Mechanisms and Effects of Acid Rain on Environment
Received: 02-May-2014 / Accepted Date: 27-May-2014 / Published Date: 30-May-2014 DOI: 10.4172/2157-7617.1000204
Abstract
This paper focuses on mechanisms and effects of air pollution on atmosphere. It’s been mentioned that the most causes of our world’s global climate change are totally different circumstances. Among them, acidic rain is one among the chronic problems for the global climate change and ecological deformation of our surroundings. It's been finished that usually, precipitation that includes a pH 5.6 is taken into account as air pollution. It’s fashioned, once sulphur oxides and gas oxides reacted with water throughout rain and as gases or fine particles. This air pollution affects a spread of plants and animals in our surroundings. because it is mentioned higher than below ways of hindrance acidic rain on environment; it's been reduced by pack up smokestacks and exhaust pipes furthermore as victimization alternatives energy sources for vehicles, fuel station and electricity generation for various purpose so as to measure in an exceedingly safe and appropriate atmosphere without concern of worldwide warming and inexperienced house gases.
Keywords: Acid rain; Mechanisms; Environments
8026Introduction
Acid rain is a broad term used to describe several ways that acids fall out of the atmosphere. A more precise term is acid deposition, which has two parts: wet and dry. Wet deposition refers to acidic rain, fog, and snow. As this acidic water flows over and through the ground, it affects a variety of plants and animals. Dry deposition refers to acidic gases and particles. About half of the acidity in the atmosphere falls back to earth through dry deposition [1]. The wind blows these acidic particles and gases towards buildings, cars, homes and trees. Dry deposited gases and particles can also be washed from trees and other surfaces by rainstorms. When that happens, the runoff water adds those acids to the acid rain, making the combination more acidic than the falling rain alone. Precipitation that has a pH value of less than seven may contain acidic rain. This is due to the presence of acidic oxide emissions in the atmosphere from industries and vehicles. However, a rainfall that has a pH value of less than 5.6 is considered as acid rain [2]. It is formed when sulphur dioxides and nitrogen oxides, as gases or fine reacts with rain water. Particles in the atmosphere combine with water vapour and precipitate as sulphuric acid or nitric acid in rain, snow, or fog. Therefore, the main objective of this paper was to assess the effect of acid rain on environment and to suggest the methods of preventing acid rain. Moreover, to review what have done on acid rain before and to forecast what will have done in the future. This is the first phase of the research. It will continued more on experimental result in the second phase of the paper.
Methodology
How do we measure acid rain?
Acid rain is measured using pH meter from 1 to 14 value scales with a pH of 7.0 being neutral, 0 to 7 being acidic, and 7 to 14 basic [3]. When the PH value lowers, the acidity nature of rain increases. Pure water has a pH value of 7. However, normal rain is slightly acidic because different acidic oxide emissions react with rain that lowers the pH value about 5.6. According to 2000 report, the most acidic rain falling in the US has a pH of about 4.3 [4]. This acid rain's pH and the chemicals that cause acid rain are monitored by two networks that are supported by EPA. The National Atmospheric Deposition Program measures wet deposition, and its Web site features maps of rainfall pH (follow the link to the isopleths maps) and other important precipitation chemistry measurements. The Clean Air Status and Trends Network (CASTNET) measures dry deposition. Its web site features information about the data it collects, the measuring sites, and the kinds of equipment it uses [5].
Discussions
Components of acid rain
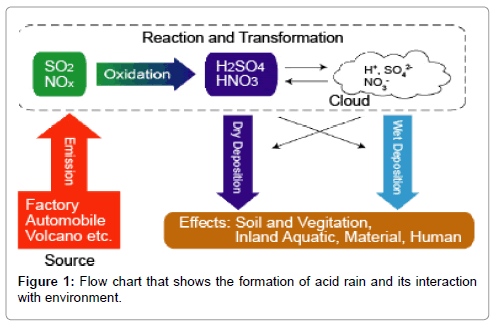
The major components of acid rains are sulphur dioxide/sulphur trioxide, carbon dioxide and nitrogen dioxide dissolves in rain water. These components are deposited as dry and wet depositions. When these pollutants are dissolved in water during rain it forms various acids (Figure 1). The chemical reactions of these pollutants are discussed as follows.
