Maturity Classification of Sugarcane (Saccharum officinarum L) Genotypes Grown Under Different Production Environments of Ethiopia
Received: 10-Aug-2017 / Accepted Date: 21-Aug-2017 / Published Date: 02-Sep-2017 DOI: 10.4172/2329-8863.1000304
Abstract
Sugarcane (Saccharum officinarum L.) yield productivity is potentially affected by maturity which is genotypic and environment dependent. The limited information about the effects of genotypes, maturity and climate on sugarcane yield has been the main concern in sugarcane production of Ethiopia. The objectives of this study were to characterize sugarcane production environments of Ethiopia using meteorological and maturity data, classify genotypes using maturity data collected over growing seasons and across locations (Sugar Estates) and identify the major climatic conditions affecting the variability in sugarcane maturity. The design of the experiment was simple lattice design replicated across five locations and three seasons. Recoverable sucrose percentage and Brix Data of 49 genotypes evaluated across environments were collected over crop ages and subjected to ANOVA, Principal Component Analyses (PCA) and AMMI2 analyses. Results from ANOVA revealed brix percentage measurements were highly significantly (P<0.01) affected by environment, genotype and their interaction effects. Both principal component and additive main and multiplicative interaction (AMMI2) bi-plots generated similar genotype adaptations to maturity and successfully classify the genotypes in to early, medium and late under specific environments. Most of the CIRAD advanced lines were early maturing with wide range of maturing periods while those genotypes that were introduced at mid-way selection state were medium maturity. The correlation analysis made between environmental covariates and environmental IPCA scores demonstrated mean, maximum and minimum temperature and relative humidity regimes were the major climatic conditions affecting genotype × location interactions for brix percentage within 14-16 months of crop ages while minimum relative humidity was the major environmental factor that affect maturity across locations within 10-18 months crop ages. Further applications of these techniques will involve the inclusion of covariates derived from soil, climatic and management factors over diverse regions.
Keywords: Brix measurement; Crop age; Early; Late; Medium; PCA Biplot; AMMI2
Introduction
Sugarcane (Saccharum officinarum L.) is a complex aneu-polyploidy plant, with chromosome number in somatic cells, ranging from 2n=8x=80-124 in cultivated to 2n=10x=48-150 in wild types that propagates asexually through planting of vegetative cuttings (setts) of mature stalks. The importance of sugarcane has increased in recent years because it is a source of industrial raw material for sugar allied industries such as acetic acid, paper, plywood, industrial enzymes, animal feed and as source of renewable energy [1]. Moreover, sugarcane is one of the most efficient species in the plant kingdom in terms of biomass production [2].
Maturity is one of the factors that affect yield in sugarcane. Harvesting of sugarcane at the appropriate age i.e., peak maturity with acceptable tonnage, is necessary to realize maximum yield with the least possible field losses under the given growing environment [3]. This is because harvesting either under-aged or over-aged cane without proper timing leads to loss in cane yield, sugar recovery, poor juice quality and problems in milling [4]. From the grower’s point of view, maturity, age and ripening have become closely linked in such a way that the ripening process has been conveniently descripted as the culmination of sugarcane maturity [5]. However, the potential impact of different growing and plant physiological conditions during maturation complicates the identification of appropriate harvest period where peak sucrose content of sugarcane can be attained [6]. Research results reported by Ramburan [7] suggested that harvest age is affected by the environment and is the one of the causes of genotype × environment interaction in sugarcane. Gilbert et al. [8] reported cultivar × harvest age interactions and pointed out cultivar characterization with respect to harvest are helpful to design breeding programmes in sugarcane. Moreover, climatic variables have significant correlations with sugarcane ripening and must be analyzed considering long periods of time preceding the harvest (120-150 days) because their actions on the plants metabolism and physiology are not immediate [9].
According to Legendre and Burner [10], early-generation sugarcane hybrids have great potential for high biomass production. This means that development of varieties for certain purpose depends on selection stages (early, mid-way and advanced). To date, various sugarcane producing countries have intensified their interests in the creation of sugarcane varieties for multipurpose use [10,11]. Of the traits considered in sugarcane, sucrose content, fiber content, sugar yield, fiber yield and total biomass yield along with other important desirable morphological traits have received more attention. This necessitates the study of the relationships among traits and their relative importance and contribution to total variation. Such data, in some cases are handled by univariate data analysis which is not very informative. Multivariate techniques are suitable for analyzing many variables simultaneously and have been widely used to measure the diversity in germplasm collections assess the relative contributions that various traits [12] and classify variables based on a specific factor [13]. These multivariate techniques have also been used in the classification of germplasm collections, multi-environment trials and analysis of factors contributing to yield [14,15].
In Ethiopian sugar estates, cane maturity is customarily determined by taking the crop age and appearance as criteria for several years. In addition, the estates use a wide range of harvest periods which extend from 18 to 24 months after planting for all varieties. However, more resources could be saved if early maturing varieties harvested at their appropriate age. Moreover, there is lack of information on maturity status and exact time of ripening during the length of growing period for recently released varieties as these genotypes are introduced from different origin. This results in improper use of materials and improper supply of quality cane in some crushing periods in all Ethiopian Sugar factories and projects. Thus, before conducting research on determining appropriate harvest age, the maturity status of the genotypes has to be studied. Therefore, this investigation was initiated to (a) Characterize sugarcane production environments of Ethiopia using meteorological and maturity data (b) classify genotypes using maturity data collected over growing seasons and across locations (Sugar Estates), and (c) identify the major climatic conditions affecting sugarcane maturity.
Materials and Methods
Description of experimental sites
Thirteen METs conducted across five locations or sugarcane production environments (Wonji Sugar Estate, Metahara Sugar Estate, Tendaho Sugar Project, Belles Sugar project and Finchaa Sugar Estate) during 2013-2014, 2014-2015 and 2015-2016 crop years (two successive plant cane crops plus first ratoon crop trials) (Table 1). For location Tendaho, however, only one plant cane crop trial was conducted.
| Location | Code | Soil Type | Latitude | Alt | AEP (mm) | AT (0C) | RH (%) | Days to harvest | Season |
|---|---|---|---|---|---|---|---|---|---|
| (m.a.s.l) | |||||||||
| Wonji | W | Vertisol | 8o31’ N and 39o12’ E | 1500 | 6.6 | 17.5 | 55 | ||
| Finchaa | F | Luvisol | 9o30’ -10o00’ N and 37o15’ -37o30’ E | 1350-1600 | 6.8 | 22.7 | 62 | ||
| Metahara | M | Luvisol (F3a, C2) | 08°54′N and 39°55′E | 947 | 6.9 | 25.3 | 56 | ||
| Belles | B | Vertisol | 11°30’ N and 36°41’ E | 1110 | 8.5 | 24.7 | 54 | ||
| Tendaho | T | Luvisol | 110 20’ - 110 50’ N and Longitude 400 55’to 410 E. | 340-400 | 6 | 32.5 | 59 | ||
| Crop age (months) |
|||||||||
| 10 | 10 | 210 | Feb./Mar. | ||||||
| 12 | 12 | 150 | May./Jun | ||||||
| 14 | 14 | 90 | Sept. | ||||||
| 16 | 16 | 30 | Nov. | ||||||
| 17 | 17 | At harvest | Nov./Dec. | ||||||
| 18 | 18 | 30 AH | Dec./Jan. | ||||||
Table 1: Description of Locations and crop ages. *PC1=First Plant cane Crop Trial; PC2=Second Plant Cane Crop Trial; R1=First Ratoon Crop Trial; RF=Rainfall; AT=Average temperature; AEP=Average pan evaporation; ARH=relative humidity; SN=serial number; Sugar Estate= Old Sugar Factory which is under production; Sugar Project= new project which is under establishment; AH=after harvest; RS= Recoverable Sucrose percentage; Alt=Altitude; AEP=Average Pan Evaporation; AT=Average Temperatures; RH=Average Relative Humidity.
