Research Article Open Access
Maternal Gestational Diabetes and Fetal Congenital Heart Disease: An Observational Study
| Lindsey E Hunter1 and Gurleen K Sharland2* | |
| 1Lindsey E Hunter, Specialist Registrar in Paediatric and Fetal Cardiology, UK | |
| 2Gurleen K Sharland, Consultant in Fetal Cardiology, Department of Congenital Heart Disease, Evelina London Children’s Hospital, London, UK | |
| Corresponding Author : | Gurleen Sharland Department of Congenital Heart Disease Evelina London Children’s Hospital Westminster Bridge Road, London SE1 7EH, UK Tel: 020 718 82308 Fax: 020 718 82307 E-mail: gurleen.sharland@gstt.nhs.uk |
| Received October 12, 2014; Accepted January 26, 2015; Published January 26, 2015 | |
| Citation: Hunter LE, Sharland GK (2015) Maternal Gestational Diabetes and Fetal Congenital Heart Disease: An Observational Study. J Preg Child Health 2:132. doi: 10.4172/2376-127X.1000132 | |
| Copyright: © 2015 Hunter LE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Background: Maternal type 1 diabetes mellitus (DM) is a recognised risk factor for the development of fetal congenital heart disease (CHD). However the risk of CHD in babies of women with gestational diabetes mellitus (GDM) is less well known.
Objectives: The aim of this 10 year, retrospective, observational study was to compare the occurrence of congenital heart disease in these two referral groups and to evaluate whether detailed fetal echocardiography should be offered to women with GDM.
Results: During the study period 1401 women with type 1 DM were referred for fetal echocardiography and a fetal congenital heart defect was diagnosed in 44 cases (3.1%). In the GDM cohort 543 women were referred for screening and 15 cases (2.76%) of fetal CHD were detected.
Conclusions: Based on our data we consider women with GDM to be at an increased risk of having a baby with CHD. This risk might be due to a combination of hyperglycaemia, insulin resistance, an elevated BMI and more importantly, undiagnosed pre-gestational diabetes. Until more detailed data from larger studies is available, we will continue to accept these women as referrals for detailed fetal echocardiography.
| Keywords |
| Congenital heart disease; Fetal heart; Gestational diabetes mellitus |
| Abbreviations |
| AoV – Aortic Valve; AVSD - Atrioventricular Septal Defect; BMI - Body Mass Index; CHD - Congenital Heart Disease; DILV - Double Inlet Left Ventricle; DM – Diabetes Mellitus; DORV - Double Outlet Right Ventricle; ECA - Extra Cardiac Abnormality; GDM - Gestational Diabetes Mellitus; HbA1c - Glycated Haemoglobin A1c; HLHS - Hypoplastic Left Heart Syndrome; IDDM – Insulin Dependent Diabetes Mellitus; IVS - Intact Ventricular Septum; MAPCA - Multiple Aorto Pulmonary Collaterals; MV – Mitral Valve; NT - Nuchal Translucency; OGT Test - Oral Glucose Tolerance Test; PAT - Pulmonary Atresia; PS - Pulmonary Stenosis; SVT - Supraventricular Tachycardia; TAPVD - Total Anomalous Pulmonary Venous Drainage; TGA - Transposition of the Great Arteries; VSD - Ventricular Septal Defect; WHO - World Health Organization |
| Introduction |
| It is widely reported that women with pre-gestational diabetes mellitus, in particular those with Type 1 diabetes mellitus (DM), have an increased risk of having a child with congenital abnormalities, including congenital heart disease (CHD) [1-4]. In a previous publication we reported that 3.1% of women with Type 1 diabetes mellitus referred to our unit for fetal echocardiography between 1990-1994, had a baby with CHD [5]. |
| The occurrence of fetal CHD in pregnant women with gestational diabetes mellitus (GDM) is less well documented, but has generally been thought to be lower than those with pre-existing DM. Gestational diabetes is defined as ’any degree of glucose intolerance with onset or first recognition during the present pregnancy’ and can in some cases, inadvertently include women with pre-existing, undiagnosed DM [6]. International consensus dictates that the diagnosis of GDM is based on the oral glucose tolerance test, but the threshold for diagnosis and screening methods vary between professional bodies [7-9]. It is known to occur in at least 1-5% of all pregnancies and is associated with an increase in perinatal and maternal morbidity [10,11]. The combination of physiological, lifestyle, ethnic and genetic factors predisposes some women to develop gestational diabetes mellitus (GDM) [12]. Risk factors include a previous infant with a birth weight >4kg, maternal BMI >30, age > 25 years, previous GDM, family history of DM, certain ethnic groups, essential or pregnancy related hypertension, unexplained stillbirth/miscarriages and glycosuria. However, there is an absence of risk factors in approximately 50% of women. |
