Measurement of Long-Term Diet Adherence Following a Randomized Controlled Trial of a 6-Month Low-Carbohydrate Intervention on Disease Progression in Men with Recurrent Prostate Cancer
Received: 08-Feb-2022 / Manuscript No. jowt-22-53958 / Editor assigned: 10-Feb-2022 / PreQC No. JOWT-22-53958(PQ) / Reviewed: 20-Feb-2022 / QC No. JOWT-22-53958 / Revised: 25-Feb-2022 / Manuscript No. JOWT-22-53958(R) / Published Date: 02-Mar-2022 DOI: 10.4172/2165-7904.1000481
Abstract
We previously completed a 6-month randomized trial of ≤20g carbohydrates/day low carbohydrate diet (LCD) intervention vs. no dietary change in men with a rising prostate specific antigen (PSA) after failed local therapy (CAPS2). Men in the LCD group lost significant weight and exploratory analysis suggested slowed tumor growth as measured by longer PSA doubling time. Though patients were not asked to continue the diet post-study, we hypothesized the benefits of weight loss would encourage men to maintain some level of LCD relative to their pre-study diet. Of the 45 participants who completed the CAPS2 trial, 17 (5 control, 12 LCD) participants completed all on-study and follow-up measurements. The median carbohydrate intake for the LCD group at follow up was slightly lower than that of the control group (145g vs 186g; p=0.8). Caloric, macronutrient intake, weight and BMI were similar between groups at follow-up (all p≥0.2). Men randomized to a 6-month LCD, but not advised to maintain it long-term, tend to revert to their pre-study diet after study completion. Considering the benefits that consuming an LCD may have for PC patients, more effective strategies are needed to ensure long-term behavior change and improve diet adherence.
Keywords: Diet; Carbohydrate restriction; Lifestyle; Prostate cancer; Weight
Introduction
Prostate cancer (PC) treatment alternatives are needed given that local therapies are not always curative and systemic therapies oftentimes have unwanted side effects. In PC xenografts, both weight loss and low carbohydrate diets (LCD) without weight loss prolong survival [1, 2]. Previously, our team conducted CAPS2, a 6-month randomized trial of ≤20g carbohydrates/day low carbohydrate diet (LCD) intervention vs. no dietary change in men with a rising prostate-specific antigen (PSA) after failed surgery or radiation [3]. The LCD intervention was delivered weekly by a registered dietitian using telehealth strategies, primarily phone calls. The intervention was well-tolerated, resulted in significant weight loss (median of 12.3kg), and suggestively slowed tumor growth as measured by PSA doubling time. The purpose of this follow-up study was to determine if participants assigned to the LCD intervention in CAPS2, in comparison to the control participants, maintained the LCD and/or weight loss that occurred during the 6-month intervention period despite not being asked to continue the diet after the 6-month study. We hypothesized that the benefits of weight loss would encourage men from the LCD group to maintain some level of LCD relative to their pre-study diet.
Methods
Once follow-up measures were approved by each site’s Institutional Review Board (IRB), all participants (n=45) who completed the original 6-month intervention between 2014 to 2018, regardless of randomization, were invited to participate in this follow-up study in 2020. Since CAPS2 was a multisite study, the participants from Cedars Sinai Medical Center (CSMC) were contacted and consented by a CSMC coordinator. Once informed written consent was obtained, the lead study dietitian called and conducted 24-hour food recall and collected self-reported weights from CSMC participants. For the Duke and Durham Veterans Affairs sites, the lead study dietitian consented the participants and concurrently collected one 24-hour food recall and self-reported weight. Participants from every site also completed a 2-day food diary which was mailed to their coordinating office. All diet data were entered by the study dietitian and analyzed with ESHA food processor (ESHA, version 10.14, Salem, OR) which estimated the macro- and micronutrient intake of every participant. Long-term diet adherence was examined as absolute carbohydrate change from baseline to follow-up and differences between groups were tested using the Wilcoxon rank sum exact test. We also examined the change in weight from baseline to follow-up and from 6-month to follow-up.
Results
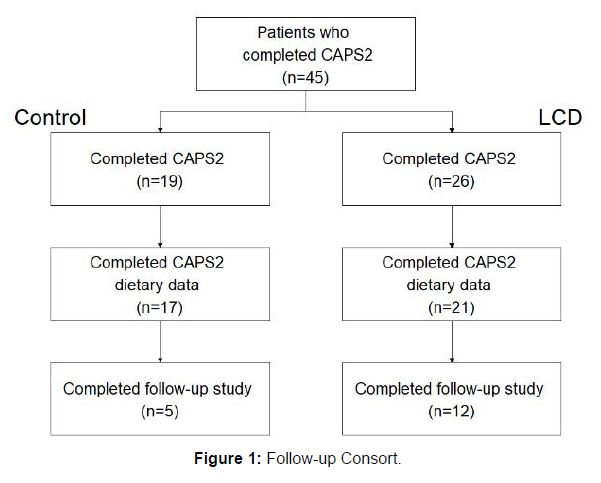
Of the 45 participants who completed the original CAPS2 trial, 20 completed this follow up study (CSMC=3; VA=6; Duke=11). Participants not consented were either lost to follow-up (n=16), or uninterested (n=9). Of the 20 who completed the follow-up, 3 participants had missing diet data from the original study and were excluded from the analysis. Thus, only 17 participants had complete dietary data from baseline, 3-month, 6-month and follow-up visits (5 control, 12 LCD) and were included in the analysis (Figure 1).