Description of experimental materials
Forty-three (43) introduced sugarcane genotypes along with six locally grown varieties were evaluated across environments. Out of the introduced materials from France, those whose name starts with PG, are clones that have been screened half way at the sugarcane breeding scheme in Cirad (France) and those varieties whose name starts with other than PG are advanced breeding clones at the final testing stage before a possible commercial release. Detail descriptions of the experimental genotypes are provided in Table 2.
| Code | Genotypes (G) | Origin | Code | Genotypes (G) | Origin |
|---|---|---|---|---|---|
| 1 | PSR97 092 | PHILSURIN (Philippines) | 26 | VMC95 212 | USA |
| 2 | DB70047 | WICSCBS (Barbados) | 27 | NCO-334 | South Africa |
| 3 | DB66 113 | WICSCBS (Barbados) | 28 | FG03 418 | CIRAD (France) |
| 4 | FG06 700 | CIRAD (France) | 29 | CO449 | India |
| 5 | FG06 729 | CIRAD (France) | 30 | FG03 204 | CIRAD (France) |
| 6 | PSR97 087 | Cuba | 31 | FG02 553 | CIRAD (France) |
| 7 | PSR97 051 | PHILSURIN (Philippines) | 32 | FG03 103 | CIRAD (France) |
| 8 | HO95 988 | USDA (Louisiana) | 33 | FG03 318 | CIRAD (France) |
| 9 | Cp99 1534 | USDA (USA) | 34 | FG04 708 | CIRAD (France) |
| 10 | FG04 829 | Cirad (France) | 35 | FG04 705 | CIRAD (France) |
| 11 | DB71 060 | Cirad (France) | 36 | FG02 551 | CIRAD (France) |
| 12 | TCP93 4245 | USDA (Texas/Canal Point) | 37 | FG03 173 | CIRAD (France) |
| 13 | CP001 252 | USDA (USA) | 38 | FG04 187 | CIRAD (France) |
| 14 | VMC95 173 | USA | 39 | FG03 372 | CIRAD (France) |
| 15 | FG03 447 | CIRAD (France) | 40 | FG03 214 | CIRAD (France) |
| 16 | CO 740 | India | 41 | C86-56 | Cuba |
| 17 | CP99 1894 | USDA (USA) | 42 | SP70-1284 | Cuba |
| 18 | FG03 425 | CIRAD (France) | 43 | C86-165 | PHILSURIN |
| 19 | FG05 408 | CIRAD (France) | 44 | B78-505 | Barbados |
| 20 | FG03 520 | CIRAD (France) | 45 | C132-81 | Cuba |
| 21 | FG04 754 | CIRAD (France) | 46 | C86-12 | Cuba |
| 22 | FG04 466 | WICSCBS (Barbados) | 47 | C90-501 | Cuba |
| 23 | FG03 526 | CIRAD (France) | 48 | B52-298 | Barbados |
| 24 | Mex54/245 | Mexico | 49 | CO- 678 | India |
| 25 | FG03 396 | CIRAD (France) |
Table 2: Description of 43 introduced sugarcane genotypes and 6 commercial varieties, and their origin. *FG= CIRAD/Guadeloupe (FRANCE); PSR=PHILSURIN (Philippines); CP= USDA (Canal Point/Florida), USA; TC=USDA (Texas/Canal Point), USA; HO=USDA (Houma, Louisiana); USA, BD=WICSCBS (Barbados/Guyana); varieties B52-298, MEX 54/245, NCo-334, Co 449, CO-678 and CO-740 are the existing commercial varieties in Ethiopia while B 78505, C132-81, SP 70-1284, C 86-165, C86-12, C90501and C86-56 are introduced from Cuba and are recently commercialized; FG03 520, FG04-466, PSR97 092, FG04 187, FG03 418, DB71 060, TCP93 4245, DB66 113, FG04 754 and FG03 372 are currently promoted for commercial use and are under verification.
Experimental design and layout
The experimental design was a 7 × 7 simple lattice square. The size of the experimental plot was 8.7 × 6 meters (52.5 m2) with four test rows and two border rows. Moreover, the design contained 7 blocks per replication and each block had an area of 8.7 m (width) × 48 m (length)= 417.6 m2 and the total experimental area per location was 0.78 hectares. Each replication was defined as replication nested in each location because the replications were unique for all locations and crop years, and each block was nested within both replications and location.
At planting, 18 two budded sets were laid on a furrow with 6 m length and milleable cane was harvested at 14 and 17 months cane age for ratoon and plant cane crops, respectively. All recommended agronomic and cultural practices were uniform to raise the crop across all the sugar estates.
Data collection
As harvest data of ratoon and plant cane crop trials were obtained at 14 and 17 months crop ages, respectively, we used these crop ages as cutting point for our decision in determining the three categories of sugarcane maturity under this investigation. Accordingly, 10 and 12 months of crop ages were categorized as early crop ages while 14-17 months crop ages were categorized as medium crop ages; the late crop age category being determined at 18 months crop age. For all crop ages, metrological data obtained from weather stations of each Sugar Estates was included.
Maturity data: Data of field brix measurements recorded at 10 (Brix10) and 12 (brix12) months crop ages from the averages of top, medium and bottom portions of each 10 randomly selected milleable stalks. Brix percentage is easy to measure at field condition using hand refractrometer. Moreover, corrected brix values (Cbrix14, Cbrix16 and Cbrix18) sampled at 14, 16 and 18 months cane age, respectively was analyzed from the diluted Brix measured at laboratory. Moreover, adjusted mean data for recoverable sucrose% analyzed at 14 (RS14) and 17 (RS17) months crop ages for ratoon and plant cane crops, respectively was included in the PCA analysis.
Meteorological and soil data: Minimum and maximum temperature and relative humidity of sampling day were recorded over crop ages (10, 12, 14, 16, 17 and 18 months) and across Wonji, Finchaa, Tendaho and Metahara Sugar Estates was included (Table 3). Moreover, monthly mean temperature, relative humidity and pan evaporation was collected during the sampling month.
