Research Article Open Access
Mass Cultivation from a Korean Raceway Pond System of IndigenousMicroalgae as Potential Biofuel Feedstock
Hong JW1,2, Kim OH1, Kim H1, Jo SW3, Cho HW2 and Yoon HS1-3*
1Advanced Bio-resource Research Center, Kyungpook National University, Daegu 41566, South Korea
2Department of Biology, Kyungpook National University, Daegu 41566, South Korea
3Department of Energy Science, Kyungpook National University, Daegu 41566, South Korea
- *Corresponding Author:
- Yoon HS
Advanced Bio-resource Research Center
Department of Biology
Department of Energy Science
Kyungpook National University, South Korea
Tel: +82 539505348
E-mail: hsy@knu.ac.kr
Received date: December 14, 2015; Accepted date: December 28, 2015; Published date: January 28, 2016
Citation: Hong JW, Kim OH, Kim H, Jo SW, Cho HW, et al. (2016) Mass Cultivation from a Korean Raceway Pond System of Indigenous Microalgae as Potential Biofuel Feedstock. Oil Gas Res 2:108. doi: 10.4172/2472-0518.1000108
Copyright: © 2016 Hong JW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Oil & Gas Research
Abstract
Naturally occurring freshwater microalgae were mass cultivated in continuous mode at a large-scale facility. From June 2014 to August 2015, biomass productivity, lipid content, and calorific value data were obtained from two 675.0 m2 raceway ponds. The collected biomass had an overall average productivity of approximately 7.0 g dry weight/m2/day and a lipid content of 12.2%. Ultimate analysis incorporated with thermal analysis indicated that the average calorific value was 17.5 MJ/kg. The dominant genera found were Chlorella, Coelastrella, Acutodesmus, and Pseudopediastrum. This pilot-scale study demonstrated the potential of microalgal biomass produced on a largescale as a biofuel under Korean geoclimatic conditions.
Keywords
Biofuel; Calorific value; Elemental analysis; Mass cultivation; Microalgae
Introduction
The world’s demand for renewable and sustainable energy resources is increasing exponentially because of changes in climate and energy shortage problems. This situation has given rise to the development of numerous new technologies such as biomass, geothermal, solar, tidal, and wind energies. Among these resources, microalgae are now considered one of the most attractive candidates for biofuel production due to their higher photosynthetic efficiency and oil yield compared to terrestrial sources [1,2]. A number of microalgae strains with desired characteristics for biofuel production have been found and/ or developed [3,4]. However, the attempts to grow these microalgae in outdoor open pond systems have not always been successful due to rapid contamination with bacteria, predatory zooplanktons, and other algal species [5,6]. To overcome these challenges, indigenous microalgae strains have been cultivated for large scale production since these endemic wild types are well adapted to their local conditions and, therefore, they are able to outcompete other indigenous algal strains [7-9]. In this study, biomass productivity and characterization data obtained from commercial-scale microalgal cultivation from outdoor raceways over 1.25 years are presented.
Materials and Methods
Raceway (RW) pond system
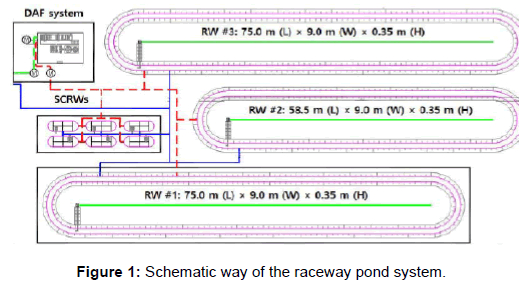
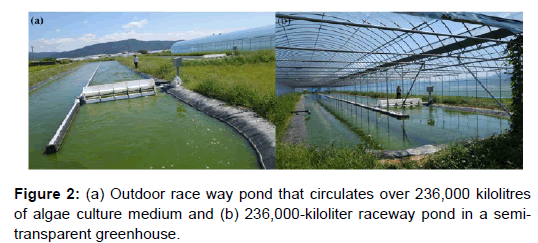
A freshwater microalgal cultivation system was constructed in August 2013 at the Chilgok-gun agricultural Technology Center (36°02’N,128°22’E); Dongan-ri, Yakmok-myeon, Chilgok-gun, Gyeongsangbukdo, South Korea). Two identical 675.0 m2 open raceway ponds (RW #1 and #3) were constructed (Figures 1 and 2). A semitransparent film cover was added to RW #1 to compare the RWs in terms of biomass yield and consumption of resources under unfavorable Korean weather conditions (monsoon and winter seasons). A 526.5 m2 RW (uncovered, RW #2) also was built as an auxiliary pond for seed culture cultivation and harvest purposes. A coagulation-flotation system was added to the facility for large-scale microalgae harvesting.
