Mechanical, Color and Barrier, Properties of Biodegradable Nanocomposites Polylactic Acid/Nanoclay
Received: 31-Oct-2018 / Accepted Date: 21-Nov-2018 / Published Date: 23-Nov-2018 DOI: 10.4172/2155-6199.1000455
Keywords: Polylactic acid; Nanoclay; Mechanical test; Barrier test; Color test
Introduction
PLA is aliphatic linear polyester thermoplastic derived from monomer lactic acid, which is obtained from the fermentation of 100% renewable and biodegradable plant sources, such as corn or rice starches and sugar feed stocks. It can be produced by chemical conversion of corn or other carbohydrate sources into dextrose. Dextrose is fermented to lactic acid followed by polycondensation of lactic acid monomers or lactide. However, the most common way to produce PLA is the Ring Opening Polymerization (ROP) of lactide monomer formed from lactic acid [1].
There are three different stereo chemical forms exist for lactide: either L-, D- or both L, D-Lactide (meso-lactide), each one having their change melting properties. PLA is insoluble in water, ethanol, methanol and aliphatic hydrocarbons but it is soluble excellent in chloroform. Its degradation half-life goes from six months to two years, depending on its stereochemistry and molecular weight [2]. PLA exhibits many advantages; it is biodegradable, renewable and highly transparent to light and has good water vapor and humidity barrier properties, comparable to those of petroleum-based plastics, such as polyethylene terephthalate (PET) or polystyrene (PS). Nonetheless, PLA has limited gas barrier capacity due to its hydrophobic in nature; it is very brittle, with less than 10% of elongation at break. Some important characteristic properties of pure PLA such as weak thermal stability, low gas barrier properties, and low ductility and toughness are inadequate for food packaging applications [3,4].
PLA has good mechanical properties, thermal plasticity, and biocompatibility and Eco friends. However, PLA is a comparatively brittle and stiff polymer with low elongation. Therefore, PLA is needed in order to compete with other flexible polymers such as polypropylene or polyethylene. There are many techniques to modify PLA such as copolymerization, blending with other polymers, the addition of plasticizers, the addition of nucleating agents, and forming composites with fiber or nanoparticles materials. The improved packaging refers to addition of nanomaterials to promote barrier and mechanical properties like nanoclay and nano silver [5].
The weight of polymer based on nanocomposites has a strong effect on the recycling of products and environmental concerns to reduce the consumption of gasoline by up to 1.5 billion litres, which in turn decreases CO2 emissions. The barrier properties play an important role in the advantages of nanocomposites materials. For example, the packing of food is one of the applications for nanocomposites [6]. In addition, the transparency of the nanocomposites is the same as virgin polymer, clays seems to behave as a nucleating agent, which induces a larger level of polymer crystallinity beside the crystallization rate. In addition, the incorporation of layered silicate enlarges the biodegradation of the resulting materials. This action can be ascribed to the hydrophilicity of layered silicate which attracts water molecules to the mixture and ends up speeding up the hydrolytic degradation of the polymer [7].
Improvement in barrier properties is one of the most notable successes of polymer/clay nanocomposites. Some quite dramatic reductions in permeability with relatively low additions of nanoclay. The objective of this study was to investigate the effects of nanoclay on barrier, mechanical and color properties of the films.
Materials
Pure grade PLA AI-1001 with density 1.25 g/cm3 supplied by (Shenzhen Esun Industrial Co., Ltd. Chain). Nanoclay supplied by Nanoshel LLC (USA) with average diameter of the particles (as recorded by the company) was about <80 nm. Chloroform Solution was purchased from Applied Chemistry (Darmstadt, Germany).
Preparation Method
Polylactide films were prepared by casting method by using 3 gm in 30 ml of chloroform than was putted in glass tubes on a magnetic stirrer at temperature 60°C for one hour. After that, the solution was stay at room temperature for one day to ensure complete solvent removal.