Table 1 shows the characteristics of the follow-up data, including caloric intake, dietary intakes of carbohydrates, protein, and fat, weight, body mass index (BMI), and time to follow-up in months. The median time to follow-up were 37.1 and 37.3 months for the control and LCD groups, respectively. The median carbohydrate intake for the LCD group at follow up was slightly lower than that of the control group (145g vs 186g) though this was not significant (p=0.8). There were no significant differences in caloric, macronutrient intake, weight or BMI between groups at follow-up (all p≥0.2).
When diet adherence was examined as absolute (g) change from 6-month to follow-up, LCD participants significantly increased their carbohydrate intake after the intervention (+118g; p=0.009 and +281%; p=0.002; Table 2). Although not significant, LCD participants’ absolute carbohydrate intake remained slightly lower at follow-up than that at baseline (median -47g; p=0.6; Table 3). Notably, participants from the LCD group regained the majority of weight lost between the 6-month visit and the subsequent follow-up (median +15lbs; p=0.009; Table 2).
Weight in the LCD group remained lower than that of the baseline measures, but this was not statistically significant (median -7 lbs; p=0.4; Table 2).
| Characteristic | Control, N = 51 | LCD, N = 121 | p-value2 |
|---|---|---|---|
| Calories (Kcals) | 1,683 (1,632, 1,834) | 1,716 (1,153, 1,985) | 0.9 |
| Carbohydrates (g) | 186 (144, 201) | 145 (88, 247) | 0.8 |
| Fat (g) | 64 (60, 70) | 62 (40, 95) | 0.6 |
| Protein (g) | 77 (55, 131) | 87 (64, 97) | >0.9 |
| Weight (lbs) | 212 (182, 238) | 177 (164, 190) | 0.2 |
| BMI (kg/m2) | 28.9 (28.8, 32.2) | 27.2 (25.6, 29.0) | 0.4 |
| Follow-up time from end of study (mo) | 37.1 (35.7, 39.8) | 37.3 (34.2, 39.8) | >0.9 |
| 1Median (IQR); 2Wilcoxon rank sum exact test. | |||
Table 1: Characteristics at Follow-up.
| Characteristic | Control, N = 51 | LCD, N = 121 | p-value2 |
|---|---|---|---|
| % calories | -11 (-12, -9) | 2 (-16, 37) | 0.2 |
| Calories (kcal) | -223 (-271, -126) | 27 (-207, 510) | 0.2 |
| % carbohydrates | -7 (-20, 7) | 281 (121, 459) | 0.002 |
| Carbohydrates (g) | -12 (-59, 12) | 118 (46, 172) | 0.009 |
| % fat | 1 (-17, 5) | -28 (-46, -1) | 0.4 |
| Fat (g) | 1 (-14, 3) | -26 (-38, -1) | 0.6 |
| % protein | -24 (-45, 10) | -39 (-45, 0) | >0.9 |
| Protein (g) | -25 (-26, 15) | -39 (-66, 0) | 0.6 |
| Weight (lbs) | 0 (-10, 22) | 15 (13, 21) | 0.5 |
| BMI (kg/m2) | -0.07 (-1.51, 2.97) | 2.32 (2.05, 3.38) | 0.6 |
| 1Median (IQR); 2Wilcoxon rank sum exact test. | |||
Table 2: Change from 6-months to Follow-up.
| Characteristic | Control, N = 51 | LCD, N = 121 | p-value2 |
|---|---|---|---|
| % calories | 16 (4, 25) | -3 (-13, 3) | 0.2 |
| Calories (kcal) | 253 (78, 333) | -62 (-281, 57) | 0.2 |
| % carbohydrates | 14 (-31, 15) | -19 (-48, 11) | 0.4 |
| Carbohydrates (g) | 26 (-63, 28) | -47 (-72, 23) | 0.6 |
| % fat | 34 (-8, 51) | 3 (-23, 18) | 0.2 |
| Fat (g) | 15 (-5, 20) | 0 (-14, 15) | 0.2 |
| % protein | 1 (-4, 83) | -1 (-19, 16) | 0.5 |
| Protein (g) | 1 (-3, 29) | -1 (-15, 11) | 0.4 |
| Weight (lbs) | -2 (-15, 27) | -7 (-12, -2) | 0.4 |
| BMI (kg/m2) | -0.3 (-2.4, 3.9) | -1.1 (-1.9, -0.3) | 0.4 |
| 1Median (IQR); 2Wilcoxon rank sum exact test | |||
Table 3: Changes from Baseline to Follow-up.
