Research Article Open Access
Maternal Obesity and Placental Oxidative Stress in the First Trimester
| Mark C. Alanis1*, Elizabeth M. Steadman1, Yefim Manevich2, Danyelle M. Townsend3 and Laura M. Goetzl1 | |
| 1Department of Obstetrics and Gynecology, Medical University of South Carolina, 96 Jonathan Lucas Street, CSB 634, Charleston, South Carolina 29425, USA | |
| 2Department of Cell and Molecular Pharmacology and Experimental Therapeutics, Medical University of South Carolina, 173 Ashley Avenue, Charleston, South Carolina 29425, USA | |
| 3Department of Pharmaceutical and Biomedical Sciences, Medical University of South Carolina, 280 Calhoun Street, Charleston, South Carolina 29425, USA | |
| Corresponding Author : | Mark C. Alanis Department of Obstetrics and Gynecology Medical University of South Carolina, 96 Jonathan Lucas Street CSB 634, Charleston, SC 29425, USA Tel: (843) 792-1241 Fax: (843) 792-0533 E-mail: alanis@musc.edu |
| Received June 20, 2012; Accepted August 06, 2012; Published August 12, 2012 | |
| Citation: Alanis MC, Steadman EM, Manevich Y, Townsend DM, Goetzl LM (2012) Maternal Obesity and Placental Oxidative Stress in the First Trimester. J Obes Wt Loss Ther 2:143. doi:10.4172/2165-7904.1000143 | |
| Copyright: © 2012 Alanis MC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Objective: Maternal obesity is associated with adverse pregnancy outcomes affected by placental dysfunction. We sought to compare levels of placental oxidative stress between obese and lean women in the first trimester.
Study Design: Obese and lean women matched 1:1 for baseline variables and gestational age were enrolled between 8 and 13 weeks of gestation at the time of voluntary surgical abortion. The global cellular redox status was determined by measuring the total protein thiol content in placental homogenates and serum (ThioGlo-1).
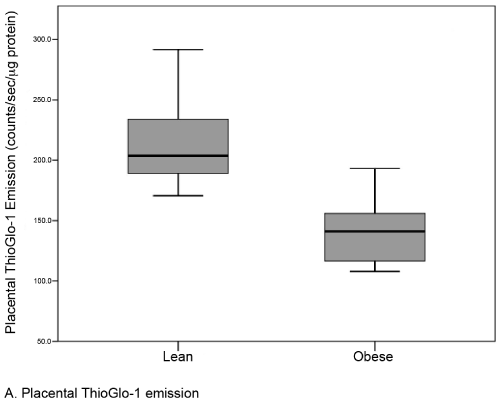
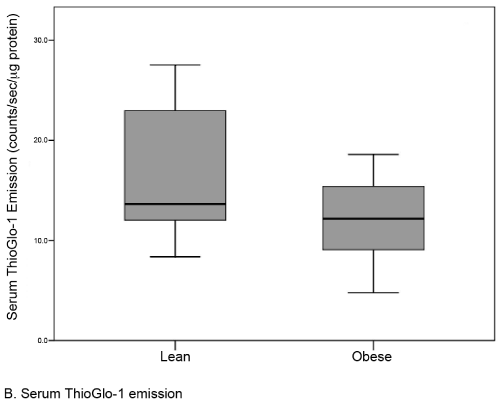
Results: There were no differences in baseline variables between obese (n=22, median BMI 35.0) and lean controls (n=22, median BMI 22.0). The median level of placental oxidative stress was 31% greater in the obese group compared to the lean group (141.1 [117-156] vs. 203.7 [189-234] counts/sec/μg protein, respectively; p <0.001). A similar but statistically insignificant difference was noted in the serum (12.2 [9-15] vs. 13.6 [12-23] counts/sec/μcg protein; p=0.09).