Cultivation of microalgae
Initially, indigenous Coelastrella grown in small-scale raceways was inoculated into the 675.0 m2 RWs and species succession was monitored both microscopically and molecularly. Commercial water soluble fertilizers, Eco-Sol (N-P-K: 25-9-18, Dongbu Farm Hannong, Ulsan, South Korea) and monopotassium phosphate (N-P-K: 0-52-34, Sang Rok Chemical, Daegu, South Korea), were added to each RW for a final concentration of 15.0-30.0 mg total nitrogen (TN)/L and 3.0-6.0 mg total phosphorus (TP)/L. A continuous mode of cultivation was carried out at a velocity of 25.0-30.0 cm/sec approximately two thirds of the algal culture was harvested from the pond and replaced with the same volume of underground water. The remaining culture was used as inoculum and appropriate amounts of nutrients were added. From October 2014 on, approximately 10.0 L/min carbon dioxide (CO2) was aerated into the RWs through Venturi tubes to enhance biomass productivity.
Microalgal culture monitoring
The microalga cultures were sampled every three days and inspected at 1000X magnification on a Nikon Eclipse E100 Biological Microscope (Tokyo, Japan). In addition, optical density at 680 nm on an X-ma 1200 V spectrophotometer (Human, Seoul, South Korea) and dry mass were measured. Representative species of each season were axenically isolated and added to our laboratory culture collection. Molecular identification was carried out using NS1/NS8 and ITS1/ITS4 primer sets [10]. Consumption of TN and TP (Tables 1-4) was analyzed by using HS-TN(CA)-L and HS-TP-L water test kits (Humas, Daejeon, South Korea). In addition, dissolved oxygen, pH, and temperature in the RWs (Tables 2-4) were monitored every three hours with an automatic water quality meter (WQC-24, DKK-TOA, Tokyo, Japan).
| Microalga | Marker gene | Accession No. | Length (bp) | Closest match (GenBank accession No.) | Overlap (%) | Sequence similarity (%) | Taxonomic affinity |
|---|---|---|---|---|---|---|---|
| KNUA036 | 18S rRNA | KT883906 | 1771 | Micractinium sp. KNUA034 (KM243325) | 100 | 99 | Micractinium sp. |
| ITS | KT883910 | 721 | Micractinium sp. KNUA034 (KM243327) | 100 | 99 | Chlorella sp.a | |
| KNUA037 | 18S rRNA | KT883907 | 1768 | Coelastrella sp. SAG 2471 (KM020087) | 99 | 99 | Coelastrella sp. |
| ITS | KT883911 | 699 | Chlamydomonas moewusii (JX290025)b | 100 | 99 | - | |
| KNUA038 | 18S rRNA | KT883908 | 1767 | Acutodesmusobliquus GS3e (AB917118) | 99 | 100 | Acutodesmus sp. |
| ITS | KT883912 | 695 | Acutodesmus nygaardii CCAP 276/50 (JQ082320) | 100 | 99 | Acutodesmus sp. | |
| KNUA039 | 18S rRNA | KT883909 | 1765 | Pseudopediastrum integrum Mj2008/86 (HM021309) | 98 | 99 | Pseudopediastrum sp. |
| ITS | KT883913 | 707 | Pseudopediastrum integrum Mj2008/86 (HM021309) | 97 | 99 | Pseudopediastrum sp. |
aThe key compensatory base changes in the ITS2 secondary structure confirmed that strain KNUA036 belonged to the genus Chlorella (data not shown).
bThe second closest match was Coelastrella sp. shy-188 (AB762691).
