Research Article Open Access
Maternal Factors Affecting Serum Leptin Levels in Preeclampsia and Normotensive Pregnant Women and Outcome of Pregnancy
| Kharb S*, Panjeta P, Ghalaut VS, Bala J and Nanda S | |
| Department of Biochemistry, Sharma University of Health Sciences, Rohtak, Haryana, India | |
| Corresponding Author : | Kharb S Department of Biochemistry Sharma University of Health Sciences Rohtak, Haryana, India Tel: 919812016036 E-mail: simmikh@gmail.com |
| Received: January 29, 2016 Accepted: February 19, 2016 Published: February 26, 2016 | |
| Citation: Kharb S, Panjeta P, Ghalaut VS, Bala J, Nanda S (2016) Maternal Factors Affecting Serum Leptin Levels in Preeclampsia and Normotensive Pregnant Women and Outcome of Pregnancy. J Preg Child Health 3:223. doi:10.4172/2376-127X.1000223 | |
| Copyright: © 2016 Kharb S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Leptin has been measured in the fetal circulation and plasma leptin may also originate from a range of other fetal and placental tissues. The present study was designed to analyze leptin levels in women with preeclampsia and to assess its relationship with renal functions, gestational age and outcome of pregnancy. Twenty five normotensive pregnant women and 25 age and gestation matched preeclamptic women. Routine investigations and serum leptin levels were analyzed in maternal blood by ELISA kit. Serum leptin levels were significantly higher in preeclamptics as compared to normotensive pregnant women and the leptin levels increased with rise in proteinuria in preeclamptic women. Systolic blood pressure in preeclamptic group showed a significant positive correlation with serum leptin. Placental weight, baby weight and Apgar score (at 1 min and 5 min) had a negative correlation with leptin levels in preeclamptic women. Serum leptin levels were negatively correlated with serum uric acid levels in normotensive pregnant women which was reversed in preeclamptics. Serum leptin levels were positively correlated with urea levels in preeclamptic women. Leptin expression in the fetus is altered by different intrauterine conditions, and these responses vary according to the nature of the stressor. Findings of present study suggest that leptin is associated with rise in blood pressure and adverse pregnancy outcome in preeclampsia. Mechanisms responsible for this increase and role played by leptin in the development of preeclampsia require further study.
| Introduction |
| Preeclampsia is characterized by intense vasospasm affecting mainly the renal, uterine and cerebral vasculature that results due to increased vasopressor substances like angiotensin II, thromboxane A2, endothelin-1 and a decrease in vasodilator substances such as nitric oxide and prostacyclin due to endothelial cell dysfunction [1]. Leptin is synthesized and secreted by adipocytes. It is also synthesized by placenta, contributing to circulating levels of leptin during pregnancy. Pregnancy is associated with increased appetite, fat mass, body weight and metabolism and rise in levels of reproductive hormones that decline after delivery. During pregnancy, there is significant increase in leptin levels in blood possibly due to marked changes in maternal weight, energy expenditure and hormone status. |
| During pregnancy, circulating maternal concentrations of leptin increase to maximal values in the second trimester, plateau in the third trimester and fall to below pre-pregnancy concentrations around birth [2]. Leptin appears in placenta, amniotic fluid, and in the fetal plasma it is detectable by 18 weeks of gestation. During the pregnancy period a considerable amount of leptin is secreted from the placental trophoblastic cells into the maternal circulation [3]. |
| Leptin is regulated by 17 β-estradiol (E2) by both genomic and nongenomic regulatory pathways through nuclear estrogen receptor α and by an unknown membrane bound receptor respectively [4]. During pregnancy, E2 levels increase as a result of placental production that is of help in maintaining pregnancy and contributing to leptin production [5]. |
| Leptin regulates processes as placental nutrient transport, placental angiogenesis, trophoblastic mitogenesis and immunomodulation that are crucial for fetal development and adequate placental function. In human umbilical vein endothelial cells, leptin through binding of the membrane bound OB–Rb present on endothelial cells induces phosphorylation of vascular endothelial growth factor receptor 2 (VEGF-R2). Activated VEGF-R2 signals through the p38 MAPK and Akt (alpha-serine/threonine protein kinase) pathways to induce proliferation, motility and angiogenesis [6]. |