| Women with type 1 DM are often referred for detailed fetal echocardiography because of the recognised risk for fetal CHD [13,14]. Women with GDM are also often referred to our unit for assessment of the fetal heart. Our initial hypothesis was that GDM was an inappropriate referral reason and women with GDM do not have an increased risk of CHD in their offspring. Therefore this observational study was undertaken to assess the occurrence of fetal CHD in women with reported gestational diabetes referred to our fetal cardiology unit and to evaluate whether fetal echocardiography should continue to be routinely offered to these women. |
| Subjects and Methods |
| This retrospective, observational study of data that had been prospectively acquired was undertaken in a tertiary fetal cardiology unit. Included in the study were all women with GDM referred for detailed fetal echocardiography between the 1st January 2003 and 31st December 2012. Patients were identified by a retrospective review of the fetal cardiology database, Filemaker Pro (Claris Corp, CA, USA), the fetal cardiology notes, correspondence from referring obstetric teams and the ‘hand held’ maternal maternity case notes. During this time period 543 women with reported GDM were referred for fetal echocardiography. Women with pre-existing Type 2 diabetes were excluded from the initial data collection. The data of all the women with type 1 diabetes mellitus (DM) referred for fetal cardiology assessment in the same 10 year period was reviewed for comparison. During this time, 1401 women with confirmed type 1 DM were referred for fetal cardiology assessment. Fetal echocardiographic studies were performed using the Toshiba Xario and Aplio ultrasound systems (Toshiba, Crawley, UK) and GE Voluson E8 system (GE Milwakee, USA). In all cases a detailed assessment of the intra-cardiac anatomy was undertaken, including cardiac Doppler studies. Confirmation of the antenatal diagnosis was made by postnatal echocardiography. We did not correlate maternal glycaemic control with the presence of congenital heart disease, as there was insufficient data available in this retrospective data collection. |
| Study Approval |
| This study was approved as an internal retrospective audit within our hospital. It was initiated to review our departmental referral criteria and improve service provision. |
| Results |
| Pregnant women with Gestational Diabetes Mellitus (GDM) |
| During the 10 year study period, 543 women reported to have GDM were referred for a detailed fetal echocardiogram. The median maternal age at referral was 33 years (Range 17-50 years, mean 22 years) and the median gestational age at the initial fetal echocardiogram was 26 weeks (Range 14-37 weeks, mean 22 weeks). The maternal BMI was not routinely recorded during the study period, but frequent reference was made to maternal habitus, often limiting the acoustic windows and therefore image quality. |
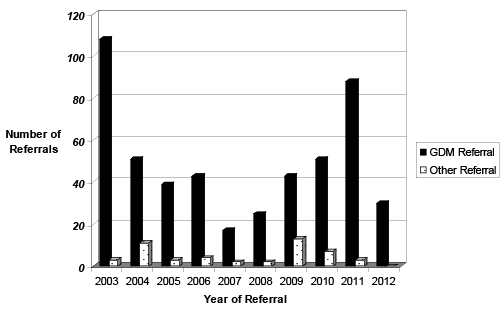
| Indication for Referral: In the GDM group, GDM was the most common indication for referral (n=506), (Figure 1). Other referral reasons included: ‘inadequate views of the fetal heart’ (n=4) which was defined as an inability to achieve the normal screening views of the fetal heart during the obstetric fetal anomaly scan [15]; family history of CHD (n=11); raised nuchal translucency (NT) (n=4); suspected CHD (n=9); fetal abnormality (n=3); fetal drug exposure (n=1) and fetal arrhythmia (n=5) (Figure 1). |
| GDM therapy: Of the 543 women with GDM, 229 (42%) were managed by diet alone; 45 (8%) by metformin; 261 (49%) by insulin and 8 (1%) by a combination of insulin and metformin. In all cases, insulin therapy and metformin was started during pregnancy and not in the pre conception phase. Over the 10 year period there was a rise in the number of women receiving metformin, or insulin and metformin in combination, with a reduction in the administration of insulin therapy in isolation [16]. |