| Crop age (Months) | Location | Climatic Conditions | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Code | MXT | MIT | MXRH (%) | MIRH | MT | MRH | EP | ||
| (C0) | (C0) | (%) | (C0) | (%) | (mm) | ||||
| Wonji | WBrix10 | 28.3 | 11.7 | 86.1 | 48.7 | 21.27 | 5.75 | 53 | |
| Finchaa | FBrix10 | 30.4 | 14.63 | 86.3 | 40 | 21.8 | 4.89 | 59.33 | |
| 10 | Metahara | MBrix10 | 33.26 | 16.6 | 84.3 | 28 | 23.08 | 5.64 | 57.67 |
| Belles | BBrix10 | 30.56 | 17.22 | 86.72 | 49.6 | 25.17 | 9.02 | 49.13 | |
| Tendaho | TBrix10 | 40 | 24.9 | 63 | 54 | 36.5 | 5 | 46 | |
| Wonji | WBrix12 | 28.8 | 9.6 | 83.6 | 47.3 | 17.47 | 6.53 | 54.93 | |
| 12 | Finchaa | FBrix12 | 31 | 13.64 | 85.5 | 40 | 20.06 | 5.3 | 59.79 |
| Metahara | MBrix12 | 32.37 | 17.3 | 81.3 | 31.3 | 21.78 | 5.73 | 60.55 | |
| Belles | BBrix12 | 31.88 | 13.79 | 78.33 | 44.22 | 23.57 | 4.95 | 65.68 | |
| Tendaho | TBrix12 | 41 | 24 | 64.3 | 53.6 | 37 | 2.6 | 50 | |
| Wonji | WBrix14 | 29.1 | 6.58 | 77.5 | 44.1 | 69.67 | 6.5 | 22.42 | |
| 14 | Finchaa | FBrix14 | 31.5 | 13.25 | 84.5 | 30.25 | 65.71 | 5.77 | 22.1 |
| Metahara | MBrix14 | 31.8 | 17.95 | 84.4 | 27.5 | 72.91 | 5.57 | 22.56 | |
| Belles | BBrix14 | 34.6 | 16 | 69.44 | 39.43 | 60.33 | 6.6 | 23.39 | |
| Tendaho | TBrix14 | 39 | 25 | 64 | 54.5 | 52 | 5.2 | 40 | |
| Wonji | WBrix16 | 26.5 | 8.5 | 75.58 | 33.6 | 22.53 | 77 | 6.5 | |
| Finchaa | FBrix16 | 28.58 | 11.61 | 79.7 | 36.85 | 21.2 | 67.6 | 4.7 | |
| 16 | Metahara | MBrix16 | 30.05 | 13.57 | 80.53 | 34.33 | 24 | 84.3 | 4.5 |
| Belles | BBrix16 | 30.05 | 13.57 | 77.98 | 35.17 | 23.23 | 60 | 7.6 | |
| Tendaho | TBrix16 | 40 | 24.9 | 63 | 54 | 40 | 52 | 5.2 | |
| Wonji | WRS17 | 26.5 | 8.5 | 75.58 | 33.6 | 17.5 | 55 | 6.6 | |
| Finchaa | FRS17 | 30.66 | 14.72 | 83.82 | 40.1 | 22.69 | 62 | 6.84 | |
| 17 | Metahara | MRS17 | 33 | 17.5 | 82.2 | 29.3 | 25.25 | 56 | 6.9 |
| Belles | BRS17 | 30 | 16 | 70.3 | 37.7 | 24.74 | 54 | 8.51 | |
| Tendaho | TRS17 | 40 | 24.9 | 63 | 54 | 32.45 | 59 | 6 | |
| Wonji | WBrix18 | 28.73 | 9.29 | 82.4 | 46.7 | 19.1 | 64.27 | 6.71 | |
| 18 | Finchaa | FBrix18 | 29.85 | 11.57 | 83.92 | 41.73 | 20.75 | 62.73 | 5.9 |
| Metahara | MBrix18 | 30.73 | 13.47 | 83.72 | 37.46 | 21.72 | 65.28 | 6.21 | |
| Belles | BBrix18 | 31.13 | 14.02 | 82.33 | 39.2 | 22.29 | 64.28 | 6.74 | |
| Tendaho | TBrix18 | 37 | 23.2 | 67 | 51 | 29.4 | 56 | 6.5 | |
Table 3: Mean Metrological Data Recorded at Different Crop Ages and soil Parameters. *Monthly Mean Relative Humidity (MRH); Maximum Relative Humidity during sampling day (MXRH), Minimum Relative Humidity during sampling day (MIRH), Monthly Mean Pan Evaporation (EP), Monthly Mean Temperature (MT); Maximum Temperature during sampling day (MXT) (Co), Minimum Temperature during sampling day (MIT); NA=Not Available.
Data analyses
The data were subjected to analysis of variance using SAS statistical software Version 9.2 [16]. Cluster analysis was used to determine the existence of discernible distinct groups with respect to growth rate and maturity status of the genotypes and Dendrogram was constructed using was done using Minitab 17 Statistical Software [17] and clustering was analyzed based on the Euclidean distance by average linkage method. For cluster analysis using growth parameters and brix measurements, the numbers of clusters were determined by looking in to the Pseudo F, Pseudo T2 and Cubic clustering criteria (CCC) in such a way that the CCC and Pseudo F-statistics clustered with small values of Pseudo T2 and larger value of T2 statistics as suggested by [18]. However, for clustering of genotypes based on maturity status, the number of clusters was decided based on the three possible maturity categories (early, medium and late). Moreover, Principal component analysis (PCA) was used to reduce the dimension of genotype × crop age, genotype × crop year × crop age and genotype × location × crop age two way table for classifications of genotypes using Gen Stat 17th edition [19]. For ease of visualization of three effects in one PCA biplot, crop year was nested in to crop age effect and mean data for brix and stalk elongation rate arranged as genotype × crop age was subjected to PCA analysis. Separately, mean data for brix and recoverable sucrose percentage arranged as genotype × environment (the term “environment” here is represents for location × crop age) was included in the PCA analysis. Moreover, AMMI2 analysis was conducted for brix percentage using Gen Stat 17th edition [19] while the location and environment IPCA scores obtained from AMMI2 were made correlated with mean of the climatic conditions (covariates) using SAS statistical software Version 9.2 [16].
Results and Discussion
Analysis of variance
Data of brix recordings collected at different ages of the crop was subjected to pooled analysis of variance (Table 4). Results of the analysis showed crop age, location and genotypes effects were highly significant (P ≤ 0.01) for Brix% which is consistent with results reported by Tahir et al. [20]. The significant effect of crop age indicated the brix accumulation of the genotypes was variable over crop ages while the significance of the genotype effect entails genotypes were variable in terms of brix accumulation. The significance of the genotype × location interaction indicated that genotypes accumulated brix across locations inconsistently while the significant effect of genotype × crop age illustrated genotypes varied in brix accumulation over crop ages. Gilbert et al. [8] reported similar results in which interaction of genotype, environment, and time of harvest were significant Moreover, the significant effect of location × sampling month entails the effect of crop age depended on location.
| Sources of Variations | DF | MSE |
|---|---|---|
| Location (L) | 4 | 766.54** |
| Crop Age (A) | 4 | 657.6** |
| Genotype (G) | 48 | 131.14** |
| Rep (A*L) | 25 | 31.61* |
| Block (Rep*A*L) | 300 | 2.5ns |
| G*A | 192 | 4.56* |
| A*L | 16 | 655.34** |
| G*L | 192 | 6.94* |
| A*G*L | 768 | 4.3* |
| Error | 899 | 2.94 |
| CV (%) | - | 7.38% |
| Mean | - | 18.39 |
Table 4: Means Squares for Brix Accumulation of 49 Sugarcane Genotypes Evaluated across Locations and over crop ages.
**=Significant at P ≤ 0.01; *=significant P ≤ 0.05; rep=replications.
The highly significant effect of the genotype × location × crop age interaction suggested the brix accumulation of the genotypes studied depends on crop age which is much governed by location. As the genotypic effects were significant means of brix percentage were subjected to principal component analyses.
Characterizations of locations using meteorological and maturity data
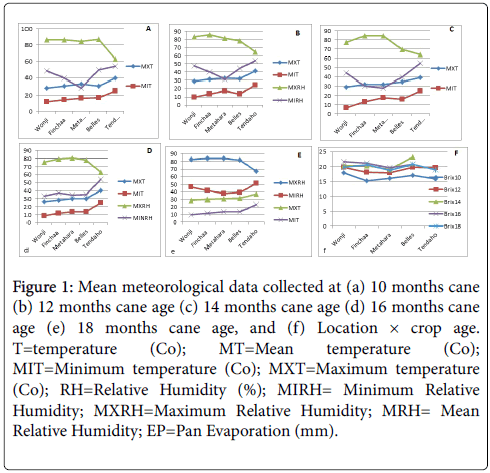
Results depicted in Figure 1a-1f indicated the characterization of locations based on the meteorological and soil data presented in Table 3.