Table 1: Results from BLAST searches using the 18S rRNA and ITS sequences of the dominant microalgae from the raceway ponds.
| Season | Month | Productivity (g DW/m2/day) | Lipid (%) | CV (MJ/kg) | Avg. Temp. (°C) | Nutrient consumption (mg/kg) | |
|---|---|---|---|---|---|---|---|
| TN | TP | ||||||
| Summer | June | 7.1 | 7.5 | 13.2 | 25.5 | 8.4 | 4.2 |
| 2014 | July | 5.4 | 5.4 | 8.5 | 28.1 | 8.6 | 3.3 |
| August | 4 | 6.8 | 11.9 | 25.9 | 6.9 | 3.2 | |
| Avg. | 5.5 | 6.6 | 11.2 | 26.5 | 7.9 | 3.5 | |
| Autumn | September | 1.1 | 6.8 | 10.7 | 25.9 | 9.5 | 4.5 |
| 2014 | October | 2.6 | 11.5 | 17.1 | 16.4 | 8.1 | 1.4 |
| November | 3.4 | 13.9 | 20.1 | 13.7 | 10.7 | 1.4 | |
| Avg. | 2.4 | 10.7 | 16 | 18.7 | 9.4 | 2.4 | |
| Winter | December | 3.6 | 17 | 21.9 | 7.3 | 7.8 | 1 |
| 2014-15 | January | 4.2 | 16.1 | 21.1 | 9 | 7.5 | 1 |
| February | 3.8 | 13.6 | 23.1 | 12.2 | 25.9 | 2.9 | |
| Avg. | 3.9 | 15.5 | 22 | 9.5 | 13.7 | 1.6 | |
| Spring | March | 4.3 | 16.1 | 23 | 14.7 | 29.5 | 4.8 |
| 2015 | April | 9.8 | 14.5 | 20.3 | 19.4 | 15.2 | 1.4 |
| May | 10.7 | 13.2 | 17.8 | 21.5 | 11.1 | 1.5 | |
| Avg. | 8.2 | 14.6 | 20.4 | 18.5 | 18.6 | 2.6 | |
| Summer 2015 | June | 10.3 | 16.5 | 19.4 | 24.9 | 13.9 | 2.3 |
| July | 8.8 | 14 | 19.3 | 28.6 | 14.9 | 2.4 | |
| August | 7.8 | 14.7 | 20.7 | 27 | 19.1 | 1.6 | |
| Avg. | 9 | 15.1 | 19.8 | 26.8 | 16 | 2.1 | |
| Overall average | 5.9 | 12.6 | 18 | 19.8 | 13.4 | 2.5 | |
Table 2: Microalgal cultivation results from RW#1.
| Seasona | Month | Productivity (g DW/m2/day) | Lipid (%) | CV (MJ/kg) | Avg. Temp. (°C) |
|---|---|---|---|---|---|
| Summer | June | 6 | 6.6 | 12.7 | 25.1 |
| 2014 | July | 8.7 | 5.8 | 9.1 | 28.9 |
| August | 4.5 | 6.6 | 10.6 | 26.3 | |
| Avg. | 6.4 | 6.3 | 10.8 | 26.7 | |
| Autumn | September | 8.9 | 6.5 | 10.4 | 24.2 |
| 2014 | October | 10 | 7 | 11 | 16.9 |
| November | 4.5 | 12.2 | 17.6 | 7.7 | |
| Avg. | 7.8 | 8.6 | 13 | 16.3 | |
| Spring | March | 7.8 | 13 | 20.7 | 13.9 |
| 2015 | April | 8.5 | 13.6 | 19.4 | 18.5 |
| May | 10.3 | 13.3 | 18.8 | 23.5 | |
| Avg. | 8.9 | 13.3 | 19.6 | 18.6 | |
| Summer | June | 15.1 | 15.8 | 19.4 | 25.7 |
| 2015 | July | 10.4 | 13.9 | 19.1 | 27.4 |
| August | 17.1 | 14.4 | 18.6 | 28 | |
| Avg. | 14.2 | 14.7 | 19 | 27 | |
| Overall average | 9.2 | 10.8 | 15.8 | 21.8 | |
Table 3: Microalgal cultivation results from RW#3.