| During normal pregnancy glomerular filtration rate and renal plasma flow increases 40% to 65% and 50% to 85%, respectively. In preeclampsia, renal plasma flow and glomerular filtration rate are at most only modestly decreased reduced GFR leads to decreased filtered load of uric acid, and serum uric acid concentrations are increased mainly as a consequence of reduced renal clearance. Kidneys account for 80% of the overall systemic removal of leptin from plasma. Placental stress occurs during pregnancy resulting in increased leptin levels to support the nutrient supply to the fetus [5]. Also, excessive maternal inflammation coupled with other placental factors may mediate excessive leptin expression in preeclamptic women. Although epidemiological studies have shown significant associations between leptin and preeclampsia, some studies have found no association after adjustment for maternal characteristics, including body mass index (BMI). Dysregulation of leptin metabolism and/or function in the placenta have been implicated in the pathogenesis of preeclampsia [2-5]. It has been reported that leptin levels decline during pregnancy late in third trimester and a rapid increase occurs during the same periods in the subjects who develop preeclampsia [4,5]. |
| Hence the present study was designed to analyze leptin levels in women with preeclampsia and compare them with normotensive pregnant and to assess its relationship with renal functions, gestational age, BMI and outcome of pregnancy in terms of mode of delivery, placental weight, Apgar score and birth weight of baby delivery. |
| Materials and Methods |
| The present study was conducted in the Department of Biochemistry in collaboration with Department of Obstetrics and Gynaecology, Pt. B.D. Sharma, PGIMS, Rohtak. Leptin levels were analyzed in blood of preeclamptic women and compared with those of normotensive pregnant women. An informed consent was taken from all the patients. Women with history of chronic hypertension, any metabolic disorder before or during pregnancy or presence of high risk factors like anemia, heart disease, diabetes, renal disease were excluded. |
| Fifty pregnant women attending the Outpatient department of Obstetrics and Gynaecology were enrolled and divided into two groups namely, Group I (control, n=25) comprising of normotensive pregnant women with singleton pregnancy at the time of delivery; and Group II (study, n=25) comprising of age and gestation matched preeclamptic women with singleton pregnancy and systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg with or without proteinuria at the time of delivery. |
| Five mL blood was drawn aseptically and serum was separated by centrifugation. Routine investigations (carried out enzymatically by autoanalyzer) and serum leptin levels were analyzed in maternal blood. Leptin was analysed by ELISA Kit (RayBio® Human Leptin ELISA Kit, Catalog #: ELH-Leptin) [7]. SPSS 20, unpaired‘t’ test and two-tailed Pearson correlation test between variables was applied. |
| Results |
| Table 1 depicts the clinical characteristics of women in both the groups. |
| The mean age of pregnant women ranged from 19-37 years (25.68 ± 4.46 in group I 25.48 ± 3.96 years in group II). Mean gestational age of group I and group II at time of delivery was 37.86 ± 0.99 and 37.89 ± 0.93 weeks respectively. Eleven women were primipara in normotensive group (44%) as compared to 14 in hypertensive group II (60%). |
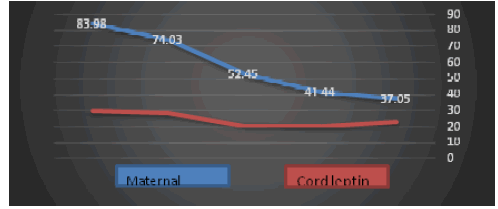
| Serum leptin levels were significantly higher in preeclamptics as compared to normotensive pregnant women (p<0.001). Serum leptin levels increased with rise in proteinuria in preeclamptic women. The leptin levels increased with rise in proteinuria from traces to 4+. Two cases had proteinuria in traces with mean leptin levels of 37.05 ± 3.47 ng/mL; nine had 1+ proteinuria with mean leptin levels of 41.44 ± 4.63 ng/mL; four had 2+ proteinuria with leptin levels 52.45 ± 11.29 ng/mL; six had 3+proteinuria with leptin levels of 74.03 ± 4.07 ng/mL and four had leptin levels of 83.98 ± 1.96 ng/mL. |
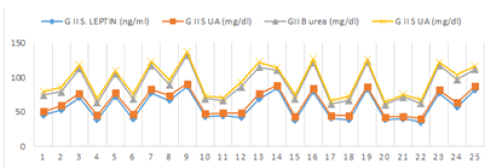
| Leptin had a strong positive correlation with blood pressure in preeclamptics. There was non-significant positive correlation between serum leptin and blood pressure levels in group I. Systolic blood pressure in preeclamptic group showed a significant positive correlation with serum leptin (r=0.642, p<0.01, Figure 1) and normotensive group was also positively correlated with serum leptin levels (r=0.026, p>0.05), however, it was not statistically significant. Diastolic blood pressure was negatively correlated with serum leptin levels in normotensive mothers (r=-0.053, p>0.05) which was reversed in preeclamptic women (r=0.271, p<0.05, Figure 1). |