Figure 1: Mean meteorological data collected at (a) 10 months cane (b) 12 months cane age (c) 14 months cane age (d) 16 months cane age (e) 18 months cane age, and (f) Location × crop age. T=temperature (Co); MT=Mean temperature (Co); MIT=Minimum temperature (Co); MXT=Maximum temperature (Co); RH=Relative Humidity (%); MIRH= Minimum Relative Humidity; MXRH=Maximum Relative Humidity; MRH= Mean Relative Humidity; EP=Pan Evaporation (mm).
Meteorological data recorded in all sampling months (cane ages) suggested Tendaho was the location characterized with higher temperature regimes while Wonji was characterized lower temperature values. Except for 12 and 14 months of cane age where Belles and Metahara, respectively were characterized by higher averages of relative humidity, location Metahara followed by Belles was characterized by higher values of maximum and mean relative humidity, and lower minimum relative humidity.
Location Belles was characterized with higher value of pan evaporation throughout the growing season. In the late crop ages (14, 16 and 18 months of crop ages), locations Finchaa and Belles were similar for most of the climatic conditions, which is consistent with results reported by Mebrahtom [21] in which these locations were classified in one mega-environment. On the contrary, the higher brix accumulation at early and late crop ages observed at location Wonji suggested the slow growth rate as a result of lower mean temperatures favor maturity and sucrose accumulation. The reason could be due to decrease of acid invertase activity in cold conditions, likely due to the increased activity of sucrose phosphate synthetase and neutral invertase, with a consequent increase in sucrose concentration as reported by Terauchi et al. [22]. Mean brix percentage data arranged as location × crop age two way table was included to characterize the locations in terms of cane maturity and the maximum brix accumulation was determined for each location and crop age (Figure 1f). Our result indicated maximum brix percentage accumulations in cane were recorded at 14 months crop age for Belles, Metahara and Finchaa Sugar Estates. On the contrary, the same genotypes evaluated at Tendaho and Wonji Sugar Estates accumulated maximum brix accumulation at 12 and 16 months crop ages, respectively. The lower brix accumulation in the late crop age under this study (18 months crop age) could be attributed to the minimum temperature prevailed in the location (Figure 1d and 1e). Cardozo and Sentelhas [5] reported similar results where minimum temperature values recorded 30 days prior to harvest were negatively correlated with sucrose accumulation.
Principal component analysis
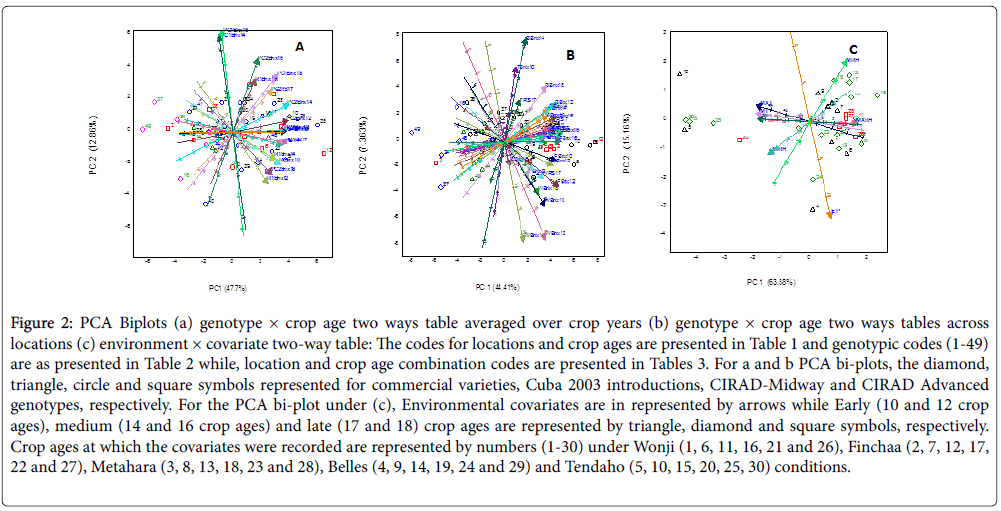
Maturity Classification of Sugarcane: Genotypes evaluated over crop years (Growing Seasons): The PCA bi-plot constructed using brix data recorded over five crop ages (Brix10, Brix12, Brix14, R1RS14, Brix16, PC1RS17, PC2RS17 and Brix18) and three crop years (First plant cane, second plant cane and first ratoon crops) (Table 5) indicated the whole data set could safely be compressed into the first two PCs without much loss of information (Figure 2b). It illustrated 60.52% of the variability was explained with the first two axes (PC1=47.7% & PC2=12.86%). Our results were nearly similar to the findings of previous reports except Tahir et al. [19] and James et al. [23] whom reports were more included to first few components. The first two PCA axes for the variables or crop ages accounted for 60.52% of the total variation, with most of the variation accounted for by the first axis (47.7%). Hence, variables PC1Brix14 and PC1Brix16 located along the second axis and thus, classification of genotypes based on these variables could be misleading. The brix data recorded over the crop ages from the first ratoon crop were positively correlated, despite the brix data recorded at 18 months of crop age, R1Brix18 showed small deviation from the other brix measurements for the same ratoon crop. Except for PC1Brix14 and PC1Brix16, which positioned along the second axis and not useful for classification, the crop years were not substantially separated .It suggested similar maturity status of the genotypes over crop years.
Figure 2: PCA Biplots (a) genotype × crop age two ways table averaged over crop years (b) genotype × crop age two ways tables across locations (c) environment × covariate two-way table: The codes for locations and crop ages are presented in Table 1 and genotypic codes (1-49) are as presented in Table 2 while, location and crop age combination codes are presented in Tables 3. For a and b PCA bi-plots, the diamond, triangle, circle and square symbols represented for commercial varieties, Cuba 2003 introductions, CIRAD-Midway and CIRAD Advanced genotypes, respectively. For the PCA bi-plot under (c), Environmental covariates are in represented by arrows while Early (10 and 12 crop ages), medium (14 and 16 crop ages) and late (17 and 18) crop ages are represented by triangle, diamond and square symbols, respectively. Crop ages at which the covariates were recorded are represented by numbers (1-30) under Wonji (1, 6, 11, 16, 21 and 26), Finchaa (2, 7, 12, 17, 22 and 27), Metahara (3, 8, 13, 18, 23 and 28), Belles (4, 9, 14, 19, 24 and 29) and Tendaho (5, 10, 15, 20, 25, 30) conditions.
| Sources of variance |
Across locations | across environments (crop age is nested) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Early crop ages | Medium crop ages | Late crop age | |||||||
| DF | %TSS | MSE | %TSS | MSE | %TSS | MSE | %TSS | MSE | |
| Locations (L) | 4 | 68.49 | 504.34** | 58.65 | 233.4** | 64.72 | 487.9** | 57.71 (22) | 672.18** |
| Genotype (G) | 48 | 17.08 | 10.47** | 19.73 | 6.54** | 10.96 | 6.9** | 6.10 (48) | 35.53** |
| Block | 5 | 152.18* | 7.47* | 6.8* | -23 | 130.24ns | |||
| GxL | 192 | 14.43 | 2.21** | 21.61 | 1.79** | 12.16 | 1.9** | 13.76(1056) | 3.34** |
| IPCA1 | 51 | 38.12P | 3.19** | 50.32 | 3.39** | 59.02 | 4.25** | 30.37(69) | 16.45** |
| IPCA2 | 49 | 36.94P | 3.2** | 28.98 | 2.03** | 24.32 | 1.8* | 17.76(67) | 8.78** |
| Residual | 92 | 24.71P | 1.15ns | 20.7 | 0.77ns | 16.63 | 0.66ns | 53.9(920) | 2.05ns |
| Error | 240 | 1.51 | 0.9 | 1.28 | -1771 | 2.02 | |||
Table 5: AMMI2 analysis of variance for Brix percentage arranged across environments (when crop age and location effects are combined) and locations (in early, medium and late crop ages). P=Percent of G × L; **=Significant at P ≤ 0.01; *=significant P ≤ 0.05; ns=nonsignificant at P>0.05; numbers in brackets are degrees of freedoms for data arranged across environments; IPCA1=first interaction principal component axis=IPCA2=second interaction principal component axis: TSS=treatment sum of squares; MSE=Means Sum of Squares.
