Measurement of Protease Activity and Concentration of a Broad Spectrum Protease Inhibitor; Alpha 2-Macroglobulin (A2m) in Plasma of Severely Chronic Ill Patients in Bangladesh
Received: 30-Jun-2016 / Accepted Date: 26-Jul-2016 / Published Date: 29-Jul-2016 DOI: 10.4172/2161-0681.1000288
Abstract
Different types of proteases may play influential roles across a spectrum of diseases. Correspondingly, protease inhibitors have been known to play crucial roles in the patho-physiology of diseases. We hypothesize the activity of the broad spectrum protease inhibitor, alpha-2-macroglobulin (A2M) may hold unexplored roles in diseases as well. Clinically measuring levels of variant proteases in combination with the monitoring of A2M, in plasma, can be a novel approach to further our understanding of diseases related to protease activity. There is evidence showing administration of purified human A2M to animal models with bacteria induced septic shock using Pseudomonas aeruginosa resulted in the reversal of the pathological effect. In addition, recent evidence has also shown the beneficial effects of treatment with autologous A2M in post-traumatic osteoarthritis patients. We investigated the relationship of protease activity and concentration of A2M in plasma of chronic ill patients in Bangladesh. Blood was collected to prepare plasma from patients (n=20) of different categories: severe burn, cancer of different types, enteric fever with psychosis, chronic pancreatitis, chronic liver diseases, different types of kidney diseases, different types of pulmonary diseases, diabetes and hypertension. As control, plasma was prepared from healthy volunteers (n=20) without any known disease(s). Plasma concentrations of A2M were measured by sandwich ELISA (Enzyme– Linked Immunosorbent Assay) and protease activity assay were done by protease activity assay kit. In this study, we have found that all chronically ill patients (n=20) with a verity of diseases, A2M concentration went down significantly (p<0.001) compared to the concentration of normal healthy individuals (n=20). On contrary, the protease activity level were increased significantly (p<0.001) in all chronically ill patients (n=20) compared the activity of normal healthy individuals (n=20). These results clearly indicated an inverse relationship of A2M to the protease activity in chronically ill patients in Bangladesh which may be a novel approach to further our understanding of pathophysiology of diseases related to protease activity.
Keywords: Autologous protein; Immune system; Chronic diseases; Prevention of diseases; Patho-physiology of diseases
314173Introduction
Microorganism invasion, metabolism and virulence are dependent on protease(s) which are secreted by prokaryotic and eukaryotic microorganism [1,2]. To protect from adverse environment and for survival, all living creatures were born with innate immune system [3]. A2M is a plasma protein that traps and acts as major components of the innate immune system by inhibiting a broad range of proteases [2,4]. A2M provide immediate defense against infection, and is an evolutionarily older defence strategy, found in plants, fungi, insects, bacteria and primitive multi-cellular organisms [5]. Low levels of A2M in protease-induced diseases are well documented in animal research [6-9], and these models may also indicate a possible relationship of disproportion of protease and protease inhibitor, A2M in the pathophysiology of many disease processes. In animal research it was also clearly documented that A2M worked as a life-saving medicine and saved animal life [8,9].
Proteases are secreted by microorganisms to penetrate into the body [10]. It is now known that single amino acid mutations in over 500 human proteases result in hereditary/genetic diseases [11]. Proteases play pivotal regulatory roles in conception, birth, digestion, growth, maturation, ageing, and death of all organisms [11]. Proteases regulate most physiological processes by controlling the activation, synthesis and turnover of proteins. Proteases are also essential in viruses, bacteria and parasites for their replication and the spread of infectious diseases, in all insects, organisms and animals for effective transmission of disease, and in human and animal hosts for the mediation and sustenance of diseases. Also, other genetic or environmental conditions can result in an over- or under- abundance of a particular crucial protease or abnormal levels of natural inhibitors/ activators of proteases, leading to abnormal physiology and disease [11].
A2M is a broad spectrum protease inhibitor in plasma, an extraordinary multifunctional blood protein, inhibits and clears many types of proteases as well as several growth factors and cytokines, including TNF-α, IL-1β, IL-6 and TGF-β from the body [4,5] had never been given any attention for a possibility as a life-saving therapeutic molecule in the field of medicine. We investigated the relationship of proteases and A2M in chronic ill patients in Bangladesh.