Conclusion: Maternal obesity is associated with placental oxidative stress in the first trimester. Oxidative stress in the first trimester may reflect or contribute to impaired placentation and placental dysfunction in obese women.
| Keywords |
| Obesity; Oxidative stress; Placenta; Redox; Protein sulfhydryls; Thiols; ThioGlo-1 |
| Introduction |
| Pregnancy has been described as a state of oxidative stress due to increased metabolic activity of the placenta and decreased antioxidant capacity during normal pregnancy [1]. Rapidly dividing placental cells produce large amounts of Reactive Oxygen Species (ROS), including superoxide anion, as a byproduct of aerobic respiration by complexes I and III of the mitochondrial electron transport chain [2]. NAD(P)H oxidase (Nox) is the other main source of superoxide anion generation in the placenta and is expressed in the cell membrane of the syncytial layer, the vascular endothelium, and maternal granulocytes found at the maternal-fetal interface [3,4]. Collectively, these observations suggest that normal pregnancy is a state close to the limit at which oxidative stress may become pathological. Decreased antioxidant capacity and increased ROS are associated with placental dysfunction resulting in preeclampsia, intrauterine fetal hypoxia and growth restriction, and stillbirth [1-3,5-7]. |
| Oxidative stress markers such as levels of plasma lipid peroxidation and urinary F2 isoprostanes are known to be increased in obese, nonpregnant women [8,9]. However, the relationship between maternal obesity and placental oxidative stress is unclear. Maternal obesity is associated with placental dysfunction, characterized clinically by preeclampsia, second trimester spontaneous abortion, and stillbirth, in a dose-dependent fashion with body mass index (BMI) [10-15]. More than 1 in 3 women in the United States are obese at the time of conception, defined as a BMI equal to or greater than 30 kg/m2[16]. Thus, there is a broad interest among public health experts, physicians, and researchers in elucidating mechanistically based interventions to reduce the impact of maternal obesity on pregnancy outcome. The aim of this study was to determine the effect of maternal obesity on placental oxidative stress in the first trimester. |
| Methods |
| Subjects |
| Obese (BMI ≥ 30) and lean (18.5 ≤ BMI <35) patients undergoing voluntary surgical abortion were recruited from a single reproductive services clinic in this IRB approved study. Inclusion criteria included a gestational age between 8(0/7) and 13(6/7) weeks and signed informed consent. Exclusion criteria included co-existing diabetes mellitus, renal disease, and use of antibiotics in the previous 6 weeks, use of any nonsteroidal anti-inflammatory drug in the previous 24 hours, use of oral or systemic steroids, or recent (<6 weeks) pelvic inflammatory disease. BMI was determined by height and weight measured immediately prior to the procedure. Obese subjects were enrolled consecutively, and a matched lean controls was subsequently enrolled for each obese subject. Matching was 1:1 by race/ethnicity, smoking status (yes/no in pregnancy), and gestational age (± 3 d). All gestational ages were confirmed by measurement of the crown-rump-length with ultrasound prior to the procedure. |
| Blood and placenta collection |
| Blood samples were obtained at the time of intravenous access and prior to initiation of the procedure. Blood samples were placed on ice and allowed to clot for at least 30 minutes. Immediately following dilation and curettage, products of conception were floated in ice-cold phosphate buffered saline (PBS, pH 7.4). Placenta was identified by the consistent frond-like appearance of villi (Figure 1). Placental specimens were washed with ice-cold PBS then flash-frozen in liquid nitrogen. The entire tissue collection process was completed in less than 20 minutes after dilation and curettage in all cases. Placental specimens were stored at -80° C until batched testing. Clotted blood samples were centrifuged at 4,000 rpm for 20 minutes at 4° C and serum fractions were kept for analyses. |
| Sample Preparation |