The recoverable sucrose percentage data obtained from the first plant cane crop and analyzed at 17 months crop age (PC1RS17) was tightly overlapped with its respective ratoon crop analyzed at 14 months crop age (R1RS14). It is an indication of similar trend in sucrose accumulation over crop types (plant cane and ratoon crops) regardless of the age difference. This could happened because ratoon crops are expected be matured earlier than plant cane crops of the same crop from which the ratoon crop raised. With respect to the classification of the genotypes based on the variables included, we used 14 months crop age as reference point as the highest average brix accumulation was observed at 14 months crop age (table not shown). Based on this reference point, the genotypes studied were classified in to early, medium and late maturing genotypes. Those genotypes which accumulated relatively better brix% in between 10-14 months crop age and in all crop ages are regarded as early maturing genotypes while those genotypes that accumulate better brix percentage in between 14-18 months crop age are categorized as medium maturing genotypes. Moreover, genotypes that mature beyond 18 months crop age (those genotypes that loosely correlated with all crop ages) can be classified as poorly performed or and late maturing genotypes. Cardozo et al. [24] reported similar classifications by analyzing the quality variables by months in which patterns of similarity among sugarcane cultivars were identified, which allowed three ripening groups to be established and classified as early, middle and late.
Based on the above reference point, TCP93 4245 (12) and FG04 705 (35) positioned far right side of the arrows of all crop ages, accumulated relatively higher brix% in all crop ages and crop years, and are categorized as early maturing genotypes. The genotypes were characterized as promising genotypes for quality traits [25]. Moreover, FG04 829(10), FG03 396 (25), FG06 729 (5) and DB70047 (2) are classified as early maturing genotypes. On the contrary, most of the commercial varieties genotypes CO-678 (49), CO 740 (16), NCO-334(27), DB66 113(3), Mex54/245 (24), FG03 103 (32), C86-56 (41) and C132-81 (45) positioned far left sides of the arrows of all crop ages. These genotypes accumulate relatively low brix% at all brix measurements (sampling months); could be late maturing genotypes (mature later than 18 months cane age) or can be poor performing genotypes. The rest genotypes located around the origin and late crop ages and cane be classified as medium maturing genotypes.
Maturity classification of sugarcane genotypes grown across sugar estates: Genotype × location × cane age interactions: As indicated in the analysis of variance (Table 4), Genotype × Location × Cane age Interaction was highly significant (P<0.01) and the above classifications might be misleading as the classifications do not take account the location and interaction effects in to consideration. Hence, it would be more reliable if the classification is location specific. The PCA bi-plot depicted based on brix and recoverable sucrose percentage data recorded over crop ages (Brix10, Brix12, Brix14, Brix16, RS17 and Brix18) and across locations (Wonji, Finchaa, Metahara, Belles and Tendaho Sugar Estates) explained 61.77% of the total variability with the first two dimensions (Figure 2c). Of the crop ages included as variables, TBrix18 and TRS17 has relatively shorter vector lengths, suggesting genotypes were least discriminated in late crop ages at location Tendaho while TBrix10, BBrix14, WBrix12 and WBrix14 positioned explicitly along PC2, could not be useful for classification. The rest of the variables which are positioned along the first axis at similar vector lengths from the origin, discriminate the genotypes similarly.
From the 28 variables, four clusters were formed. TBrix10, BBrix14, TRS17 and BBrix14 are relatively more correlated and formed one cluster while WBrix12 and WBrix14 formed the second group. Crop ages namely; WBrix10, WBrix18, F Brix12, TBrix12, TBrix18, WRS17, MBrix12 and FBrix10, most which are early crop ages, closely correlated and formed the third group while the remaining variables (most of late crop ages) were tightly correlated and formed the last group. Most of the crop ages were grouped regardless of locations, except for the second group. Such clustering could be attributed to the significant genotype × location × crop age interaction effects. The first two PCA axes for the variables or crop ages accounted for 61.77% of the total variation, with most of the variation accounted for by the first axis (44.41%). This suggests that the wide spread of crop age variables along the PC2 axis in Figure 2c is rather misleading when considering the total variation in the genotype × location × crop age table. It also suggests that crop ages spread along the PC1 axis have a greater effect on variability among the genotypes. Thus, classification of genotypes based on BBrix14, TBrix10, WBrix12 and WBrix14 could be misleading. Genotypes TCP93 4245 (12), FG04 705 (35), FG04 829(10) and FG06 729 (5) positioned far right side of the arrows of all crop ages (highly discriminated in all crop age and location effects) and accumulated relatively higher brix percentage in all crop ages and across all locations, and are categorized as early maturing genotypes. Moreover, HO95 988(8), DB70047 (2), CP99 1894 (17) and C86-12 (46) accumulate better brix within 10-16 crop ages of all Sugar Estates (except for Tendaho condition), and are classified as early maturing genotypes. Genotype 39 better matured at late crop ages (17 and 18 months crop age) at Wonji Sugar Estate. Genotypes that located near the origin are least discriminated by crop age and location effects, and can be classified as medium maturing genotypes. Genotypes FG03 173, FG04-466 (22) and FG04 187(38) adapted to Tendaho and Belles conditions within 14-18 months of crop age. On the contrary, most of the commercial varieties genotypes CO- 678 (49), NCO-334(27), DB66 113(3) and Mex54/245 (24) negatively correlated with all locations and crop ages and located far left sides of the arrows of all crop ages. These genotypes accumulate relatively low brix percentage at all brix measurements (sampling months); could be late maturing genotypes (mature later than 18 months cane age) or can be poor performing genotypes. Under this investigation, of the released genotypes by Mebrahtom [21], genotype TCP93 4245 (12) is identified as early maturing genotype while PSR97 092 ,FG03 520 (20), FG04-466 (22), FG04 187(38), FG03 418 (28), FG03 372 (39), FG04 754 (21) and DB71 060 (11) classified as medium maturing genotypes. DB66 113 (3) being late maturing genotype or accumulate lower brix percentage.
From germplasm point of view most of the genotypes selected from advanced lines were early maturing (HO95 988, DB70047, CP99 1894, PSR97 087, TCP93 4245) and medium maturing (DB71 060, CP001 252 and VMC95 173) while FG06 729, FG04 829(10) and FG04 705 (35) which were introduced at mid-way selection state were early maturity genotypes. The reason could be attributed to the selection intensities imposed at successive selection states. Thus, advanced lines are more probably characterized by sucrose percentage since more attention is given for sucrose content when genotypes are selected at early generations. Similar results were reported by Legendre and Burner [8] where early-generation sugarcane hybrids had great potential for high biomass production than for sucrose content. This investigation addresses only about the maturity trends. These trends do not reflect the harvest age patterns but serve as preliminary information for further studies aimed at determining the appropriate harvest age for each promoted genotype. Moreover, the maturity patterns displayed in the PCA Biplot (Figure 2b and 2c) could not be only attributed to genotypic effects as the genotype x environment interaction was significant for all brix measurements. This is because sugarcane ripening depends on a complex combination of climate variables, the genetic potential of cultivars and crop management [5]. Thus, research studies that will engage in determining appropriate harvest age for each promoted genotype should be environment specific.