Discussion
The primary finding of CAPS2 suggested that LCD is not only safe for PC patients but also did not adversely affect tumor growth. On the contrary, an LCD may slow PC progression and may benefit other metabolic risk factors [3]. Although no significant difference was observed, our follow-up study showed a slight trend that the LCD participants consumed slightly less carbohydrates at follow-up than at baseline and their weights remained slightly lower than pre-study weights. However, overall, our follow-up suggests that diet adherence achieved during the 6-month intervention was not satisfactorily sustained and effective strategies are needed to ensure long-term adherence.
Given the potential benefit of weight loss and LCDs on PC, longterm adherence strategies are warranted. A 2016 meta-analysis noted factors that affect weight loss and dietary adherence, which include but are not limited to age, income, education, and social support systems [4]. The same analysis also reported that supervised attendance programs had greater adherence rates (68.6%), while self-monitoring programs had the lowest adherence rate (41.5%) [4]. Furthermore, a 2018 paper by Hall, et al. suggested several relevant strategies for promoting long-term weight loss maintenance including, but not limited to, post program weight loss specific counseling, cognitive restructuring (referring to participant bingeing and negative thoughts), developing cognitive flexibility (getting rid of the all or nothing attitude), and managing expectations [5]. For our CAPS2 population, no support was provided as it was designed to be a 6-month only study. We hypothesized that in a highly motivated cohort of patients with recurrence cancer, the weight loss benefits alone would be sufficient to maintain some level of diet adherence long-term. While there was slight evidence to support this in this very small study (carbohydrate intake and weight were both lower than baseline), it appears that consuming a very LCD was a challenge long-term and more support is needed beyond the 6-month intervention. Long-term maintenance strategies for our population could have included post study dietitian check-ins, innovative technology-based tracking and reporting tools, text message support, meal planning education, or hard copy materials if the patient prefers (handouts, food diaries, etc) [6, 7].
A limitation to this follow-up is that this analysis only includes less than half of the patients included in the original study, so the randomization/balance may no longer hold. Furthermore, follow-ups were conducted at a different time frames for each participant. In terms of data collection, the study dietitian collected both diet and weight data from the participants which may present an opportunity of bias, though this bias should be comparable between groups. Additionally, participants’ weights were self-reported which could be a source of error in the weight data.
A major strength of this analysis is that our team has conducted a rigorous RCT of LCD intervention and this follow-up extends those efforts. This follow-up shows how participants may tend to return to habitual intakes after completing a strict 6-month dietary intervention without additional intervention to promote adherence; a common challenge among dietary intervention research.
Our finding suggests that while men randomized to a 6-month LCD may have made some slight long-term diet changes, the overall changes were modest and not significant. Among cancer survivors, it does not appear that dramatic weight loss is sufficient of a motivator to maintain long-term diet adherence. In light of the various potential health benefits of LCDs, effective strategies are needed for long-term adherence. Further research is needed to implement and test various adherence strategies to inspire long term behavior change.
Acknowledgement
This work was supported by NCI K24 CA160653 and the Hartford Foundation and the Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center. The Clinical Trial Registry number is NCT01763944. The authors thank all the participants for their time and effort in completing the study.
Declaration of Interest Statement
The authors report no conflict of interest.
References
- Freedland SJ, Mavropoulos J, Wang A, Darshan M, Demark-Wahnefried W, et al. (2008) Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate 68(1): 11-9.
- Mavropoulos JC, Buschemeyer WC, Tewari AK, Rokhfeld D, Pollak M, et al. (2009) The effects of varying dietary carbohydrate and fat content on survival in a murine LNCaP prostate cancer xenograft model. Cancer Prev Res (Phila) 2(6): 557-65.
- Freedland SJ, Allen J, Jarman A, Oyekunle T, Armstrong AJ, et al. (2009) A Randomized Controlled Trial of a 6-Month Low-Carbohydrate Intervention on Disease Progression in Men with Recurrent Prostate Cancer: Carbohydrate and Prostate Study 2 (CAPS2). Clin Cancer Res 26(12): 3035-3043.
- Lemstra M, Bird Y, Nwankwo C, Rogers M, Moraros J (2016) Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence 10: 1547-59.
- Hall KD, Kahan S (2018) Maintenance of Lost Weight and Long-Term Management of Obesity. Med Clin North Am 102(1): 183-197.
- Lewis E, Huang HC, Hassmen P, Welvaert M, Pumpa KL (2019) Adding Telephone and Text Support to an Obesity Management Program Improves Behavioral Adherence and Clinical Outcomes. A Randomized Controlled Crossover Trial. Int J Behav Med 26(6): 580-590.
- Ducrot P, Mejean C, Aroumougame V, Ibanez G, Alles B, et al. (2017) Meal planning is associated with food variety, diet quality and body weight status in a large sample of French adults. Int J Behav Nutr Phys Act 14(1): 12.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2968
- [From(publication date): 0-2022 - Dec 16, 2025]
- Breakdown by view type
- HTML page views: 2351
- PDF downloads: 617