| Gestational age, placental weight, baby weight and Apgar score (at 1 min and 5 min) had a positive correlation with leptin levels in normotensive mothers (r=-0.172, 0.168, 0.226, 0.009 and 0.076 respectively, p>0.05 at all levels). On the other hand, it was negative in preeclamptics (r=-0.291,-0.277,-0.356 and -0.203 respectively, p>0.05 at all levels), except for gestational age which was positive (r= 0.048, p>0.05). |
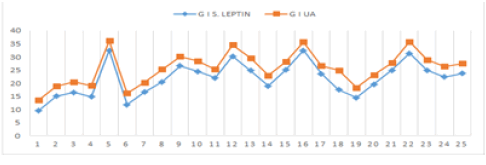
| In normotensive pregnant women a non-significant correlation was observed between serum leptin and urea levels (r=0.148, p>0.05). Serum leptin levels were negatively correlated with serum uric acid levels in normotensive pregnant women which was reversed in preeclamptics (r=0.866, p<0.001, Figures 2-3b). Serum leptin levels were positively correlated with urea levels in preeclamptic women (r=0.563, p<0.05). |
| Discussion |
| In the present study, leptin showed a strong positive correlation with blood pressure in preeclamptics that was non-significant in group I. Similar positive correlation has also been reported by Iftikhar et al between serum leptin and systolic and diastolic blood pressure in preeclamptic group as compared to control [8]. |
| In preeclamptics, significant correlation was observed between serum leptin and BMI (r=0.5, p<0.01 vs. r=0.08, p NS) for preeclamptics and normotensive pregnant women respectively. Leptin levels in preeclamptics were independently related to maternal body mass index (BMI) in few studies. Highman et al failed to observe a relationship during normal pregnancy. This may be due to weight gain during fat deposition. However, it may be appreciated here that weight gain during pregnancy is solely not due to fat deposition [9]. In pregnancy, maternal plasma leptin concentrations are elevated, and lack the well-established correlation with body fat energy stores that are observed in non-pregnant women, indicating an alternative function for leptin during pregnancy and fetal development. Maternal and fetal plasma leptin levels are dysregulated in pathological conditions such as gestational diabetes, pre-eclampsia and intrauterine growth retardation, representing an effect or a cause of disturbances in the feto-placenta-maternal unit [10,11]. Hypoxia is involved in the regulation of leptin expression, and may therefore contribute to elevated plasma leptin levels in pre-eclampsia [9]. Elevated pro-inflammatory activity in preeclampsia promotes augmented leptin production, and proinflammatory cytokines (e.g. interleukin-1, interleukin-6) are involved in pathophysiology of preeclampsia and stimulatory effects of interleukin-1 and interleukin-6 on leptin production have been observed, suggesting that [9]. |
| The present study also determined serum leptin levels according to the level of proteinuria. It also showed a rise in serum leptin levels with level of proteinuria. Urea in preeclamptic group showed positive correlation (r=0.563, p<0.05) when compared to normotensive group (r=0.148, p>0.05). Uric acid showed significant positive correlation with leptin in preeclamptic group (r=0.866; p<0.001), while it was negative in normotensive mothers (r=0.221, p>0.05). Other studies have also reported increased leptin levels with proteinuria in preeclampsia [8]. The kidney is directly involved in leptin clearance and the rate of leptin clearance from serum determines leptin levels. Studies have reported a leptin gene in the kidneys in rats. Studies on arterial-venous differences in leptin concentrations in humans suggest a major role of kidney in leptin elimination. Serum leptin levels have been shown to be elevated in uraemia depending on catabolic defect [10]. |
| There could be several possible causes for elevated leptin levels. Impaired renal function is a pathophysiological component of preeclampsia. The measured increase in plasma leptin concentration may reflect reduced renal clearance in preeclamptics [10]. Also, high leptin levels may be due to the possible hem concentration in preeclampsia caused by association of preeclampsia with reduced plasma volume [11]. However, this was not measured in the present study. |
| Leptin has also been shown to serve as a cofactor of TGF-beta activation, promote renal endothelial cell proliferation, and potentially may play a role in renal glomerulosclerosis [12,13]. Recent studies have reported that infusion of recombinant leptin into normal rats for 3 weeks fosters the development of focal glomerulosclerosis [11]. Also, leptin is also reported to be related to insulin resistance [14] and high C-reactive protein levels,15 both of which have been shown to be related to chronic kidney disease [15-17]. |