PLA/nanoclay composite films were prepared by add nanoclay swollen in chloroform by mixing for one hours while polylactide dissolved in chloroform at 60°C then nanoclay solution was sonicated from homogenizer Soniprep-150 MSE for an hour to increase distribution. The amount of nanoclay in PLA was varied between weight percentage (1, 2, 4 and 6) wt%.
Characterizations
Thickness: Determination of thickness of pure PLA and PLA/ nanoclay composites calculated by electronic digital micrometer type (293-821, Mitutoyo) sensitivity was used to measure the thickness of composites films and find that is 105 μm.
X-Ray Diffraction (XRD) analysis: The structure curve pure of polylactic acid and PLA/nanoclay composites film were characterized by Phillips X‘Pert Pro MRD (Cu Kα radiation (λ=1.54 nm) in (40 kV, 40 mA) between 5° and 60°.
Fourier Transform Infrared (FTIR) analysis: The infrared spectra were recorded with the help of Shimadzu type FTIR -7600 in range 400 to 4000 cm-1.
Mechanical properties
Tensile strength: According to ASTM D-882 [8] standard modulus of elasticity, tensile strength, and elongation equipped with a 5 kg load cell in tensile mode. Tested films were cut into 10 mm width and 80 mm in length and the initial gauge length and the speed were fixed at 10 mm/min. Tensile strength (σs), Young’s modulus (E) were determined according to the following equation:
σs=F/A (1)
E=F L0/A ΔL (2)
Where: F: force exerted on an object under tension, L0: original length, A: cross section area, ΔL: length of the object changes.
Tear strength: Tear strength of films was determined on the same Universal Electronic Dinamometer according to ASTM D-1922 [9] by the trouser tear method. The sample size was 100 mm long and 63 mm wide having a cut of 50 mm at the center of one end. A pendulum impact tester is used to measure the force required to propagate slit a fixed distance to the edge of the test sample.
Optical properties
Color test and Brightness: Color properties were evaluated measuring color coordinates in the CIELAB color space L* (lightness), a*(redness-greenness) and b* (yellowness-blueness) were analyzed using a Konica CM-3600d color. Average values for samples were calculated by the color differences (ΔE) was evaluated by Equation (3) [7]
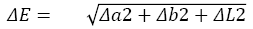 (3)
(3)
Where: ΔL=L stander*-L sample, Δa=a stander*– a sample, Δb=b stander*-b sample, Stander values for white plate were L=96.86, a=-0.02 and b=1.99 respectively for pure polylactic acid.
Barrier properties
Oxygen Transmission Rate (OTR): Oxygen Transmission Rate of the films was measured according to the ASTM D-3985 [10] using gas permeation instrument Qualities. The diffusing oxygen is measured by a gas detector that is sensitive only to oxygen, after 12-16 hours test was completed. Before each measurement, the samples were kept dry in vacuum oven in nitrogen gas and the gas permeability rate expressed in cm3/m2. day that keep at temperature (23°C) and relative humidity (0% RH) with test area 50 cm2 and thickness ≤ 3 mm.
Water vapor transmission rate: The water vapor transmission rate of the films was measured according to the ASTM E-96 [11] using water permeation instrument Qualities. Film specimens were mounted horizontally on poly (methylmethacrylate) cups filled with distilled water up to 1 cm under the film. The cups were weighed every hour for a period of value was noted usually after 6-8 hours and measure the weight loss versus time in unit gm/m2·24 h at 37°C and (90%RH) with 50 cm2 area and thickness is ≤ 3 mm, with.