| Anomaly screening: Nuchal translucency (NT) thickness was not routinely documented by all referring centres during the early period of the study and therefore only documented in 66 (12%) of the 543 GDM referrals. In 10/66 (15%) documented the NT was greater than 2.5mm, and in two cases it was above 3.5mm. The presence or absence of extra cardiac abnormalities (ECA) was documented in 416 (76.6%) of the 543 fetuses. Of the cases where it was documented, 20 (4.8%) had an ECA detected. |
| GDM and fetal CHD |
| Demographics: In the GDM cohort (n=543), 15 (2.76%) of the fetuses had CHD. In these 15 affected pregnancies, the median maternal age was 34 years (Range 23-41 years, mean 33.5 years). The referral reason in a third of the 15 cases was maternal gestational diabetes in isolation. The other referral reasons included: abnormal views of the heart (n=6); increased NT (n=2); inadequate views of the fetal heart (n=1) and fetal arrhythmia (n=1). In this cohort of 15 women, five were managed by diet, two by metformin and eight by insulin therapy in isolation. |
| Anomaly screening: In 10 (66.6%) of the 15 CHD cases, a NT measurement was documented, and of these, two were abnormally elevated (4.6mm; 8.1mm). Four of the 15 CHD cases were noted to have extra-cardiac anomalies, which included: polyhydramnios; fetal hydrops secondary to fetal tachycardia, in a baby with a confirmed ventricular septal defect; a two vessel umbilical cord and extra digits (noted postnatally to be dysmorphic with low set ears and ambiguous genitalia). |
| CHD detected: The defects identified in the GDM cohort are summarised in Table 1. The type of CHD was varied and included right and left sided abnormalities; obstructive lesions; potential shunting lesions; complex and simple lesions(Table 1). The defects identified in the GDM cohort are summarised in Table 1. The type of CHD was varied and included right and left sided abnormalities; obstructive lesions; potential shunting lesions; complex and simple lesions(Table 1). |
| Outcome: Of the 15 pregnancies diagnosed with GDM and fetal CHD, two resulted in a termination. Three of the 15 resulted in a neonatal death: one post-surgical intervention; one due to complications linked to multiple ECAs and one family opted for comfort care postnatally. Nine of the 15 children are alive to date: five have undergone cardiac surgery; one a cardiac catheter intervention and three continue to be monitored as outpatients, with no intervention to date. One of the 15 was lost to follow up postnatally, following transfer of care to another cardiac centre. |
| Pregnant women with Type 1 Diabetes Mellitus |
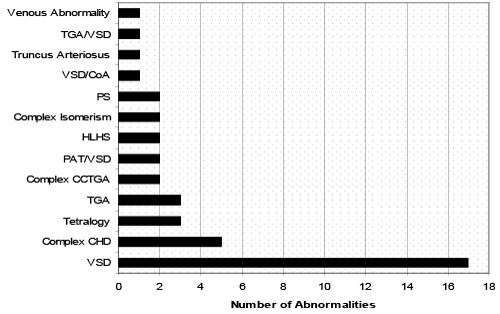
| During the study period, 1401 women with confirmed type 1 DM were referred for fetal cardiology assessment. The median maternal age at the time of referral was 31 years (Range 19-42 years, mean 30.2 years) and the median gestational age at initial echocardiogram was 20 weeks (Range 14-28 weeks, mean 21.5 weeks). Of the 1401 women referred, a fetal congenital heart defect was detected in 44 cases (3.1%). Figure 2 demonstrates the wide spectrum of congenital heart defects diagnosed, the most common diagnosis being a ventricular septal defect (VSD). In addition, eleven (25%) of the 44 with CHD had a known ECA (Figure 2). |
| The comparisons between the GDM and type 1 DM cohorts are summarised in Table 2. |
| Discussion |
| Fetal exposure to hyperglycaemia during embryogenesis, in women with poorly controlled diabetes mellitus, is known to be associated with congenital abnormalities, including congenital heart disease (CHD) [2,4,8,17]. A small increase in circulating blood glucose levels may be detrimental to the developing embryo and good glycaemic control has been shown to reduce the rate of congenital abnormalities, preterm delivery and stillbirths [18-20]. Animal models have demonstrated that diabetic embryopathy is a complex process influenced by metabolic signalling, cell signalling, maternal and fetal genotypes and environmental factors as well as exposure to hyperglycaemia[21]. In non-diabetic pregnancies there is an increase in maternal insulin resistance due to maternal physiological adaptations which occur to ensure adequate fetal growth and development [22]. These adaptations include maternal glucose intolerance, altered glucose metabolism, cortisol/growth hormone levels and may be compounded by reduced physical activity and increased calorific intake during pregnancy. GDM, which tends