Materials and Methods
After obtaining approval from Institutional ethical committee of Mymensingh Medical College, Mymensingh, Bangladesh, voluntary consent was obtained from patients with chronic diseases and healthy volunteers. An amount of 1.6 ml of venous blood was obtained in 3.8% sodium citrate containing vacutainer blood collection tubes in room temperature from patients of different category (n=20) and healthy individuals (n=20). Plasma was prepared by conventional method [12]. The inclusion criteria for adult patients who were admitted to hospital were (1) Males or females age 12 to 80 years of any race or ethnicity (2) Chronically ill patients suffering from any of the diseases mentioned above for at least 6 months and at the end stage of the disease (3) Informed consent/assent as applicable. The exclusion criteria were (1) Substance abuse or dependence within 12 months prior to enrollment, (2) use of medication known to alter immune function within four weeks prior to enrollment, such as antiretroviral medications, (3) pregnancy, lactation, or the use of oral contraceptives (4) hemoglobin <9 μg/dl.
Plasma concentration of A2M were measured by sandwich ELISA (Enzyme–Linked Immunosorbent Assay) assay kit from abcam, ab 108888-alpha 2 Macroglobulin Human ELISA Kit, USA and protease (trypsin as a representative of proteases) assay by Protease assay kit from Creative BioMart, USA. Both assays were performed according to the manufacturer’s instruction.
Study design
A pilot study with cross-sectional design conducted over a short period with the following objectives:
General objectives: The study was conducted to assess the risk factor of protease activity in chronic patients, and develop understanding of common disease etiology, generate hypothesis for future study.
Specific objectives: 1) To investigate the association between protease activity and plasma level of alpha 2-macroglobulin among patients of chronic disease in Bangladesh. 2) To estimate the prevalence of the protease activity of interest for the same population.
Rationale: In Bangladesh and many other developed and developing countries the role of proteases from microorganisms in disease processes have not been extensively studied yet. We would like to study the involvement of proteases and its inhibitor, A2M in different diseases.
Statistical basis of sample size: Total patients: 20 and Normal subjects: Control: 20 are very nice sample size for following statistical analysis procedures for this study. Statistical analysis was performed by parametric techniques such as t-test, analysis of variance (ANOVA) and an analysis of covariance (ANCOVA) if the data are approximately normal or can be normalized using a suitable transformation.
Statistical analysis
Student's t test was used for comparisons between control and patient’s protein levels (A2M) in ELISA assay and enzymatic activity for the analysis of protease activity (trypsin) in plasma (Table 1).
| A2M | Protease | |
|---|---|---|
| Burn (sever more than 50%) | 463.1098 | 4879.146 |
| Squamous cell carcinoma | 461.6682 | 4985.947 |
| Meningioma | 404.6826 | 5053.401 |
| Acute B Lymphoblastic Leukemia (B-ALL) | 419.4378 | 4851.04 |
| Acute myeloid Leukemia on Palliation (3 Months) | 473.4554 | 4643.058 |
| Acute Lymphoblastic Leukemia (ALL) | 477.865 | 5160.202 |
| Obstructive Jaundice, Choledocholitheasis - malignancy | 443.6906 | 5407.532 |
| Intra-Abdominal Malignancy | 435.3802 | 5297.92 |
| Enteric Fever with Psychosis | 469.7242 | 5354.132 |
| Chronic Pancreatitis | 398.7466 | 5000 |
| Polycystic Kidney Disease, Chronic Kidney Disease | 471.3354 | 4550.309 |
| Chronic Kidney Disease (Stage IV), Diabetis, hypertension, percutaneous coronary intervention | 438.5178 | 4454.75 |
| Lupus Nephritis | 569.873 | 5.36.537 |
| Chronic Obstructive Pulmonary Disease/ Corpulmonary History of Renal failure and Hypertension | 435.7194 | 5528.387 |
| Cardiovascular Disease (Infraction- Hypertension - Catatonia) | 418.4202 | 5165.823 |
| Chronic Obstructive Pulmonary Disease/Mass Lesion in Lungs | 561.626 | 4825.745 |
| Diabetes/Hypertension | 457.937 | 5112.423 |
| Chronic liver Disease, Spontaneous bacterial peritonitis decompensated | 540.617 | 4971.894 |
| Left side hemorrhage | 512.4634 | 5165.823 |
| RTI, Response to Intervention, Multiple (2months) | 401.8842 | 4980.326 |
Table 1: Concentration of A2M (mg/ml) and protease (trypsin) activity level (mg/ml) in plasma of patients of different diseases (n=20).