Results and Discussion
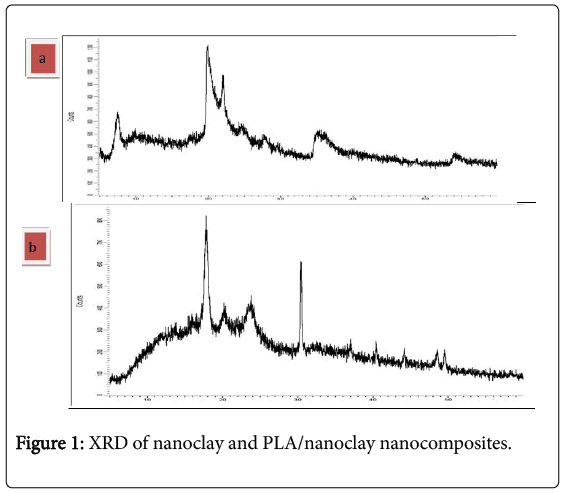
X-Ray Diffraction of powder nanoclay
XRD patterns of the clay nano powder is given in Figure 1a shows the XRD of the clay nanopowder typical peaks around 2θ=5°, 20°, 25°, 30°, and 35° of the (001), (002) (111), (003) (005), reflections corresponding to Na –montmorillonite.
In Figure 1b shows the XRD of the PLA/nanoclay nanocomposites peaks at 2θ=13°, 17°, 20°, 23°, 30°, 38°, 40° where 2θ=17°, 20° of pure PLA that mean is semicrystalline it was shown by Nadia et al. [12]. Incorporation of nanoclay in polymer matrix (PLA) causes an increase in intensity and sharpness of PLA peaks at 2θ=17°, 30° which can be attributed to improvement of matrix crystallinity.
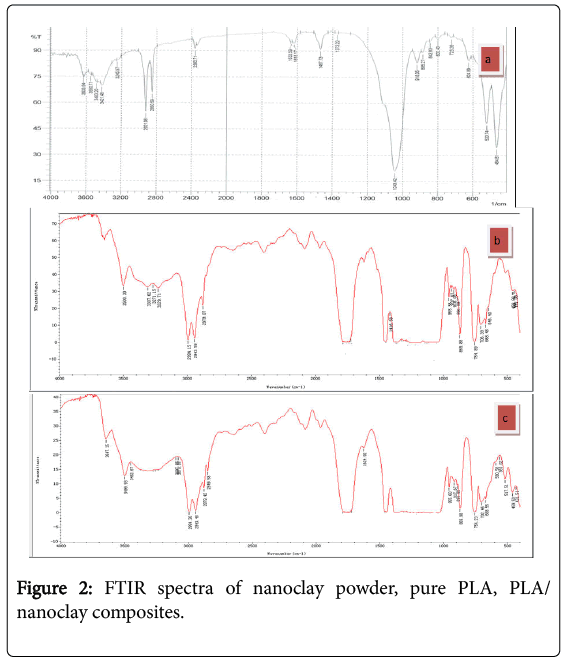
Fourier transform infrared analysis of nanoclay, pure PLA and PLA/nanoclay
FTIR analysis of a chemical substance shows marked selective absorption in the infrared (IR) region. Nanoclay is prepared by adding 1–2 mg clay in equal amount of KBr and ground to fine powder, Solid casted pellet is placed in the path of light source at wave number ranges from 400 to 4000 cm−1. In Figure 2a, it has been reported that peak intensity at 3421 cm-1, 3340 cm-1 corresponding to O-H stretching for the silica and water and 1633 cm-1 related to (O-H bending) may be due to presence of water molecules from moisture. Si- O-Si bond is confirmed from 1043 cm−1. The peaks at 920 cm-1 to from Al-OH-Al deformation of alumina, there are also unique bands due to tetrahedral SiO4 near 700 cm-1 in layer silicate that be used for specific mineral identification. Bands at 2930 cm-1, 1314 cm-1 to C-H vibrations of methylene groups (asymmetric starching and bending) and 484 and 520 cm−1 ascribe the presence of bonds such as Si-O-Al and Si-O-Mg.