Major climatic conditions that discriminate locations: The PCA biplot constructed based on the environment × covariates two-way table depicted in Figure 2d accounted for 78.74% of the variability existed among environments (locations × crop ages). It successfully identified those covariates that had the greatest impact on environmental variability. The higher variability explained by first axis (PC1=63.58%) as compared to the second axis (PC2=15.16%) suggested the spread of environmental covariates along the second axis could be misleading and those covariates spread along the PC1 axis have a greater effect on variability among the environments. Except for, pan evaporation (EP), all of the covariates spread along the first axis and were important to interpret the variability among environment. Moreover, these covariates located at similar vector lengths from any direction thereby suggesting their importance for environmental characterizations. Therefore, our discussions focused on the patterns of environments and environmental covariates along the first axis. For this reason, pan evaporation (EP) could be excluded from further analysis. Regarding with the relationships existed among the covariates studied; MXRH and MRH were positively correlated while MXT, MIT, MXT and MIRH were highly and positively correlated. MXRH located in opposite direction to MXT, MIT and MXT vectors and is negatively correlated with these covariates while none of the covariates correlated with EP.
As far as the relationship of environments (locations and crop ages) and environmental covariates is concerned, only those covariates that contributed more to the variability were considered. The overlapped clustering of environments from early, medium and late crop ages across locations around maximum relative humidity (MXRH) suggested the MXRH played great role in discriminating environments across locations over crop ages. Moreover, MRH was positively correlated with most of medium crop ages at location Metahara while all temperature regimes (MT, MIT, MXT) and MIRH positively correlated with two early, three medium and one late crop ages at Tendaho location. MIRH was negatively correlates some of early and medium crop ages while the temperature regimes were negatively correlated with most of late crop ages across all locations (except for Tendaho condition). On the contrary, EP was associated only with two environments which are from early and medium crop ages at Belles condition.
AMMI2 Analysis
The AMMI2 analysis of variance indicated locations accounted for 68.5, 58, 65 and 57.71% of the total sum of squares in the early, medium and late crop ages, respectively. 17.08, 19.73 and 10.96% of the total sum of squares was explained by genotypic effects at early, medium and late crop ages, respectively while 14.43, 21.61 and 12.6% of the total sum of squares was accounted for the genotype x location interaction effects. The larger sum of squares for locations indicated that the locations were diverse, with large differences among location means causing most of the variation in Brix percentage. AMMI2 analysis conducted based on G × L data revealed that environments (E), genotypes (G), and the G × E interactions were highly significant (P<0.001) for Brix percentage (Table 5). The E, G and G × E accounted for 77.43, 8.49 and 14.09% of the total sums of squares (SS) for brix percentage, respectively where the largest amount of variation was explained by environment. The SS for G × E was greater than the G SS. The significance of environmental effect (here “environment” represent as location × crop age) suggested the location nested in crop age effect was complex for sugarcane maturity while the significant effect of genotype × environment interaction indicated inconsistent brix percentage accumulation of genotypes across locations and over crop ages. In most of the cases, the magnitude of interactions effects were larger than the genotypes but with small difference. It suggested that the possibility of formation of mega environments for brix percentage is minimal. The IPCA1 and IPCA2 axes were also significant, and although explaining 47.54% of the G × E interactions. The relative proportions of the G × E interactions explained by IPCA1 (30.38%) and IPCA2 (17.17%) were not balanced. when the data was arranged for early, medium and late crop ages across locations, location, genotype and G × L interaction effects were all highly significant (p<0.01). The IPCA axis accounts for 75.06, 79.30 and 83.34 of the G × L for early, medium and late crop ages, respectively.
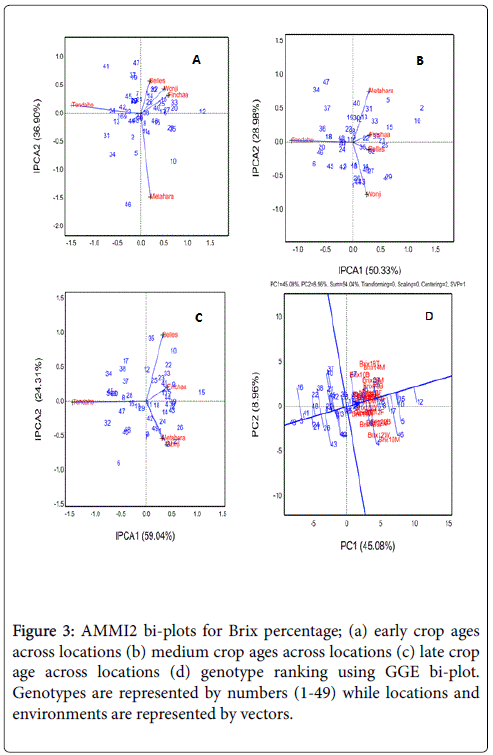
In the early crop ages, the AMMI2 bi-plot for which captured 75.06% of the total G × L interaction clearly showed the separation of locations and patterns of genotypes (Figure 3a). Location Tendaho explicitly separated along the first axis where genotypes 41, 24, 31 and 34 responded well. Locations Belles, Wonji and Finchaa showed deviations along both axes where genotypes 12, 33, 20, 28, 5, 27, 18, 25, 17, 8 22, 14 and 1 showed better adaptation. Moreover, genotypes 46, 10 and 5 showed better affinity to Metahara location which was clearly separated along the second axis. The spread of locations in three of the quadrants suggested locations were variable for maturity in the early crop ages.
Figure 3: AMMI2 bi-plots for Brix percentage; (a) early crop ages across locations (b) medium crop ages across locations (c) late crop age across locations (d) genotype ranking using GGE bi-plot. Genotypes are represented by numbers (1-49) while locations and environments are represented by vectors.
The AMMI2 bi-plot (Figure 3b) constructed based on the brix data obtained from the medium crop ages accounted for 79.06% of the G × L interaction. Location Tendaho clearly separated from other locations along the first axis while locations Finchaa and Belles located along the first axis in the opposite direction of the same axis. Locations Metahara and Wonji showed significant projection along both axes. It also indicated locations Tendaho, Metahara and Wonji showed greater variability for maturity in the medium crop ages while locations Finchaa and Belles were similar. With respect to the adaptation of genotypes to maturity across locations, genotypes 20, 6, 46, 36, 34, 37, 47, 32, 18 and 45 responded well to location Tendaho while genotypes 5, 31, 40, 13, 30, 12 and 33 showed better adaptation to Metahara. Genotypes 29, 4, 43, 44, 27, 49, 14 and 48 well responded to Wonji Condition while 15, 21, 25, 39, and 32 showed better adaptation to Finchaa and Belles conditions. Those genotypes located closely around the origin (12, 30, 19, 22, 35, 38, 11 and 23) showed consistent performance across locations (low IPCA1 and to right hand side of the grand mean) while genotypes 2 and 10 were better adaptation in all locations (except for Tendaho condition).
For the late crop ages, the AMMI2 bi-plot (Figure 3c) which captured 83.35% of the total G × L interaction showed similar trend in separations of locations where location Tendaho explicit separation along the first axis while location Finchaa located along the same axis in the opposite direction. Moreover, locations Metahara and Wonji deviated along both axes while location Belles located along the second axis with small projection to the first axis. Generally, locations Belles and Finchaa were correlated while Metahara and Wonji were closely correlated (overlapping) thereby suggesting locations which are correlated responded similarly to maturity. Regarding to the genotype adaptation to maturity across locations, genotypes, 34, 46, 45, 20, 32, 30, 17, 38, 8, 28, 47, 48, 40 and 6 adapted to Tendaho location. Genotypes 35, 12 and 7 located along the second axis and matured well in all locations. Genotypes 10, 22, 33, 25, 41, 9, 13, 5, 44 and 39 adapted to Belles and Finchaa conditions while genotypes 26, 21, 1, 24, 49, 14 and 43 showed better adaptation to Metahara and Wonji; genotypes 11, 18, 4, 39 and 15 being well adapted to Metahara, Wonji, Finchaa and Belles locations. In the first three AMMI2 biplots (Figure 3a-3c), location Tendaho had smallest (near to zero) IPCA2 scores; thus relative ranking (not absolute yields) of genotype would be fairly stable in this environment. In the AMMI2 bi-plot (Figure not presented) built based on the data obtained from the combined effect of location and crop ages for brix percentage, three overlapping clusters for the early, medium and late crop ages across locations. The first cluster includes those environments of medium and late crop ages located along the first axis. Environments which are from early crop ages and locations Wonji, Belles and Finchaa spread along the positive direction of the second axis formed the second grouping while those environments positioned along the second axis in the negative direction included early crop ages from locations Metahara and Tendaho formed the last group. The overlapping of crop ages regardless of locations suggested the maturity status of genotypes depends on location.