| Mean birth weight in group I and group II was 2.59 ± 0.46 kg and 2.48 ± 0.53 kg respectively. Majority of the babies had birth weight between 2.1-3.5 kg in 92% of normotensive pregnant and 76% in mothers with preeclampsia. 20% babies of hypertensive mother had birth weight between 1.6-2.0 kg, whereas, no babies in control group had birth weight<1.5 kg. Thus, birth weight was less in preeclamptics as compared to normotensive women though it was not statistically significant (p>0.05). The mean placental weight was comparable in both the groups (488 ± 50.58 g vs. 468 ± 59.30 g respectively). |
| Placental leptin expression patterns have been reported to coincide with maternal serum leptin levels. Gene and protein sequence of placental and adipose tissue leptin are identical but gene regulation in placenta is under a tissue specific enhancer suggesting independent and differential expression from adipose tissue [18-20]. Placental leptin mRNA and protein in humans are localized in syncytiotrophoblast and villous vascular endothelial cells [21]. Thus, placenta acts to balance maternal–fetal leptin synthesis, and can be influenced by both placental expressed leptin and circulating maternal leptin [22]. During pregnancy, there is increase in both circulating leptin and soluble leptin receptors. The higher concentrations of leptin in preeclamptics are consistent with reports showing up regulation of leptin mRNA expression in preeclamptic placentae [23]. Also, increased leptin expression in cultured human trophoblasts cell line under hypoxic conditions have been reported by the same group and increase in leptin concentration in preeclampsia could be due to placental ischemia. This could also be due to adaptive response of fetoplacental unit to impaired placental perfusion to meet the energy requirements of the fetus. Also during preeclampsia there is evidence of increased inflammatory mediators which increase the plasma leptin concentration. Placental pathological and pathophysiological changes occur in preeclampsia and it can be recommended that analysis of the biological activity of the products released by the placenta should be done which could provide a clue to the understanding placental response or adaptation. |
| Strong association between plasma leptin levels and blood pressure have been reported and possibly leptin released from placenta stimulates central receptors that regulate blood and/or heart. Strong association between leptin and uric acid levels in preeclamptics have been reported in literature [24]. Strong relationship between leptin and uric acid was noted in the present study (r=0.866, p<0.001, Figure 2). Since both leptin and uric acid seemed to be markers of preeclampsia, this strong relationship could be a simple reflection of this. In nonpregnant diabetics, leptin has been related to urate concentrations is dependent of BMI and blood pressure and they may affect the circulating levels of each other [25]. Also, leptin may contribute to increased uric acid levels as blood uric acid levels are increased by oxidative stress and leptin has been reported to increase oxidative stress in human endothelial cell cultures [26,27] suggesting its possible role in development of preeclampsia. |
| Leptin is an important physiological regulator of fetal growth and altered in pathological state of pregnancy such as diabetes and preeclampsia. Findings of present study suggest that leptin is associated with rise in blood pressure and adverse pregnancy outcome in preeclampsia. Alterations in maternal-placental-fetal leptin exchange may modify the development of the fetus and contribute to the increased risk of developing disease in adulthood including cardiovascular, metabolic, and renal disease. Mechanisms responsible for this increase and role played by leptin in the development of preeclampsia require further study. |
References
- Granger JP, Alexander BT, Llinas MT, Bennett WA, Khali RA (2001) Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension 38: 718-722.
- Christou H, Serdy S, Mantzoros CS (2002) Leptin in relation to growth and developmental processes in the fetus.SeminReprod Med 20: 123-130.
- Nakatsukasa H, Masuyama H, Takamoto N, Hiramatsu Y (2008) Circulating leptin and angiogenic factors in preeclampsia patients.Endocr J 55: 565-573.
- Laivuori H, Gallaher MJ, Collura L, Crombleholme WR, Markovic N, et al (2006) Relationships between maternal plasma leptin, placental leptin mRNA and protein in normal pregnancy, pre-eclampsia and intrauterine growth restriction without pre-eclampsia. Mol Hum Reprod 12: 551-556.
- Taylor BD, Ness RB, Olsen J, Hougaard DM, Skogstrand K, et al. (2015) Serum leptin measured in early pregnancy is higher in women with preeclampsia compared with normotensive pregnant women.Hypertension 65: 594-599.