Figure 2b shows the of pure PLA and appear peaks at 1418, 2994 and 3600 cm−1 were assigned to the C–O, C–H(double) and O–H stretching of the –CH(CH3)–OH end group of PLA, respectively. The band at –C–O bond stretching (955 cm-1) in –CH–O– groups.
In Figure 2c shows that PLA/nanoclay exhibit identical absorption peaks, suggesting lack of strong interactions between PLA molecules and nanoclay nanocomposites. In PLA/nanoclay, the C=O and O– H stretching peaks were at (955 and 3617 cm−1, respectively) and atstrong peaks are corresponding to stretching vibration of carbonyl group C=O and C-O groups at (1747, 1087 cm-1). This was attributed to the strong interactions between the PLA hydroxyl end groups and the nanoclay platelet surfaces, and/or the ammonium groups of the surfactant in the modified clay, Si–O stretching (917 cm−1) and Si–O bending vibration of organo clay (516-462 cm-1), respectively.
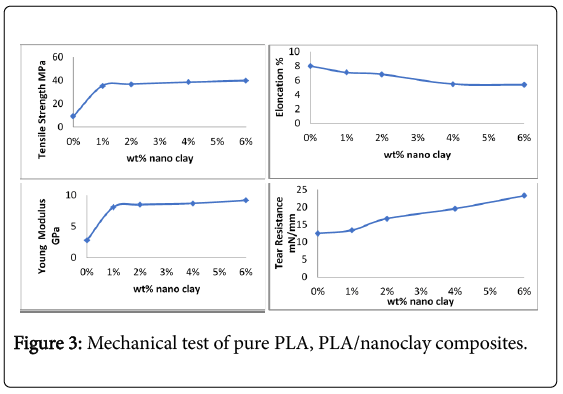
Mechanical properties PLA and PLA/nanoclay
Table 1 shows the values of mechanical properties of pure PLA and PLA/nanoclay films, tensile strength (9.34 MPa), Elongation (8%) and Young Modulus (2.8 GPa) because that PLA based materials are rigid and brittle polymer at room temperature (RT) due to its Tg ~ 55°C [6]. The pure PLA as a brittle material the reasons for this brittle behaviour for the pure PLA is due to the low entanglement density (Ve) and the high value of characteristic ratio (C∞), a measure of chain stiffness [12].
| Samples | Tensile Strength MPa | Elongation % | Young Modulus GPa | Tear Resistance mN/mm | |
|---|---|---|---|---|---|
| Pure PLA | 9.34 | 8.0 | 2.8 | 12.5 | |
| PLA/clay%1 | 35.3 | 7.11 | 8.1 | 13.4 | |
| PLA/clay%2 | 36.8 | 6.85 | 8.5 | 16.7 | |
| PLA/clay%4 | 38.6 | 5.49 | 8.7 | 19.6 | |
| PLA/clay%6 | 39.9 | 5.41 | 9.2 | 23.3 | |
Table 1: Mechanical test of pure PLA, PLA/nanoclay composite.
When add nanoclay increased in Tensile Strength, Young Modulus between (35.3-39.9)MPa and (8.1-9.2) GPa respectively appear in Figure 3 because that due to the hydrogen bonding interaction between PLA and nanoclay and the structure of PLA consists of two hydroxyl groups at the end of its polymer chains these prepared PLA/ nanoclay composites embedded in a network of PLA polymeric chains and the origin hydrogen bonds formed between the PLA molecules and nanoclay and these new hydrogen bonds would improved the mechanical properties. The main reason for this behavior may be attributed to the resistance exerted not only by the clay itself with high surface area high aspect ratio and vary high elastic modulus, but also by the stronger interfacial interaction between the matrix and layered silicate due to the vast surface exposed to the clay layers [13,14]. Good dispersion of the nanoclay in polylactic acid reduced tensile ductility and increased tensile strength compared with pure polymer that mean the complete dispersion of clay nanolayers in a polymer optimizes the number of available reinforcing elements for carrying an applied load and deflecting cracks. Nanoclay decreased in elongation between (7.11-5.41)% with increase in nanoclay concentration.