| Frozen placental samples were manually homogenized in lysis buffer containing 50 mMTris-HCl (pH 7.5), 2mM Ethylene Diaminetetraacetic Acid (EDTA), 2mM Ethylene Glycol Tetraacetic Acid (EGTA), 1% Triton X-100, 150 mMNaCl, 10 glycerol, and diluted 1:100 with Phenyl Methyl Sulfonyl Fluoride (PMSF). The lysis buffer was supplemented with a proteinase inhibitor cocktail containing 4-(2-amnioethyl) benzenesulfonyl fluoride (AEBSF), pepstatinA, E-64, bestatin, leupeptin, and aprotinin (Sigma-Aldrich, St. Louis, MO). Samples were incubated in the lysis buffer with periodic vortexing at 40C for 15 minutes. The incubated samples were then centrifuged at 15,000 rpm for 15 minutes to precipitate non-soluble material. The supernatant was collected and passed through a Biospin-6 (Bio-Rad Laboratories, Inc., Hercules, CA) size-exclusion chromatography mini-column and the protein concentration was determined using the Bradford Assay (Bio-Rad Laboratories, In., Hercules, CA). The resultant filtrate was diluted 1:100 with 20 mM PBS. |
| Fluorescent detection of oxidative stress |
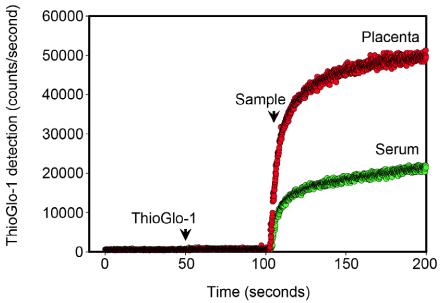
| Oxidative stress was assessed through the measurement of reduced protein thiol content using the sulfhydryl-specific maleimide fluorescent dye, ThioGlo-1 (Calbiochem Inc., San Diego, CA) as previously described [17-18]. The total reduced protein thiol content is the reciprocal of the total oxidized protein content. The fluorescent emission of ThioGlo-1-protein sulfhydryl adducts, therefore, is an accurate measure of the global protein redox status [19]. Freshly prepared placental homogenates and thawed serum were analyzed using real-time kinetics mode of a QM-4 fluorometer (Photon Technology International, Inc., Piscatway, NJ; Figure 2). Saturation ThioGlo-1 fluorescence was normalized for the total protein content of each sample. |
| Statistical Analysis |
| Continuous data were described as medians (IQR), and categorical data were described by frequencies (column percents). The primary outcome was ThioGlo-1 emission, normalized for protein content (counts/sec/μg protein). Group comparisons between obese and lean women were performed using Wilcoxon rank sum tests or Chisquare tests as appropriate. Linear relationships between placental and serum ThioGlo-1 emission and between gestational age and ThioGlo-1 emission were assessed by Spearman rank correlation coefficients. P-values <0.05 were considered statistically significant for all tests (SAS 9.1.3 (Cary, NC)). |
| Results |
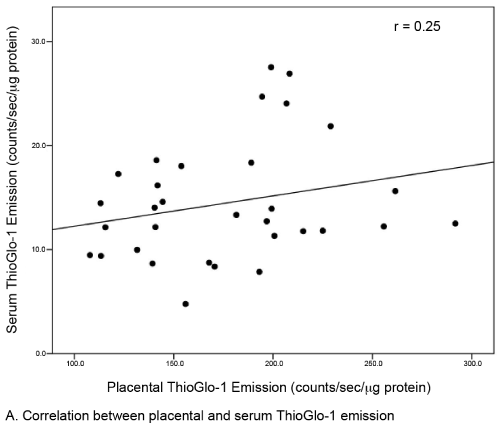
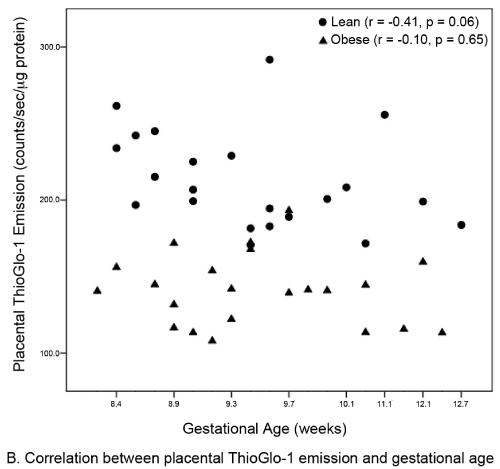
| A total of 44 subjects (22 matched pairs) were enrolled. There were no differences in background or demographic variables (Table 1). Maternal obesity was associated with a 31% median increase in placental oxidative stress compared to lean controls (Figure 3A). Thirtytwo subjects (16 in each group) also had serum available for peripheral oxidative stress assessment. A similar, but non-significant increase in serum oxidative stress was found (Figure 3B). There was no significant correlation between placental and serum oxidative stress either within groups (obese r=-0.11, p=0.67; lean r=-0.06, p=0.83) or overall (r=0.25, p=0.17, Figure 4a). There was also no correlation between gestational age and placental (r=-0.16, p=0.28) or serum (r=0.26, p=0.14) oxidative stress (Figure 4b). Overall, the level of oxidative stress measured in the placenta was far more pronounced than in the serum (Figure 3A, Figure 3B). |
| Discussion |
| Maternal obesity is an emerging public health concern of enormous clinical impact and research interest. Our data shows for the first time that maternal obesity is associated with placental oxidative stress in the first trimester. This finding supports a first trimester origin for the observed increased rate of placental dysfunction noted in obese women later in pregnancy, although a direct relationship cannot be established from this investigation [10-15]. Oxidative stress is increasingly viewed as an upstream process resulting in inflammation and cellular injury. Indeed, maternal obesity is associated with robust placental inflammation at term Challier et al. [20] demonstrated that mRNA expression of TNF-α and other pro-inflammatory cytokines are elevated in placentas of obese women compared to lean women at term [20]. These investigators found that an accumulation of activated CD14+ macrophages in the placenta was considered the primary source for these pro-inflammatory cytokines [20]. Other investigators have found that placental mitochondrial ROS production is stimulated by TNF-α [21]. Therefore, increased obesity-related placental inflammatory cytokines may promote further production of ROS and induce a feed forward cycle of placental cellular damage [22]. |