• CO2+H2O → H2CO3 (carbonic acid)
• SO2+H2O → H2SO3 (sulphorous acid)
• NO2+H2O → HNO2 (nitrous acid)+HNO3 (nitric acid)
Causes for the formation of acid rain
Natural sources and human activities are the main causes for the formation of acid rain in the world. Natural source causes are emissions from volcanoes and biological processes that occur on the land, in wetlands, and in the oceans contribute acid-producing gases to the atmosphere; and Effects of acidic deposits have been detected in glacial ice thousands of years old in remote parts of the globe. Whereas, activities of human beings are burning of coal, using Oil and natural gas in power stations to produce electricity, cooking purpose and to run their vehicles are giving off oxide of sulphur, oxides of carbon, oxides of nitrogen, residual hydrocarbons and particulate matters to the environment. These emissions mix with water vapour and rainwater in the atmosphere producing weak solutions of sulphuric and nitric acids, which fall back as acid rain to the ocean, lake and land.
Areas affected by acid rain due to power plant
Canada and USA: Acid rain is a problem in Eastern Canada and the Northeastern USA. Large smelters in western Ontario and steel processing plants in Indiana, Ohio historically used coal as a source of fuel. The sulfur dioxide produced was carried eastward by the jet stream. Acid rain from power plants in the Midwest United States has also harmed the forests of upstate New York and New England. In many areas water and soil systems lack natural alkalinity such as lime base cannot neutralize acid.
Sulfur dioxide is emitted from industrial processes and the burning of fossil fuels. In particular, ore smelting, coal-fired power generators, and the processing of natural gas result in the greatest emissions of sulfur dioxide. In 2000, Canada emitted 2.4 million tons of sulfur dioxide. Moreover, the primary causes of oxides of nitrogen are a vehicle, which accounts about 60% of all nitrogen oxide emissions. However, emissions also come from furnaces, boilers and engines. In 2000, Canada emitted 2.5 million tones of nitrogen oxide [6]. Therefore, these emissions are the main causes of acid rain all over the world.
Europe and Asia: Industrial acid rain is a substantial problem in China, Eastern Europe and Russia and areas down-wind from them. The effects of acid rain can spread over a large area, far from the source of the pollution. Research carried out in North America in 1982, revealed that sulphur pollution killed 51,000 people and about 200,000 people become ill due to this emissions.
Over the past decades, Norway has suffered a great damage due to the effect of acid rain. While Norway’s sulphur dioxide emissions have decreased significantly since the 1970s and 1980s, and nitrogen oxide emissions have decreased slightly, the damages from acid rain appear to be worsening in southern Norway. This is because it takes years for the ecosystems and the environment to recover from the effects of acidification. According to the State of the Environment in Norway, 18 salmon stocks have been lost and 12 are endangered, and have been wiped out of all of the large salmon rivers in southern Norway [7].
Hydrodesulphurization (HDS)
Hydro treating is a catalytic chemical process widely used to remove sulfur compounds from refined petroleum products such as gasoline or petrol, jet fuel, diesel fuel, and fuel oils. One purpose for removing the sulfur is to reduce the sulfur dioxide emissions resulting from using those fuels in automotive vehicles, aircraft, railroad locomotives, ships, or oil burning power plants, residential and industrial furnaces, and other forms of fuel combustion.
Another important reason for removing sulfur from the intermediate product naphtha streams within a petroleum refinery is that sulfur, even in extremely low concentrations, poisons the noble metal catalysts platinum and rhenium in the catalytic reforming units that are subsequently used to upgrade the of the naphtha streams [8].
Effects of acid rain on environment
Harmful to aquatic life: This is due to increasing the acidity character in water bodies that Stops eggs of certain organisms (e.g. fish) to stop hatching, Changes population ratios and affects their ecosystem.
Harmful to vegetation: Vegetables are destructed due to increased acidity in soil, Leeches nutrients from soil, and slowing plant growth, poisoning plants, creates brown spots in leaves of trees, impeding photosynthesis, allows organisms to infect through broken leaves.
Affects human health: Causes respiratory problems, asthma, dry coughs, headaches and throat irritations; Leeching of toxins from the soil by acid rain can be absorbed by plants and animals. When consumed these toxins it affect human’s life severely ,which cause brain damage, kidney problems and Alzheimer's disease have been linked to people who eat meat of "toxic" animals/plants by these pollutant.
Effect on transport: Currently, both the railway industry and the aeroplane industry have to spend a lot of money to repair the corrosive damage done by acid rain. Furthermore, bridges have collapsed in the past due to acid rain corrosion. Acid rain dissolves the stonework and mortar of buildings (especially those made out of sandstone or limestone). It reacts with the minerals in the stone to form a powdery substance that can be washed away by rain.
How do we prevent our environment from acidic rain?