Tear resistance is the force it takes to rip a plastic film, generally plastic sheet with a property of brittleness will have very low tear resistance; this is clearly proved from the Table 1, pure PLA is a brittle material it shows low tear resistance is 12.5 mN/mm. When addition of nanoclay improvement in tear resistance shown between (13.4-23.3 m N/mm) by considering that the nanoclay contain silicate layers are able to inhibit or at least to slow down crack propagation by deviating their tear path. The higher tear strength of the samples appar in 6% nanoclay because the interfacial bonding between the clay and the PLA matrix suffices to produce a strengthening effect as demonstrated.
Optical properties PLA, PLA/nanoclay
Color is very important factors to be considered in food packaging since it could influence consumer acceptance and commercial success of a food product. Table 2 shows Color test of pure PLA, PLA/nanoclay composites.
| Sample | L | a | b | Brightness% |
|---|---|---|---|---|
| Pure PLA | 90.05 | -14.38 | -2.76 | 80.88 |
| PLA/CLAY%1 | 88.85 | -19.18 | -1.61 | 74.63 |
| PLA/CLAY%2 | 86.8 | -21.1 | -0.76 | 71.62 |
| PLA/CLAY%4 | 85.06 | -22.01 | -0.07 | 69.32 |
| PLA/CLAY%6 | 84.64 | -22.6 | -0.05 | 68.75 |
Table 2: Color Properties of pure PLA and PLA/nanocomposite.
When nanoclay is added to the film sample, slight decrease in lightness values (L) was observed when increasing nanoclay content and about 84.64 for nanocomposites with 6% nanoclay and 90.05 for pure PLA. While, a parameter reduced that mean the red color value of samples decrease and green color value is increased, b parameter did not show regular changes this results mean nature of nanoclay agreed with visual observation.
Brightness is percentage reflectance of light at wavelength 457 nm show that high brightness in pure PLA that is 80.88% and for PLA/ nanoclay is ranged (74.63-68.75) % that appear high transparency of PLA compare PLA/nanoclay composites because the structure of nanocomposites willbe optically clear which is traced to the thickness of each nanoclay layer that is smaller than the wavelength of visible light.
Barrier properties (OTR, WVTR) of PLA and PLA/nanoclay
The main limitations of PLA as a packaging material are a high gas permeability (O2, and water vapor) that show in Table 3. Higher oxygen permeability coefficients and water vapour transmission rate are an indication of lower barrier protection that appear in pure PLA. The high molecular weight glassy polymers (PLA) with rigid chains have very high oxygen permeability. Polylactic acid is showing that OTR and WVTR taking of (488.96 cm3. mm/m2. day. atm) and (181.818 g. mm/m2. day) respectively that mean pure PLA suffers from some serious drawbacks such as its great sensitivity towards moisture and its poor water vapour barrier property because is hydrophobic significantly tend to be non-polar, that mean exhibit a high contact angle but is sensitive to water vapour that appear in Table 3.
| Samples | Oxygen Permeability Coefficients (cm3. mm/m2. day. bar) | Water Vapour Transmission Rate (g. mm/m2. day) |
|---|---|---|
| Pure PLA | 488.9 | 181.8 |
| PLA/clay%1 | 239.4 | 97.2 |
| PLA/clay%2 | 209.3 | 87.7 |
| PLA/clay%4 | 183.6 | 72.3 |
| PLA/clay%6 | 186.1 | 58.1 |
Table 3: Barrier test of PLA, PLA/nanoclay composites.
In PLA/nanoclay composites shown in Table 3 samples found that significant reduce in OTR and WVTR with increasing clay content at 6 weight %, due to the ‘tortuous path’ that improvement in barrier properties, nanoclay contain platelets forming complex barriers to gases and water vapor. As more tortuosity is present in a polymer structure, higher barrier properties will result in 1-6% of nanoclay to polymer matrix due to the impermeable clay layers distributed in polymer matrix consequence increasing the effective diffusion path length [15].