| Our finding that global placental redox status in obese women is independent of gestational age was surprising. A common belief is that placental oxidative stress occurs only after 10 weeks of gestation, based on the observation that only after this time point does the fetal circulation come into direct contact with the uterine spiral arteries and the intervillous oxygen tension rises sharply (pO2=50 mmHg) [23,24]. Prior to this period, the fetal environment is very hypoxic (pO2<20 mmHg) [24].The reason for independence of oxidative stress for gestational age in obese women is unclear. There are two potential explanations for this phenomenon. The first is hypoxiainduced production of ROS from the placental mitochondrial electron transport chain [2]. The second involves a priori increased NADPH oxidase activity in the placenta of obese women. In fact, NADPH oxidase appears to be the major contributor of ROS production in non-pregnant obese women [8,25,26]. Our findings differ from those earlier reported by Roberts et al. [27] who found no direct relationship between lean, overweight, and obese BMI classes and oxidative stress [27]. The disagreement between our two studies most likely reflect that their study: 1) was limited to term gestations without pregnancy complications; 2) contained a very limited number of subjects (7 lean, 5 overweight, and 8 obese), which may have limited the power to detect increases in oxidative stress; 3) used quasi-quantifiable methods, such as western blot, to measure placental oxidative stress; and 4) recruited both laboring and non-laboring women [27]. In contrast, our study examines the induction of placental oxidative stress at the critical time period following placentation. Further, our study was not confounded by the increase in oxidative stress known to be associated with parturition [17,28]. Finally, Roberts et al. [27] selected for uncomplicated pregnancies [27], which may have reduced the likelihood of detecting placental oxidative stress, while our sample is unselected. Another advantage of our study is that our subjects were matched for important baseline variables that could potentially affect oxidative stress. Consistent with non-pregnant studies [8], the level of oxidative stress measured in the serum was slightly increased in our obese subjects. However, only a subset of subjects had serum available for analysis, limiting our power to establish statistical significance. |
| There are numerous advantages to the assessment of placental oxidative stress as described and performed in our study. First, Hansen et al. demonstrated the appropriateness of determination of the global cellular sulfhydryl status as an indicator of oxidative stress [19]. Protein cysteine residues (e.g. sulfhydryls) are considered to be redox switches and mediate oxidative and nitro sative stress-induced signaling events that are critical to cell fate. Rather than assaying a specific component of the oxidative stress (e.g. antioxidant levels), which may fail to detect oxidative stress, tissue assessment of protein redox status is highly sensitive. Second, this direct measurement of the total protein redox status is straightforward and reproducible. Third, samples can be stored and assayed in batch, further reducing variability within the assay. |
| We anticipate that our approach affords the opportunity to identify obesity-related mechanisms of placental oxidative stress in the first trimester. Further, our study implicates pre-pregnancy maternal obesity as a potential first-trimester marker for selection of those whom may be candidates for antioxidant trials for the prevention of adverse pregnancy outcome. |
References
- Wisdom SJ, Wilson R, McKillop JH, Walker JJ (1991) Antioxidant systems in normal pregnancy and in pregnancy-induced hypertension. Am J Obstet Gyneco 165: 1701-1704.