to develop in the 2nd or 3rd trimester of pregnancy, has been attributed to an increase in perinatal morbidity and mortality, although pregestational diabetes is known to have a greater association with fetal anomalies than GDM [23-27]. Our preliminary data suggests that the risk of CHD in a GDM pregnancy is 2.76% with a 26.7% risk of a concomitant extra cardiac abnormality. This is similar to the incidence of CHD in our type1 DM pregnancies, at 3.1%, with a risk of a concomitant extra cardiac abnormality of 25%. The occurrence of CHD in our GDM population may reflect a combination of hyperglycaemia, insulin resistance, an elevated BMI and possibly and perhaps most significantly, undiagnosed pre-gestational diabetes. During the study period there was an increase in the number of women being managed with metformin or metformin and insulin in combination, which is perhaps a reflection of the high safety profile of metformin in pregnancy, and a more acceptable method of therapy [16]. |
| A record of maternal BMI has become routine within our unit, although this was not the scenario in the early era of this study. The reasons for our change in practice are related to published data suggesting an association between maternal BMI and birth defects and the association between a raised BMI and GDM. An increase in sedentary living, poor diet and obesity in the UK and USA has contributed to the increasing incidence of type 2 diabetes and with an earlier age of onset [28]. Associations have been made between maternal BMI at conception, pre gestational insulin resistance and an increased incidence of birth defects. Although the relationship between GDM and congenital heart disease in the fetus remains largely unreported, studies have suggested a link between increased maternal BMI and fetal CHD [23,29,30]. In addition, an elevated maternal BMI can result in poorer acoustic windows, compounding the difficulty in diagnosing CHD prenatally [3,31-34]. A study examining the diurnal, glycaemic profiles of non-diabetic obese women compared to normal weight women, demonstrated that the obese population had a significantly higher postprandial glycaemic value, but there was no recorded difference in fasting blood glucose levels [35]. Another UK study suggested that up to 4.7% of the obese population investigated had undiagnosed type 2 diabetes and 18% had impaired glucose metabolism [36]. In population studies, maternal obesity during pregnancy has been linked to an increased risk of morbidity and mortality from cardiovascular disease in adult offspring, later in life [37,38]. |
| Our data raises several issues. Firstly, in women who are at higher risk of developing type 2 diabetes or GDM, screening and initiating treatment at antenatal booking may be a step too late to prevent the adverse effects of altered glucose metabolism on embryogenesis. If this is the case, perhaps more women should be made aware of the association between increased BMI, undiagnosed pregestational diabetes and adverse pregnancy outcomes, including increased risk of abnormalities in the baby. There may be benefits from screening at risk women for diabetes before they conceive.In recent years the WHO have advised that a glycated haemoglobin A1c (HbA1c) level can be measured to diagnose diabetes, replacing the oral glucose tolerance (OGT) test which takes 2 hours and requires the patient to fast. One of the advantages of the HbA1c is it can be taken with routine bloods and a level above 6.5% confirms the diagnosis of diabetes [39,40]. Could this be a quick screening tool? This may allow for provision of preconception advice, weight loss, and subsequent good diabetic control during pregnancy to potentially reduce the risk of congenital anomalies. Secondly, as paediatric cardiologists we have traditionally dealt with congenital heart disease primarily as a de novo occurrence, in some cases in the context of chromosomal abnormalities or in pregnancies considered ‘high risk’. If our preliminary observational data reflects the prevalence of undiagnosed pregestational, type 2 diabetes, perhaps women with risk factors for type 2 diabetes should be offered fetal echocardiography screening, similar to those with pre-existing type 1 diabetes. |
| Study Limitations |