For ranking of genotypes, GGE bi-plot is better than the AMMI2 model. In the present study, genotypes are ranked based on mean value for brix percentage. The GGE bi-plot depicted in Figure 3d accounted for 54.04% of the Genotype+genotype × environment interaction variability for brix percentage ranked the genotypes according to their mean performance and stability. Based on Yan [26], genotypes positioned to the right side of the abscissa ordinate (vertical line) which separates those genotypes that are below average from genotypes that yielded above average are high yielder. Moreover, the stability of the genotypes can be measured by the projection of their markers on the derived axis green line (AEC ordinate) that is perpendicular to the ATC axis (green single headed arrow) or to the origin and perpendicular lines are drawn from the genotype. Markers to this axis and points at which they intersect this axis define the stability of genotypes. Accordingly Genotypes 12, 35, 10, 5, 46, 17, 28, 19, 8, 2, 20, 14 and 4 located to the right side of the ordinate and accumulated in order better brix% in cane while genotypes 12, 35, 10, 17, 28, 19, 8 and 2 had short projections and are both high yielder and stable genotypes in brix accumulation over crop ages and across locations. The result is consistent with the results of maturity classification displayed using PCA bi-plots for using (Figure 2a and 2b). Similar results were reported by Mebrahtom [21] where the 12, 35, 17, 28 and 8 showed better yield performance and stability in recoverable sucrose%.
Effect of climatic conditions on maturity of sugarcane genotypes grown across different production environments
The correlation of climatic conditions with location and environmental AMMI2 IPCA scores for brix% is presented in Table 5. AMMI2 IPCA scores partitioned from Genotype × location interaction for each crop age and genotype × environment interaction (crop age nested in to location effect) were made correlated with maximum and minimum temperature and relative humidity recorded during sampling day and mean temperature, relative humidity and pan evaporation of sampling month. Significant correlations of the climatic conditions (covariates) with IPCA scores suggested that any separation of the environments on the AMMI2 biplots was attributed to the relevant climatic condition. At the early crop ages, covariate MIRH significantly and negatively correlated with IPCA1 and positively correlated IPCA2 scores. It indicated MIRH was one of the major climatic conditions that contributed more to the separation of location Tendaho along the first axis. Its negative correlation with IPCA1 suggested it lowered the maturity of genotypes at this location as Tendaho was characterized with higher MIRH (Figure 2c) and showed lower yield in AMMI2 bi-plot (Figure 3a). Moreover, its positive and significant correlation with the second axis indicated MIRH was the major factor for clear separation of Metahara along the second axis characterized with the lowest values of MIRH (Figure 2c) and favors better maturity at this location.
The negatively and significantly (p<0.01) correlation of temperature regimes (MXT, MIT and MT) with IPCA1 in medium crop ages suggested these covariates played a major role in the explicit separation of location Tendaho along the first axis (Figure 3b) which showed poor performance (less than grand mean). Therefore, the higher monthly mean, daily maximum and minimum temperatures disfavored the maturity trend at Tendaho in the medium crop ages (14-17 months crop ages). On the contrary, the MXRH and MIRH positively and significantly (p<0.01) correlated with the same axis (IPCA1). The close correlation of the maximum and minimum relative humidity with first axis can be related to the separation of Finchaa and Belles locations in AMMI2 bi-plot with greater mean performance (Figure 3b). Moreover, the strong, negative and non-significant (p>0.05) correlation of MT and MIRH with IPCA2 might be attributed to the separation of Wonji and Metahara locations along the second axis; with better mean performance (means greater than grand mean) and characterized with lower MIRH and MT. It suggests the lower values of these covariates did not cause to yield reduction at these locations.
In the late crop age (18 months crop age), the temperature regimes are still negatively and significantly (p<0.05) correlated with the first axis where Tendaho was separated with significant deviation along the same axis with lower maturity performance. Similar to the previous crop ages, higher temperature conditions expected to be the major factors that amounted to affect maturity negatively. Strong, significant and positive correlations of IPCA1 with MXRH and MRH implied the small deviation of Finchaa along this axis was attributed to the effect of these covariates on maturity under Finchaa condition. Moreover, the negative non-significant correlation (large in magnitude) of temperature regimes (MXT, MT, MIT) separated locations Metahara and Wonji along the second axis while the positive and non-significant correlation (large in magnitude) of MXRH and MRH with the same axis separated location Belles along the IPCA2. To generate more information, data of brix percentage from the same data set was arranged as genotype × location × crop age interaction and subjected to AMMI2 bi-plot (Figure 3d). MXT, MIT and MIRH were negatively and significantly correlated with the first axis, suggesting these covariates played a major role in separating the environments (late crop ages across locations) along IPCA1. In other words, most of the locations at late crop ages were characterized with lower values of temperature regimes and MIRH. Moreover, MXRH was positively correlated with location IPCA1 scores in the late crop ages which were characterized by higher MXRH PCA bi-plot. The result revealed that locations were variable for MXRH at 16 and 18 months crop age.
Generally, the maximum and minimum temperature and relative humidity regimes were the major climatic condition that caused for the separation of locations and crop ages, and existence of genotype x location and genotype x environment interactions for brix percentage in late crop ages. Furthermore, MIRH was showed great affinity with early and late crop ages for all locations which agreed with reports of Ftwi et al. [27] where MIRH affected genotype × location interaction for recoverable sucrose percentage (Table 6).
| Covariates | Genotype × locations | Genotype × environment | ||||||
|---|---|---|---|---|---|---|---|---|
| Early crop ages | Medium crop ages | Late crop ages | ||||||
| IPCA1 | IPCA2 | IPCA1 | IPCA2 | IPCA1 | IPCA2 | IPCA1 | IPCA2 | |
| MXT | 0.03ns | 0.29ns | -0.97** | -0.70ns | -0.95* | -0.88ns | -0.63** | 0.29ns |
| MIT | -0.11ns | 0.48ns | -0.95** | -0.66ns | -0.93* | -0.86ns | -0.66** | 0.23ns |
| MXRH | 0.22ns | -0.48ns | 0.95** | 0.83ns | 0.99** | 0.88ns | 0.65** | -0.35ns |
| MIRH | -0.91* | 0.95* | 0.99** | -0.82ns | -0.79ns | -0.66ns | -0.56** | 0.42* |
| MT | -0.31ns | 0.62ns | -0.98** | -0.80ns | -0.94* | -0.88ns | -0.18ns | 0.25ns |
| MRH | -0.44ns | 0.43ns | 0.71ns | 0.35ns | 0.97** | 0.89ns | 0.11ns | -0.23ns |
Table 6: Correlation coefficients of climatic conditions (covariates) with Location and Environmental (Location × Crop age) IPCA scores obtained from AMMI2 for Brix% sampled over crop ages. **=Signi icant at P ≤ 0.01;*=signi icant P ≤ 0.05; ns =nonsigni icant at P>0.05; MT=Mean temperature (Co); MIT=Minimum temperature (Co); MXT=Maximum temperature (Co); RH=Relative Humidity (%); MIRH=Minimum Relative Humidity; MXRH=Maximum Relative Humidity; MRH= Mean Relative Humidity; EP=Pan Evaporation (mm).
