Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Clinical images Open Access
Massive Gas-forming Gangrene in a Diabetic Foot Infection
| Shigeo Kono1*, Reiko Nakagawachi1, Jun Arata2 and Benjamin A Lipsky3 | ||
| 1WHO-collaborating Centre for Diabetes, National Hospital Organization, Kyoto Medical Center, Kyoto, Japan | ||
| 2Department of Plastic Surgery, National Hospital Organization, Kyoto Medical Center, Kyoto, Japan | ||
| 3University of Washington, Seattle, Washington, USA , University of Geneva, Switzerland, and University of Oxford, Oxford, UK | ||
| Corresponding Author : | Dr. Shigeo Kono MD, PhD WHO-collaborating Centre for Diabetes Kyoto Medical Center, Fukakusamukaihatacho Fushimiku, Kyoto, Japan Tel: +81-75-641-9161 E-mail: skono@kyotolan.hosp.go.jp |
|
| Received July 24, 2014; Accepted July 27, 2014; Published July 31, 2014 | ||
| Citation: Kono S, Nakagawachi R, Arata J, Lipsky BA (2014) Massive Gasforming Gangrene in a Diabetic Foot Infection. Clin Res Foot Ankle 2:i101. doi:10.4172/2329-910X.1000i101 | ||
| Copyright: © 2014 Kono S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Clinical Research on Foot & Ankle
| Clinical Image | |
| This 63-year-old woman with type 1 diabetes on hemodialysis burned her heel three months previously. Despite surgical debridement and topical silver sulfadiazine the wound worsened, with the development of purulent secretions and fever to 39°C, leukocytosis (35,900/mL), an elevated c-reactive protein (420 mg/L), but with a low creatine kinase (20 IU/L). Repeated aerobic and anaerobic cultures of wound tissue grew only Streptococcus agalactiae, Staphylococcus aureus and corynebacteria (unspeciated). She was treated with intravenous meropenem and vancomycin, but infection progressed and she was transferred to our hospital. | |
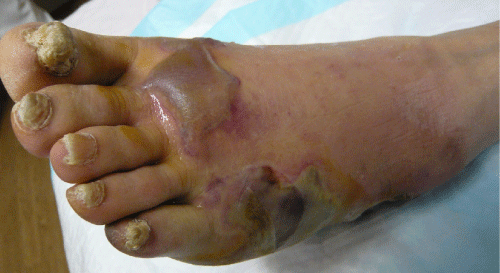
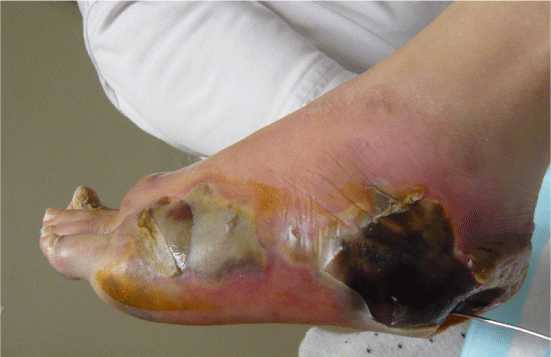
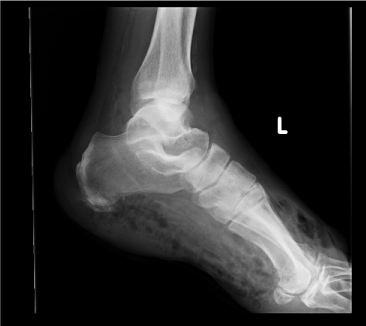
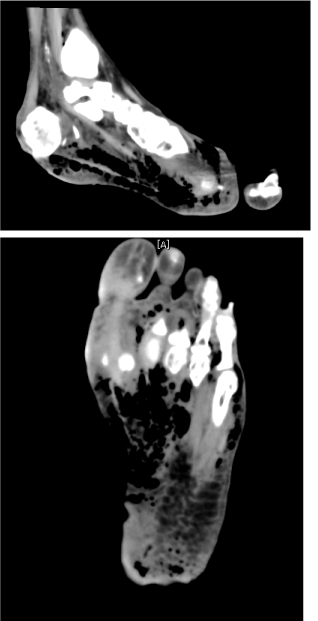
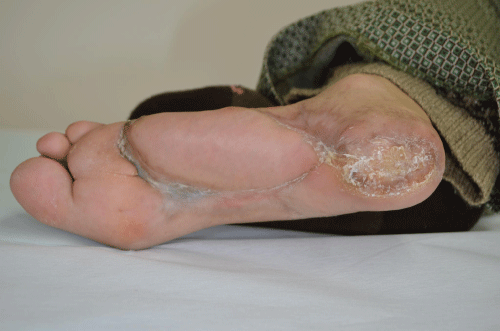
| Examination of her foot revealed erythema, warmth, purulence, crepitus, and wet gangrene (Figures 1 and 2). Examination of the affected foot revealed sensory peripheral neuropathy but no circulatory impairment. Plain radiographs showed massive gas in the entire foot (Figure 3). Computed tomography confirmed extensive gas, making the plantar aponeurosis clearly visible (Figure 4). | |
| She underwent immediate aggressive operative wound debridement, and then repeated bedside debridement. Repeat bacterial cultures of wound tissue grew only Streptococcus agalactiae and coagulasenegative Staphylococcus. Clindamycin was added to the meropenem and vancomycin therapy. Over 6 months her infection resolved and the foot healed without amputation (Figure 5). | |
| Gas-forming gangrene is an uncommon, but potentially catastrophic, clinical presentation of diabetic foot infection. It is often misdiagnosed as clostridial gas gangrene, leading to rapid, but potentially unnecessary amputation before definitive diagnosis. While gas-forming infections may be caused by Clostridium species, those in the diabetic foot are more often associated with mixed aerobic (and sometimes anaerobic bacteria) and usually have a more gradual progression and better prognosis. Successful treatment with limb preservation requires rapid and accurate clinical and microbiological diagnosis, urgent surgical debridement, appropriate antibiotic therapy and sometimes hyperbaric oxygen therapy. In our patient treatment with extensive debridement and clindamycin, which suppresses staphylococcal and streptococcal toxin production, likely helped salvage her foot. | |
| Financial support and disclosure | |
| None of the authors have any conflicts of interests or financial disclosures relevant to this work. | |
| References | |
References
- Bessman AN, Wagner W (1975) Nonclostridial gas gangrene. Report of 48 cases and review of the literature. See comment in PubMed Commons below JAMA 233: 958-963.
- Brucato MP, Patel K, Mgbako O2 (2014) Diagnosis of gas gangrene: does a discrepancy exist between the published data and practice. See comment in PubMed Commons below J Foot Ankle Surg 53: 137-140.
- Finkelstein B, Kamble R, Ferdinando E, Mobarakai N (2003) Autoamputation of the foot caused by untreated gas gangrene: a case report. See comment in PubMed Commons below J Foot Ankle Surg 42: 366-370.
- Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, et al. (2012) Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis: e132-e173
- Russell NE, Pachorek RE (2000) Clindamycin in the treatment of streptococcal and staphylococcal toxic shock syndromes. See comment in PubMed Commons below Ann Pharmacother 34: 936-939.
Figures at a glance
 |
 |
 |
 |
 |
||||
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Post your comment
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 22549
- [From(publication date):
October-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 17762
- PDF downloads : 4787
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
Journals by Subject
Clinical & Medical Journals
International Conferences 2025-26