Results
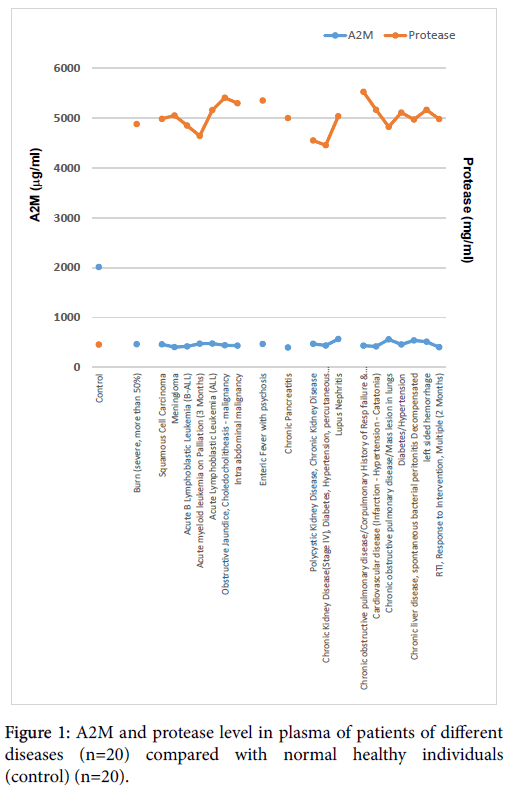
Protease activity of individual patient’s plasma were shown high compared to healthy individuals (control) 516.16 ± 17.17 (mg/ml) (n=20) (drawn in blue) (Figure 1). In contrast, A2M concentration of individual patient’s plasma were low compared to healthy individuals (control) was 2013.63 ± 52.00 (μg/ml) (n=20) (drawn in red) (Figure 1). All severely chronic ill patients showed a significant increase in protease activity (drawn in red) (Figure 1). In contrast, all severely chronic ill patients showed a significant decrease in A2M level (drawn in blue) (Figure 1).
Discussion
In animal models we have previously shown that in critical stages of septic shock models, plasma levels of a broad spectrum protease inhibitor, A2M goes down and restoring A2M, drastically improved conditions of all subjects, resulting in full recoveries [8,9]. In our present study, from our results similar phenomenon were also observed in clinical conditions. In severe to terminal ill patients, A2M, goes down and oppositely protease activity levels in plasma goes up, suggesting an inverse relationship taken on by protease and protease inhibitors which is comparable with the results found in animal models. Previously we also have observed in animal models that when A2M was depleted out for two hours, only one hundredth of the lethal dose of a protease (Pseudomonas aeruginosa elastase ) for guinea pigs is enough to kill them [8,9]. In another experimental group from the same study, we observed that injecting purified A2M at the crucial stage of protease induced septic shock model drastically improved conditions of all subjects, resulting in full recoveries [8,9]. Again, if we increased the A2M concentration in plasma by injecting purified human A2M to 150%, all of the subjects were protected from their lethal doses [8,9]. Our results from these animal models had given us the interest to investigate blood plasma A2M levels and protease activity levels in chronically ill patients with hopes of seeking a comparisonal relationship (if any) between proteases and A2M-a method of investigational thought still novel in the field of medicine.
Corresponding to previously studied animal models and proposed hypothesis, we observed that A2M levels in plasma dropped significantly in all chronically suffering ill patients while also observing an inversed increase in their protease activity. This phenomenon suggests that under normal conditions, A2M actively functions to remove proteases. It can be further inferred that maintained A2M activity confers protective immunity from various protease induced pathological symptoms by limiting protease(s) capacity to destroy different protective or defence systems (coagulation/complement/ immunoglobulin etc.).
In animal models we also observed that during the crisis-stage of septic shock pathogenicity, A2M levels came down to 30% of its original level [8,9]. Therefore under chronic and severely ill conditions, A2M, which is supplied from liver, continuously attempts to maintain normal immune conditions via protease inhibition. However, increased severity and chronicity of infection progressively limits A2M conferred immunity after an interim of prolonged and continuous secretion of proteases by micro-organisms. This inevitably depletes the ability for endogenous A2M based immunity via protease inhibition, either due to deteriorating production or exhaustion from liver, indicatively leading to the key event for the beginning of disease process [13,14].
Contrasting levels of A2M and protease(s) activity must be further investigated in order to understand the pathophysiology of many disease processes where the necessary amount of A2M needed to maintain normal immunity is jeopardized due to production depletion/exhaustion in chronic illness.