| Due to the retrospective nature of our study, our referral population was determined by the local, obstetric referral criteria at each of the hospitals that refer to our tertiary centre. Subsequently the true denominator of our regional GDM population was unknown. In addition, the obstetric diagnostic criteria for GDM were not defined. To minimise our false positive rate, we excluded women with pregestational diabetes and included only patients in whom a diagnosis of GDM was confirmed by the referring team. |
| Conclusion |
| Our retrospective study from a single center, suggests that women with GDM may have a similar risk for occurrence of fetal CHD as women with pre-existing Type 1 diabetes. The occurrence of CHD in our GDM population may reflect a combination of hyperglycaemia, insulin resistance, an elevated BMI and possibly and perhaps most significantly, undiagnosed pre-gestational diabetes. The data in this study must be considered as preliminary, but based on this preliminary data we consider these women to be at an increased risk of having a baby with CHD. Until more detailed data from larger studies is available, we will continue to accept these women as referrals for detailed fetal echocardiography. We would recommend a prospective, multicentre study to identify women at high risk, who may have undiagnosed pregestational type 2 diabetes. In addition we recommend further studies to assess an association between an increased maternal BMI and the incidence of congenital heart disease. |
| Acknowledgements |
| The authors wish to acknowledge Dr John Simpson and Dr Owen Miller, Consultants in Fetal and Paediatric Cardiology and all the fetal cardiology team at the Evelina London Children’s Hospital. We are grateful to Dr Emma Wilmot, Queens Medical Centre, Nottingham, UK, for sharing her expertise from her PhD in type 2 diabetes mellitus and the STAND research. |
References
- Becerra JE, Khoury MJ, Cordero JF, Erickson JD (1990) Diabetes mellitus during pregnancy and the risks for specific birth defects: a population-based case-control study. Pediatrics 85: 1-9.
- Lisowski L, Verheijen P, Copel J, Kleinman CS, Wassink S et al. (2010) Congenital heart disease in pregnancies complicated by maternal diabetes mellitus. An international clinical collaboration, literature review, and meta-analysis. Herz 35(1):19-26.
- Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, et al. (2008) Diabetes mellitus and birth defects. Am J ObstetGynecol 199: 237.
- Nizard J, Ville Y (2009) Thefetus of a diabetic mother: sonographic evaluation. SeminFetal Neonatal Med 14: 101-105.
- Meyer-Wittkopf M, Simpson JM, Sharland GK (1996) Incidence of congenital heart defects in fetuses of diabetic mothers: a retrospective study of 326 cases. Ultrasound ObstetGynecol 8: 8-10.
- American Diabetes A. (2006) Diagnosis and classification of diabetes mellitus. Diabetes Care. 29 Suppl1:8.
- Claesson R, Ekelund M, Berntorp K (2013) The potential impact of new diagnostic criteria on the frequency of gestational diabetes mellitus in Sweden. ActaObstetGynecolScand 92: 1223-1226.
- Avalos G, Owens L, Dunne F, for the ADIPC. (2013) Applying Current Screening Tools for Gestational Diabetes Mellitus to a European Population--Is it Time for Change? Diabetes Care. 36(10):3040-4.
- Yogev Y, Metzger B, Hod M. (2009) Establishing diagnosis of gestational diabetes mellitus: Impact of the hyperglycemia and adverse pregnancy outcome study. Semin Neonatal Fetal Med. 14(2):94-100.
- Kapoor N, Sankaran S, Hyer S, Shehata H (2007) Diabetes in pregnancy: a review of current evidence. CurrOpinObstetGynecol 19: 586-590.
- Nolan CJ (2011) Controversies in gestational diabetes. Best Pract Res ClinObstetGynaecol 25: 37-49.
- Gilmartin AB, Ural SH, Repke JT (2008) Gestational diabetes mellitus. Rev ObstetGynecol 1: 129-134.
- Simeone RM, Devine OJ, Marcinkevage JA, Gilboa SM, Razzaghi H, et al. (2015) Diabetes and congenital heart defects: a systematic review, meta-analysis, and modeling project. Am J Prev Med 48: 195-204.
- Fung A, Manlhiot C, Naik S, Rosenberg H, Smythe J, et al. (2013) Impact of prenatal risk factors on congenital heart disease in the current era. J Am Heart Assoc 2: e000064.
- Yagel S, Cohen SM, Achiron R. (2001) Examination of the fetal heart by five short-axis views: a proposed screening method for comprehensive cardiac evaluation. Ultrasound Obstet Gynecol. 17(5):367-9.
- Rowan JA, Hague WM, Gao W, Battin MR, Moore MP; MiG Trial Investigators (2008) Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med 358: 2003-2015.