- Garonna E, Botham KM, Birdsey GM, Randi AM, Gonzalez-Perez RR (2011) Vascular endothelial growth factor receptor-2 couples cyclo-oxygenase-2 with pro-angiogenic actions of leptin on human endothelial cells. PLoS One 6: e18823.
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, et al. (1996) Serum immunoreactive-leptin concentrations in normal-weight and obese humans.N Engl J Med 334: 292-295.
- Iftikhar U, Khoja A, Mehjabeen, Iqbal A, Karira KA (2008) Evaluation of serum leptin levels during normal pregnancy and in pre-eclampsia.J Ayub Med Coll Abbottabad 20: 137-140.
- Highman TJ, Friedman JE, Huston LP, Wong WW, Catalano PM (1998) Longitudinal changes in maternal serum leptin concentrations, body composition, and resting metabolic rate in pregnancy.Am J ObstetGynecol 178: 1010-1015.
- Cumin F, Baum HP, Levens N (1996) Leptin is cleared from the circulation primarily by the kidney.Int J ObesRelatMetabDisord 20: 1120-1126.
- Wolf G, Hamann A, Han DC (1999) Leptin stimulates proliferation and TGF-? expression in renal glomerular endothelial cells: potential role in glomerulosclerosis. Kidney International 56: 860-872.
- Wolf G, Chen S, Han DC, Ziyadeh FN (2002) Leptin and renal disease.Am J Kidney Dis 39: 1-11.
- Wolf G, Ziyadeh FN (2006) Leptin and renal fibrosis.ContribNephrol 151: 175-183.
- Zimmet PZ, Collins VR, de Court MP (1998) Is there a relationship between leptin and insulin sensitivity independent of obesity? A population-based study in the Indian Ocean nation of Mauritius. International Journal of Obesity and Related Metabolic Disorders 22: 171-177.
- Shamsuzzaman AS, Winnicki M, Wolk R, Svatikova A, Phillips BG, et al. (2004) Independent association between plasma leptin and C-reactive protein in healthy humans.Circulation 109: 2181-2185.
- Chen J, Muntner P, Hamm LL, Fonseca V, Batuman V, et al. (2003) Insulin resistance and risk of chronic kidney disease in nondiabetic US adults.J Am SocNephrol 14: 469-477.
- Fox ER, Benjamin EJ, Sarpong DF, Nagarajarao H, Taylor JK, et al. (2010) The relation of C--reactive protein to chronic kidney disease in African Americans: the Jackson Heart Study.BMC Nephrol 11: 1.
- Lepercq J, Challier JC, Guerre-Millo M, Cauzac M, Vidal H, et al. (2001) Prenatal leptin production: evidence that fetal adipose tissue produces leptin. J ClinEndocrinolMetab 86: 2409-2413.
- Hauguel-de Mouzon S, Lepercq J, Catalano P (2006) The known and unknown of leptin in pregnancy.Am J ObstetGynecol 194: 1537-1545.
- Bi S, Gavrilova O, Gong DW, Mason MM, Reitman M (1997) Identification of a placental enhancer for the human leptin gene.J BiolChem 272: 30583-30588.
- Lea RG, Howe D, Hannah LT, Bonneau O, Hunter L, et al. (2000) Placental leptin in normal, diabetic and fetal growth-retarded pregnancies.Mol Hum Reprod 6: 763-769.
- Challier J, Galtier M, Bintein T, Cortez A, Lepercq J, et al. (2003) Placental leptin receptor isoforms in normal and pathological pregnancies.Placenta 24: 92-99.
- Mise H, Sagawa N, Matsumoto T, Yura S, Nanno H, et al. (1998) Augmented placental production of leptin in preeclampsia: possible involvement of placental hypoxia.J ClinEndocrinolMetab 83: 3225-3229.
- Anim N, Sooranna SR, Steer PJ, Johnson MR (2000) Longitudinal analysis of maternal leptin concentration during normal pregnancy and preeclampsia. Hum Reprod 15: 2033-2036.
- Fruehwald-Schultes B, Peters A, Kern W, Beyer J, Pfützner A (1999) Serum leptin is associated with serum uric acid concentrations in humans.Metabolism 48: 677-680.
- Bouloumie A, Marumo T, Lafontan M, Busse R (1999) Leptin induces oxidative stress in human endothelial cells.FASEB J 13: 1231-1238.
- Tessier DR, Ferraro ZM, Gruslin A (2013) Role of leptin in pregnancy: consequences of maternal obesity. Placenta 34: 205-211.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3a | Figure 3b |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 10608
- [From(publication date):
February-2016 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 9645
- PDF downloads : 963
