, Koppula S, Kim IS, Kumar H, Kim BW, et al. (2012) The role of bioactive compounds on the promotion of neurite outgrowth. Molecules 17: 6728-6753.
- Li P, Matsunaga K, Yamakuni T, Ohizumi Y (2002) Picrosides I and II, selective enhancers of the mitogen-activated protein kinase-dependent signaling pathway in the action of neuritogenic substances on PC12D cells. Life Sci 71: 1821-1835.
- Haraguchi H, Saito T, Ishikawa H, Sanchez Y, Ogura T, et al. (1996) Inhibition of lipid peroxidation by sesquiterpenoid in Heterotheca inuloides. J Pharm Pharmacol 48: 441-443.
- Costa S, Utan A, Speroni E, Cervellati R, Piva G, et al. (2007) Carnosic acid from rosemary extracts: a potential chemoprotective agent against aflatoxin B1. An in vitro study. J Appl Toxicol 27: 152-159.
- Rau O, Wurglics M, Paulke A, Zitzkowski J, Meindl N, et al. (2006) Carnosic acid and carnosol, phenolic diterpene compounds of the labiate herbs rosemary and sage, are activators of the human peroxisome proliferator-activated receptor gamma. Planta Med 72: 881-887.
- Poeckel D, Greiner C, Verhoff M, Rau O, Tausch L, et al. (2008) Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress pro-inflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem Pharmacol 76: 91-97.
- Kosaka K, Mimura J, Itoh K, Satoh T, Shimojo Y, et al. (2010) Role of Nrf2 and p62/ZIP in the neurite outgrowth by carnosic acid in PC12h cells. J Biochem 147: 73-81.
- Mimura J, Kosaka K, Maruyama A, Satoh T, Harada N, et al. (2011) Nrf2 regulates NGF mRNA induction by carnosic acid in T98G glioblastoma cells and normal human astrocytes. J Biochem 150: 209-217.
- Kobayashi M, Yamamoto M (2006) Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul 46: 113-140.
- Motohashi H, Yamamoto M (2004) Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med 10: 549-557.
- Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, et al. (2008) Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem 104: 1116-1131.
- de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, et al. (2008) Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med 45: 1375-1383.
- Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, et al. (2008) The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci 1147: 61-69.
- Liu R, Gao M, Qiang GF, Zhang TT, Lan X, et al. (2009) The anti-amnesic effects of luteolin against amyloid beta(25-35) peptide-induced toxicity in mice involve the protection of neurovascular unit. Neuroscience 162: 1232-1243.
- Tsai FS, Peng WH, Wang WH, Wu CR, Hsieh CC, et al. (2007) Effects of luteolin on learning acquisition in rats: involvement of the central cholinergic system. Life Sci 80: 1692-1698.
- Lin TY, Lu CW, Chang CC, Huang SK, Wang SJ (2011) Luteolin inhibits the release of glutamate in rat cerebrocortical nerve terminals. J Agric Food Chem 59: 8458-8466.
- Lin LF, Chiu SP, Wu MJ, Chen PY, Yen JH (2012) Luteolin induces microRNA-132 expression and modulates neurite outgrowth in PC12 cells. PLoS One 7: e43304.
- Dirscherl K, Karlstetter M, Ebert S, Kraus D, Hlawatsch J, et al. (2010) Luteolin triggers global changes in the microglial transcriptome leading to a unique anti-inflammatory and neuroprotective phenotype. J Neuroinflammation 7: 3.
- Pavlica S, Gebhardt R (2010) Protective effects of flavonoids and two metabolites against oxidative stress in neuronal PC12 cells. Life Sci 86: 79-86.
- Wruck CJ, Claussen M, Fuhrmann G, Romer L, Schulz A, et al. (2007) Luteolin protects rat PC12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathway. J Neural Transm Suppl: 57-67.
- Cheng HY, Hsieh MT, Tsai FS, Wu CR, Chiu CS, et al. (2010) Neuroprotective effect of luteolin on amyloid beta protein (25-35)-induced toxicity in cultured rat cortical neurons. Phytother Res 24: S102-S108.
- Jin CM, Yang YJ, Huang HS, Kai M, Lee MK (2010) Mechanisms of L-DOPA-induced cytotoxicity in rat adrenal pheochromocytoma cells: implication of oxidative stress-related kinases and cyclic AMP. Neuroscience 170: 390-398.