There are several ways to reduce acid deposition and precipitation. These are:
Clean up smokestacks and exhaust pipes: Almost all of the electricity that powers modern life comes from burning fossil fuels like coal, natural gas, and oil. However, exhaust emission of these fuels are the main causes of acid deposition that released into the atmosphere. Coal fuel accounts for most US SO2 and a large portion of NOx emissions. Sulfur is present in coal as an impurity, and it reacts with air when the coal is burned to form SO2. In contrast, NOx is formed when any fossil fuel is burned. There are several options for reducing SO2 emissions, including using coal containing less sulfur, washing the coal, and using devices called scrubbers to chemically remove the SO2 from the gases leaving the smokestack and recycling to use as a raw material. Power plants can also switch fuels; for example burning natural gas creates much less SO2 than burning coal. Certain approaches will also have additional benefits of reducing other pollutants such as mercury and carbon dioxide. Understanding these "co-benefits" has become important in seeking cost-effective air pollution reduction strategies. Finally, power plants can use technologies that don't burn fossil fuels. Each of these options has its own costs and benefits, however; there is no single universal solution. Similar to scrubbers on power plants, catalytic converters reduce NOx emissions from cars. These devices have been required for over twenty years in the US, and it is important to keep them working properly and tailpipe restrictions have been tightened recently. EPA has also made, and continues to make, changes to gasoline that allows it to burn cleaner dioxide of sulfur (SO2) and NOx.
Use alternative energy sources: There are other sources of electricity besides fossil fuels such as nuclear power, hydropower, wind energy, geothermal energy, and solar energy. Of these, nuclear and hydropower are used most widely; wind, solar, and geothermal energy have not yet been harnessed on a large scale. There are also alternative energies available to power automobiles, including natural gas powered vehicles, battery-powered cars, fuel cells, biofuels and biodiesel and combinations of alternative and gasoline powered vehicles. All sources of energy have environmental costs as well as benefits. Some types of energy are more expensive to produce than others. Nuclear power, hydropower, and coal are the cheapest forms today, but changes in technologies and environmental regulations may shift that in the future. All of these factors must be weighed when deciding which energy source to use today and which to invest for tomorrow.
Liming: Powdered limestone added to water and soil to neutralize acid. It is commonly used in Norway and Sweden. However, it is more expensive and short-term remedy. Acid deposition penetrates deeply into the fabric of an ecosystem, changing the chemistry of the soil as well as the chemistry of the streams and narrowing, sometimes to nothing, the space where certain plants and animals can survive. Because there are so many changes, it takes many years for ecosystems to recover from acid deposition, even after emissions are reduced and the rain becomes normal again. For example, while the visibility might improve within days, and small or episodic chemical changes in streams improve within months, chronically acidified lakes, streams, forests, and soils can take years to decades or even centuries (in the case of soils) to heal. However, there are some things that people do to bring back lakes and streams more quickly. Limestone or lime (a naturally-occurring basic compound) can be added to acidic lakes to "cancel out" the acidity. This process, called liming. Liming tends to be expensive, has to be done repeatedly to keep the water from returning to its acidic condition, and is considered a short-term remedy in only specific areas rather than an effort to reduce or prevent pollution. Furthermore, it does not solve the broader problems of changes in soil chemistry and forest health in the watershed, and does nothing to address visibility reductions, materials damage, and risk to human health. However, liming does often permit fish to remain in a lake, so it allows the native population to survive in place until emissions reductions reduce the amount of acid deposition in the area.
Conclusion
Generally, rainfall that has a pH value less than 5.6 is considered as acid rain. It is formed when sulphur dioxides and nitrogen oxides reacted with water during rain and as gases or fine. Acids rain is described in terms of wet and dry depositions. The wet deposition refers to acidic rain, frog and snow whereas dry deposition refers to acidic gases and particles. This acid rain affects a variety of plants and animals (Harmful to aquatic life, Harmful to vegetation, affects human health and Transport) in our environment. As it is discussed above under Methods of prevention of acidic rain on environment; we reduce it by Clean up smokestacks and exhaust pipes as wells as using alternative energy sources for vehicles and electricity generation for different purpose in order to live in a safe and suitable environment without fare of global warming.
References
- Stephen KL (1999) Introduction to acid-base chemistry. Simon Fraser University, Canada.
- Marian B (1994) Weather and Climate. Hong Kong: Time Life Asia, Hing Kong.
Citation: Wondyfraw M (2014) Mechanisms and Effects of Acid Rain on Environment. J Earth Sci Clim Change 5: 204. DOI: 10.4172/2157-7617.1000204
Copyright: © 2014 Wondyfraw M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 71083
- [From(publication date): 8-2014 - Apr 01, 2025]
- Breakdown by view type
- HTML page views: 64706
- PDF downloads: 6377