- Myatt L, Cui X (2004) Oxidative stress in the placenta. Histochem Cell Biol 122: 369-382.
- Cui XL, Brockman D, Campos B, Myatt L (2006) Expression of NADPH oxidase isoform 1 (Nox1) in human placenta: involvement in preeclampsia. Placenta 27: 422-431.
- Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, et al. (2001) Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol 185: 792-797.
- Llurba E, Gratacós E, Martín-Gallán P, Cabero L, Dominguez C (2004) A comprehensive study of oxidative stress and antioxidant status in preeclampsia and normal pregnancy. Free Radic Biol Med 37: 557-570.
- Wang Y, Walsh SW (1998) Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta 19: 581-586.
- Potdar N, Singh R, Mistry V, Evans MD, Farmer PB, et al. (2009) First-trimester increase in oxidative stress and risk of small-for-gestational-age fetus. BJOG 116: 637-642.
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, et al. (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752-1761.
- Keaney JF Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, et al. (2003) Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol 23: 434-439.
- Alanis MC, Goodnight WH, Hill EG, Robinson CJ, Villers MS, et al. (2010) Maternal super-obesity (body mass index > or = 50) and adverse pregnancy outcomes. Acta Obstet Gynecol Scand 89: 924-930.
- Ovesen P, Rasmussen S, Kesmodel U (2011) Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol 118: 305-312.
- Cedergren MI (2004) Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol 103: 219-224.
- Salihu HM, Dunlop AL, Hedayatzadeh M, Alio AP, Kirby RS, et al. (2007) Extreme obesity and risk of stillbirth among black and white gravidas. Obstet Gynecol 110: 552-557.
- Chu SY, Kim SY, Lau J, Schmid CH, Dietz PM, et al. (2007) Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol 197: 223-228.
- Nohr EA, Bech BH, Davies MJ, Frydenberg M, Henriksen TB, et al. (2005) Prepregnancy obesity and fetal death. a study within the Danish National Birth Cohort. Obstet Gynecol 106: 250-259.
- Flegal KM, Carroll MD, Ogden CL, Curtin LR (2010) Prevalence and trends in obesity among US adults, 1999-2008. JAMA 303: 235-241.
- Goetzl L, Manevich Y, Roedner C, Praktish A, Hebbar L, et al. (2010) Maternal and fetal oxidative stress and intrapartum term fever. Am J Obstet Gynecol 202: e1-e5.
- Townsend DM, Manevich Y, He L, Hutchens S, Pazoles CJ, et al. (2009) Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J Biol Chem 284: 436-445.
- Hansen RE, Roth D, Winther JR (2009) Quantifying the global cellular thiol-disulfide status. Proc Natl Acad Sci U S A 106: 422-427.
- Challier JC, Basu S, Bintein T, Minium J, Hotmire K, et al. (2008) Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29: 274-281.
- Schulze-Osthoff K, Bakker AC, Vanhaesebroeck B, Beyaert R, Jacob WA, et al. (1992) Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem 267: 5317-5323.
- Rusterholz C, Hahn S, Holzgreve W (2007) Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin Immunopathol 29: 151-162.
- Jaffe R, Jauniaux E, Hustin J (1997) Maternal circulation in the first-trimester human placenta--myth or reality? Am J Obstet Gynecol 176: 695-705.
- Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, et al. (2000) Onset of maternal arterial blood flow and placental oxidative stress. Am J Pathol 157: 2111-2122.
- Patel C, Ghanim H, Ravishankar S, Sia CL, Viswanathan P, et al. (2007) Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J Clin Endocrinol Metab 92: 4476-4479.
- Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, et al. (2008) Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism 57: 1071-1077.
- Roberts VH, Smith J, McLea SA, Heizer AB, Richardson JL, et al. (2009) Effect of increasing maternal body mass index on oxidative and nitrative stress in the human placenta. Placenta 30: 169-175.
- Cindrova-Davies T, Yung HW, Johns J, Spasic-Boskovic O, Korolchuk et al. (2007) Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am J Pathol 171: 1168-1179.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3a |
 |
 |
 |
| Figure 3b | Figure 4a | Figure 4b |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 16042
- [From(publication date):
August-2012 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 11439
- PDF downloads : 4603