Conclusions
The nanocomposites PLA/nanoclayare studied, XRD, FTIR show strongly associated with the dispersion of nanoclay in polylactic acid.
• The nanoclay is used at (1, 2, 4, 6%) increases in mechanical, optical and improvement the barrier test for industrial manufacture and packaging application.
• Measured values of barrier properties of OTR, WVTR permeability of the PLA/nanocomposites were compared with pure PLA showing good barrier properties at 6% nanoclay by creating a maze or tortuous path that retards the diffusion of gas molecules through the polymer matrix.
References
- De Silva RT, Pasbakhsh P, Goh KL, Chai SP, Chen J (2014) Synthesis and characterisation of poly (lactic acid)/halloysite bionanocomposite films. Journal of Composite Materials 48: 3705-3717.
- Eng CC, Ibrahim NA, Zainuddin N, Ariffin H, Yunus WM, et al. (2013) Enhancement of mechanical and thermal properties of polylactic acid/polycaprolactone blends by hydrophilic nanoclay. Indian Journal of Materials Science.
- Jamshidian M, Tehrany EA, Imran M, Jacquot M, Desobry S (2010) Polyâ€lactic acid: production, applications, nanocomposites, and release studies. Comprehensive Reviews in Food Science and Food Safety 9: 552-571.
- Vroman I, Tighzert L (2009) Biodegradable polymers. Materials 2: 307-344.
- Agrawal SK, Sanabria-DeLong N, Bhatia SK, Tew GN, Bhatia SR (2010) Energetics of Association in Poly (lactic acid)-based Hydrogels with Crystalline and Nanoparticle− Polymer Junctions. Langmuir 26: 17330-17338.
- Farah S, Anderson DG, Langer R (2016) Physical and mechanical properties of PLA, and their functions in widespread applications-A comprehensive review. Advanced Drug Delivery Reviews 107: 367-392.
- McKeen LW (2014) Handbook of Polymer Applications in Medicine and Medical Devices. Elsevier Inc., Amsterdam, Netherlands.
- ASTM D882-12 (2012) Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International, West Conshohocken, PA, USA.
- ASTM D1922-15 (2015) Standard Test Method for Propagation Tear Resistance of Plastic Film and Thin Sheeting by Pendulum Method. ASTM International, West Conshohocken, PA, USA.
- ASTM D3985-17 (2017) Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM International, West Conshohocken, PA, USA.
- ASTM E96/E96M-16 (2018) Standard Test Methods for Water Vapor Transmission of Materials. ASTM International, West Conshohocken, PA, USA.
- Ali NA, Noori FTM (2014) Crystallinity, mechanical, and antimicrobial properties of polylactic acid/microcrystalline cellulose/silver nanocomposites. Int J Appl Innov Eng Manag 3: 77-81. Â
- Bhattacharya M (2016) Polymer nanocomposites-a comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 9: 262.
- Tang X, Alavi S, Herald TJ (2008) Barrier and mechanical properties of starchâ€clay nanocomposite films. Cereal Chemistry 85: 433-439.
- Dadashi S, Mousavi SM, Emam-Djomeh Z, Oromiehie A (2014) Functional properties of biodegradable nanocomposites from poly lactic acid (PLA). Int J Nanosci Nanotechnol 10: 245-256.
Citation: Mohsen AH, Ali NA (2018) Mechanical, Color and Barrier, Properties of Biodegradable Nanocomposites Polylactic Acid/Nanoclay. J Bioremediat Biodegrad 9: 455. DOI: 10.4172/2155-6199.1000455
Copyright: © 2018 Mohsen AH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 8000
- [From(publication date): 0-2018 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 6472
- PDF downloads: 1528