It is well known that until now natural defence mechanisms or the innate immune system always deals with the description about a pathogen/micro-organism when they enter the body. After entry, a number of orchestrated defence systems start playing a role to opsonize them and eliminate them from the body. However, the dynamic roles shared by protease(s) and the protease inhibitor, A2M, have never been so keenly studied in regards to its application to human diseases, especially for severe and chronically ill patients. We suggest to measure protease activity and levels of broad spectrum protease inhibitor (A2M) in plasma in chronically ill patients. An external supply of A2M to counterbalance insufficient endogenous levels of A2M in plasma could provide a promising new medical treatment in chronic ill patients after measuring their deficiency in more diseases.
Author Contributions
MMK contributed to the conception of experiments, data analysis, and writing of the manuscript. MMK performed experimental work with IH All patients’ samples were arranged by MAM. All control samples were arranged by IH. Statistical data analysis and figures were made by MEK. All authors contributed to the project.
Acknowledgment
We thank Mr. Sariful Islam of Ibn Sina laboratory at Dhaka, Bangladesh for technical help regarding blood sample collections.
References
- Somerville GA, Proctor RA (2009) At the crossroads of bacterial metabolism and virulence factor synthesis in Staphylococci. MicrobiolMolBiol Rev 73: 233-248.
- Khan MM (2015) Prevention of proteases by a multifunctional plasma protein: alpha-2-macroglobulin (A2M), can protect us from many diseases.
- Bhanothu V, Lakshmi V, Theophilus JP, Rozati R, Badhini P, et al. (2015) Investigation of Toll-Like Receptor-2 (2258G/A) and Interferon Gamma (+874T/A) Gene Polymorphisms among Infertile Women with Female Genital Tuberculosis. PLoS One 10: e0130273.
- Armstrong PB (2006) Proteases and protease inhibitors: a balance of activities in host-pathogen interaction. Immunobiology 211: 263-281.
- Armstrong PB, Quigley JP (1999) Alpha2-macroglobulin: an evolutionarily conserved arm of the innate immune system. Dev Comp Immunol 23: 375-390.
- Khan MM, Yamamoto T, Araki H, Ijiri Y, Shibuya Y, et al. (1993) Pseudomonalelastase injection causes low vascular resistant shock in guinea pigs. BiochimBiophysActa 1182: 83-93.
- Khan MM, Yamamoto T, Araki H, Shibuya Y, Kambara T (1993) Role of Hageman factor/kallikrein-kinin system in pseudomonalelastase-induced shock model. BiochimBiophysActa 1157: 119-126.
- Khan MM, Shibuya Y, Nakagaki T, Kambara T, Yamamoto T (1994) Alpha-2-macroglobulin as the major defence in acute pseudomonal septic shock in the guinea-pig model. Int J ExpPathol 75: 285-293.
- Khan MM, Shibuya Y, Kambara T, Yamamoto T (1995) Role of alpha-2-macroglobulin and bacterial elastase in guinea-pig pseudomonal septic shock. Int J ExpPathol 76: 21-28.
- Kong F1, Singh RP (2008) Disintegration of solid foods in human stomach. J Food Sci 73: R67-80.
- Puente XS1, Sánchez LM, Overall CM, López-OtÃn C (2003) Human and mouse proteases: a comparative genomic approach. Nat Rev Genet 4: 544-558.
- Thavasu PW (1979) Procedure for labeling CX sample tubes and transferring serum. In: Clinical Diagnosis and Management by Laboratory Methods 1: p60, (edn., Henry JB) WB Saunders Company, Philadelphia, PA, USA.
- Khan MM (2015) Prevention of proteases by a multifunctional plasma protein: alpha-2-macroglobulin (A2M), can protect us from many diseases.
- Rehman AA, Ahsan H, Khan FH (2013) α-2-Macroglobulin: a physiological guardian. J Cell Physiol 228: 1665-1675.
Citation: Khan MM, Muqueet MA, Hossain I, Khan ME, Shibli MH, et al. (2016) Measurement of Protease Activity and Concentration of a Broad Spectrum Protease Inhibitor; Alpha 2-Macroglobulin (A2m) in Plasma of Severely Chronic Ill Patients in Bangladesh. J Clin Exp Pathol 6:288. DOI: 10.4172/2161-0681.1000288
Copyright: © 2016 Khan MM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 11751
- [From(publication date): 8-2016 - Jul 11, 2025]
- Breakdown by view type
- HTML page views: 10878
- PDF downloads: 873