Conclusion
Results of the present study indicated the diverse nature of locations and strong genetic variability among genotypes related to maturity. The significant effect of the genotype × location × crop age interaction suggested the brix accumulation of the genotypes studied depends on crop age which is much governed by location. Both principal component and additive main and multiplicative interaction (AMMI2) bi-plots generated similar genotype adaptations to maturity. Genotypes TCP93 4245, FG04 705, FG04 829 and FG06 729 adapted to all crop age and Sugar Estates, and are categorized as early maturing genotypes while HO95 988, DB70047 , CP99 1894, FG06 700 and C86-12 accumulated better brix within 10-16 crop ages of all Sugar Estates (except for Belles condition), and are classified as early maturing genotypes. Genotypes FG03 173, FG04-466 (22) and FG04 187(38) adapted to Tendaho and Belles conditions within 14-18 months of crop age while FG05 408, FG03 418, FG04 754, FG03 526, PSR97 092 and FG03 520 are medium maturing genotypes across all locations. On the contrary, most of the commercial varieties genotypes CO- 678, NCO-334, DB66 113 and Mex54/245 accumulate relatively low brix percentage at all brix measurements (sampling months); could be late maturing genotypes (mature later than 18 months cane age) or can be poor performing genotypes. This classification can be used as preliminary information for further research studies conducted in determining the appropriate harvest age of these genotypes.
The correlation analysis made between environmental covariates and environmental IPCA scores demonstrated mean, maximum and minimum temperature and relative humidity regimes were the major climatic conditions affecting genotype × location interactions for brix percentage within 14-16 months of crop ages while minimum relative humidity was the major environmental factor that affect maturity across locations within 10-18 months crop ages. Result of this investigation highlighted that G × E interpretation using multivariate techniques can be utilized successfully for better understanding of sugarcane maturity responses to environmental conditions, and help understand the relative effects of environmental factors on sugarcane maturity. Further applications of these techniques will involve the inclusion of covariates derived from soil, climatic and management factors over diverse regions.
References
- De-Oliveira MED, Vaughan BE, Rykiel EJ (2005) Ethanol as fuel: energy, carbon dioxbalances, and ecological footprint. Bioscience 55: 593-602.
- Brumbley SM, Purnell MP, Petrasouits LA, Nielsen LK, Twine PH (2007) Developing the sugarcane biofactory for high value biomaterials. Int Sugar J 109: 5-15.
- Muchow RC, Higgins AJ, Rudd AV, Ford AW (1998) Optimizing harvest date in sugar production: A Case Study for the Mossman Mill Region in Australia-I. Sensitivity to Crop Age and Crop Class Distribution. Field Crops Research 57: 243-251.
- Khandagave R, Patil B (2007) Manipulation of cutting age, varieties and planting time to improve sugar and cane yield. Int Sugar Cane Technl 26: 212-220.
- Cardozo NP, Sentelhas PC (2013) Climatic effects on sugarcane ripening under the influence of cultivars and crop age. Sci Agric 70: 449-456.
- O’Leary GJ (2000) A review of three sugarcane simulation models with respect to their prediction of sucrose yield. Field Crop Research 68: 97-111.
- Ramburan S (2011) Sugarcane cultivar × time of harvest interactions in South Africa. S Afr J Plant & Soil 28: 75-84.
- Gilbert RA, Shine JM, Miller JD, Rice RW, Rainbolt CR (2006) The effect of genotype, environment and time of harvest on sugarcane yields in Florida, USA. Field Crops Research 95: 156-170.
- Cardozo NP (2012) Modeling sugarcane ripening as function of meteorological variables.
- Legendre BL, Burner DM (1995) Biomass production of sugarcane cultivars and early-generation hybrids. Biomass Bioenerg 8: 55-61.
- Wang LP, Jackson PA, Lu X, Fan YH, Foreman JW, et al. (2008) Evaluation of Sugarcane × Saccharum spontaneum Progeny for Biomass Composition and Yield Components. Crop Sci 48: 951-961.
- Rao MS, Weerathaworn P (2009) Diversification of breeding program to develop multipurpose sugarcane cultivars. Sugar Tech 11: 77-79.
- Yao Y, Xuan Z, He Y, Lutts S, Korpelainen H, (2007) Principal component analysis of intraspecific responses of tartary buckwheat to UV-B radiation under field conditions. Environ Exp Bot 61: 237-245.
- Ferraro DO, Riveroa DE, Ghersaa CM (2009) An analysis of the factors that influence sugarcane yield in Northern Argentina using classification and regression trees. Field Crops Res 112: 149-157.
- Santchurn D, Ramdoyal K, Badaloo MGS, Labuschagne M (2012) From sugar industry to cane industry: investigations on multivariate data analysis techniques in the identification of different high biomass sugarcane varieties. Euphytica 185: 543-558.
- Statistical Analysis System Institute (2009) SAS Users Guide, Version 9.2. SAS Ins Inc. Cary North Carolina, USA.
- Minitab 17 Statistical Software (2010). Computer software]. State College, PA: Minitab, Inc.
- Milligan G (1980) An examination of the effect of six types of error perturbation on fifteen clustering algorithms. Psychrometrika 45: 325-342.
- Payne R, Murray D, Harding S, Baird D, Soutar D (2014) Introduction to GenStat for Windows. Statistical Services Centre.
- Tahir M, Rahman H, Gul R, Ali A, Khalid M (2013) Genetic Divergence in Sugarcane Genotypes. American Journal of Experimental Agriculture. 3: 102-109.
- Mebrahtom F (2017) Genotype × Environment Interaction, In Vitro Screening for Salinity and Drought Tolerance in Sugarcane (Saccharum officinarum L.), Ethiopia. PhD Dissertation in Plant Breeding. School of Plant Sciences, Haramaya University, Ethiopia.
- Terauchi T, Matsuoka M, Kobayashi M, Nakano H (2000) Activity of sucrose phosphate synthase in relation to sucrose concentration in sugarcane internodes. Japan Journal Tropical Agriculture 44: 141-151.
- James T, Jianping W, Barry G, Sushma S, Tomas A, et al. (2014) Phenotypic characterization of the Miami World Collection of Sugarcane and related grasses for selecting a representative core. Crop Sci 61: 1-16.
- Cardozo NP, Sentelhas PC, Panosso AR, Ferraudo AS (2014) Multivariate analysis of the temporal variability of sugarcane ripening in south-eastern Brazil. Crop and Pasture Science 65: 300-310.
- Mebrahtom F, Firew M, Eyasu A (2016) Multivariate analysis of sugar yield contributing traits in Sugarcane (Saccharum officinarum .L), in Ethiopia. Afr J Plant Sci 10: 145-156.
- Yan W (2001) GGE Biplot-A Windows application for graphical analysis of multi-environment trial data and other types of two-way data. Agron J 93: 1111-1118.
- Mebrahtom F, Firew M, Eyasu A (2017) Temporal and Spatial Factors Affecting the Nature of Genotype × Environment Interaction in Sugarcane (Saccharum officinarum L.) under Ethiopian Agro-Climatic Conditions: An Integrated Approach. American Journal of Plant Sciences 8: 1721-1749.
Citation: Ftwi M, Mekibib F, Tesfa M (2017) Maturity Classification of Sugarcane (Saccharum officinarum L) Genotypes Grown Under Different Production Environments of Ethiopia. Adv Crop Sci Tech 5: 304. DOI: 10.4172/2329-8863.1000304
Copyright: © 2017 Ftwi M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 9965
- [From(publication date): 0-2017 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 8939
- PDF downloads: 1026