- Lambeng N, Michel PP, Agid Y, Ruberg M (2001) The relationship between differentiation and survival in PC12 cells treated with cyclic adenosine monophosphate in the presence of epidermal growth factor or nerve growth factor. Neurosci Lett 297: 133-136.
- Sanchez S, Jimenez C, Carrera AC, Diaz-Nido J, Avila J, et al. (2004) A cAMP-activated pathway, including PKA and PI3K, regulates neuronal differentiation. Neurochem Int 44: 231-242.
- Maruoka H, Sasaya H, Shimamura Y, Nakatani Y, Shimoke K, et al. (2010) Dibutyryl-cAMP up-regulates nur77 expression via histone modification during neurite outgrowth in PC12 cells. J Biochem 148: 93-101.
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT (2004) The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A 101: 8786-8790.
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, et al. (2004) cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med 10: 610-616.
- Kato H, Araki T, Itoyama Y, Kogure K (1995) Rolipram, a cyclic AMP-selective phosphodiesterase inhibitor, reduces neuronal damage following cerebral ischemia in the gerbil. Eur J Pharmacol 272: 107-110.
- Block F, Schmidt W, Nolden-Koch M, Schwarz M (2001) Rolipram reduces excitotoxic neuronal damage. Neuroreport 12: 1507-1511.
- Imanishi T, Sawa A, Ichimaru Y, Miyashiro M, Kato S, et al. (1997) Ameliorating effects of rolipram on experimentally induced impairments of learning and memory in rodents. Eur J Pharmacol 321: 273-278.
- Hansen TO, Rehfeld JF, Nielsen FC (2003) KCl potentiates forskolin-induced PC12 cell neurite outgrowth via protein kinase A and extracellular signal-regulated kinase signaling pathways. Neurosci Lett 347: 57-61.
- Du T, Zamore PD (2007) Beginning to understand microRNA function. Cell Res 17: 661-663.
- Ambros V (2008) The evolution of our thinking about microRNAs. Nat Med 14: 1036-1040.
- Giraldez AJ, Cinalli RM, Glasner ME, Enright AJ, Thomson JM, et al. (2005) MicroRNAs regulate brain morphogenesis in zebrafish. Science 308: 833-838.
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, et al. (2006) A brain-specific microRNA regulates dendritic spine development. Nature 439: 283-289.
- Kim J, Krichevsky A, Grad Y, Hayes GD, Kosik KS, et al. (2004) Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proc Natl Acad Sci U S A 101: 360-365.
- Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, et al. (2005) A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci U S A 102: 16426-16431.
- Klein ME, Lioy DT, Ma L, Impey S, Mandel G, et al. (2007) Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat Neurosci 10: 1513-1514.
- Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, et al. (2008) An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci U S A 105: 9093-9098.
- Nudelman AS, DiRocco DP, Lambert TJ, Garelick MG, Le J, et al. (2010) Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus 20: 492-498.
- Impey S, McCorkle SR, Cha-Molstad H, Dwyer JM, Yochum GS, et al. (2004) Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell 119: 1041-1054.
- Kawai Y, Garduno L, Theodore M, Yang J, Arinze IJ (2011) Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem 286: 7629-7640.
- Yang D, Li T, Wang Y, Tang Y, Cui H, et al. (2012) miR-132 regulates the differentiation of dopamine neurons by directly targeting Nurr1 expression. J Cell Sci 125: 1673-1682.
- Baek SH, Rosenfeld MG (2004) Nuclear receptor coregulators: their modification codes and regulatory mechanism by translocation. Biochem Biophys Res Commun 319: 707-714.
- Glozak MA, Sengupta N, Zhang X, Seto E (2005) Acetylation and deacetylation of non-histone proteins. Gene 363: 15-23.
- Chen Lf, Fischle W, Verdin E, Greene WC (2001) Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science 293: 1653-1657.
- Cesena TI, Cardinaux JR, Kwok R, Schwartz J (2007) CCAAT/enhancer-binding protein (C/EBP) beta is acetylated at multiple lysines: acetylation of C/EBPbeta at lysine 39 modulates its ability to activate transcription. J Biol Chem 282: 956-967.
- Sun Z, Chin YE, Zhang DD (2009) Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol 29: 2658-2672.
- Kang SA, Na H, Kang HJ, Kim SH, Lee MH, et al. (2010) Regulation of Nur77 protein turnover through acetylation and deacetylation induced by p300 and HDAC1. Biochem Pharmacol 80: 867-873.
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 14123
- [From(publication date):
specialissue-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9545
- PDF downloads : 4578
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