- Corrigan N, Brazil DP, McAuliffe F (2009) Fetal cardiac effects of maternal hyperglycemia during pregnancy. Birth Defects Res A ClinMolTeratol 85: 523-530.
- Shaw GM, Quach T, Nelson V, Carmichael SL, Schaffer DM, et al. (2003) Neural tube defects associated with maternal periconceptional dietary intake of simple sugars and glycemic index. Am J ClinNutr 78: 972-978.
- Yazdy MM, Liu S, Mitchell AA, Werler MM (2010) Maternal dietary glycemic intake and the risk of neural tube defects. Am J Epidemiol 171: 407-414.
- Aberg A, Westbom L, Källén B. (2001) Congenital malformations among infants whose mothers had gestational diabetes or pre-existing diabetes. Early Hum Dev. 61(2):85-95.
- Eriksson UJ1 (2009) Congenital anomalies in diabetic pregnancy. SeminFetal Neonatal Med 14: 85-93.
- Hadden DR, McLaughlin C (2009) Normal and abnormal maternal metabolism during pregnancy. SeminFetal Neonatal Med 14: 66-71.
- Balsells M, García-Patterson A, Gich I, Corcoy R (2012) Major congenital malformations in women with gestational diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Res Rev 28: 252-257.
- Sekhavat S, Kishore N, Levine JC (2010) Screening fetal echocardiography in diabetic mothers with normal findings on detailed anatomic survey. Ultrasound ObstetGynecol 35: 178-182.
- HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, et al. (2008) Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 358: 1991-2002.
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, et al. (2005) Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 352: 2477-2486.
- Reece EA, Leguizamón G, Wiznitzer A (2009) Gestational diabetes: the need for a common ground. Lancet 373: 1789-1797.
- Holden SH, Barnett AH, Peters JR, Jenkins-Jones S, Poole CD, et al. (2013) The incidence of type 2 diabetes in the United Kingdom from 1991 to 2010. Diabetes ObesMetab 15: 844-852.
- Yogev Y, Visser GH (2009) Obesity, gestational diabetes and pregnancy outcome. SeminFetal Neonatal Med 14: 77-84.
- Martínez-Frías ML, Frías JP, Bermejo E, Rodríguez-Pinilla E, Prieto L, et al. (2005) Pre-gestational maternal body mass index predicts an increased risk of congenital malformations in infants of mothers with gestational diabetes. Diabet Med 22: 775-781.
- International Association of D, Pregnancy Study Groups Consensus P, Metzger B, Gabbe S, Persson B, Buchanan TA et al. (2010) International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 33(3):676-82.
- Gilboa SM, Correa A, Botto LD, Rasmussen SA, Waller DK, et al. (2010) Association between prepregnancy body mass index and congenital heart defects. Am J ObstetGynecol 202: 51.
- Wong SF, Chan FY, Cincotta RB, Oats JJ, McIntyre HD (2002) Routine ultrasound screening in diabetic pregnancies. Ultrasound ObstetGynecol 19: 171-176.
- Eckmann-Scholz C, Hoffmann U, Kramer HH, Schollmeyer T, Schem C, et al. (2012) Perinatal management of pregnancies with severe fetal heart defects and epigenetic aspects. J MaternFetal Neonatal Med 25: 2542-2545.
- Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, et al. (2004) Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J ObstetGynecol 191: 949-953.
- Wilmot EG, Edwardson CL, Biddle SJ, Gorely T, Henson J, et al. (2013) Prevalence of diabetes and impaired glucose metabolism in younger 'at risk' UK adults: insights from the STAND programme of research. Diabet Med 30: 671-675.
- Factor-Litvak P (2013) Maternal obesity and heart disease in the offspring. BMJ 347: f4960.
- Reynolds R, Allan K, Raja E, Bhattacharya S, McNeill G et al. (2013) Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ (Clinical research ed). 347 (f4539).
- Inzucchi SE1 (2012) Clinical practice. Diagnosis of diabetes. N Engl J Med 367: 542-550.
- Katreddy MV, Pappachan JM, Taylor SE, Nevill AM, Indusekhar R, et al. (2013) Hemoglobin A1c in early postpartum screening of women with gestational diabetes. World J Diabetes 4: 76-81.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 20776
- [From(publication date):
February-2015 - Dec 18, 2024] - Breakdown by view type
- HTML page views : 16116
- PDF downloads : 4660