| Season | Month | Productivity (g DW/m2/day) | Lipid (%) | CV (MJ/kg) | Avg. Temp. (°C) | Nutrient consumption (mg/kg) | |
|---|---|---|---|---|---|---|---|
| TN | TP | ||||||
| Summer 2014 | June | 6.5 | 7.1 | 12.9 | 25.3 | 7.6 | 4.3 |
| July | 7 | 5.6 | 8.8 | 28.5 | 7.4 | 3.4 | |
| August | 4.2 | 6.7 | 11.2 | 26.1 | 8.3 | 3.2 | |
| Avg. | 5.9 | 6.5 | 11 | 26.6 | 7.8 | 3.6 | |
| Autumn 2014 | September | 5 | 6.7 | 10.6 | 25.1 | 9.1 | 4.1 |
| October | 6.3 | 9.3 | 14 | 16.6 | 9.1 | 2.1 | |
| November | 3.9 | 13 | 18.8 | 10.7 | 13.1 | 1.5 | |
| Avg. | 5.1 | 9.7 | 14.5 | 17.5 | 10.4 | 2.6 | |
| Winter 2014-15 | December | 3.6 | 17 | 21.9 | 7.3 | 7.8 | 1 |
| January | 4.2 | 16.1 | 21.1 | 9 | 7.5 | 1 | |
| February | 3.8 | 13.6 | 23.1 | 12.2 | 25.9 | 2.9 | |
| Avg. | 3.9 | 15.5 | 22 | 9.5 | 13.7 | 1.6 | |
| Spring 2015 | March | 6 | 14.5 | 21.8 | 14.3 | 22.7 | 3.8 |
| April | 9.1 | 14.1 | 19.8 | 19 | 14.6 | 2 | |
| May | 10.5 | 13.2 | 18.3 | 22.5 | 13.2 | 2.1 | |
| Avg. | 8.6 | 13.9 | 20 | 18.6 | 16.9 | 2.6 | |
| Summer 2015 | June | 12.7 | 16.2 | 19.4 | 25.3 | 14.3 | 2.5 |
| July | 9.6 | 14 | 19.2 | 28 | 14.4 | 2.3 | |
| August | 12.5 | 14.5 | 19.7 | 27.5 | 18.1 | 1.8 | |
| Avg. | 11.6 | 14.9 | 19.4 | 26.9 | 15.6 | 2.2 | |
| Overall average | 7 | 12.2 | 17.5 | 19.6 | 13.1 | 2.5 | |
Table 4: Average cultivation results from RW#1 and RW#3 (from June 2014 to August 2015).
Microalgal biomass harvest
When the microalgal culture reached late exponential or early stationary phase, biomass was harvested by chemical coagulation with 17% polyaluminum chloride (Kumsung E and C, Ansan, South Korea) followed by dissolved air flotation (Dongshin enTech, Yangsan, South Korea). The harvested biomass was stored at -20°C until utilization.
Characterization of microalgal biomass
The biomass samples were freeze-dried, pulverized with a mortar and pestle, and sieved through ASTM No. 230 mesh (opening=63 μm). Total lipid content was determined by the sulfo-phosphovanillin colorimetric method [11]. Ultimate analysis was conducted to determine the carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) contents using a Flash 2000 elemental analyzer (Thermo Fisher Scientific, Milan, Italy). Proximate analysis was carried out to measure ash content using a DTG-60A thermal analyzer (Shimadzu, Kyoto, Japan). The oxygen (O) content was calculated by subtracting the ash and CHNS contents from the total and gross calorific value (GCV, hereafter CV) was estimated by the following equation developed by Given et al. [12]: CV=0.3278C+1.419H+0.09257S-0.1379O+0.637 (MJ/kg).
Results and Discussion
Dominant microalgal species
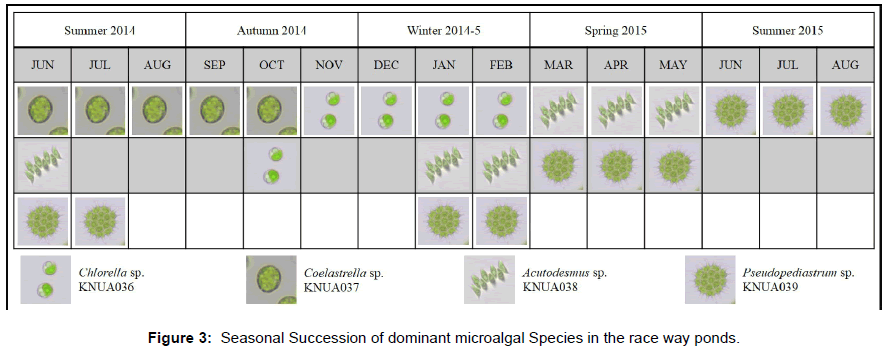
In this study, various species were allowed to dominate naturally and reach their stable states to avoid competition and increase productivity [13,14]. Since South Korea experiences four distinct seasons, the changes in the dominant species could be attributed to the daylight time and temperature variations of each season. As presented in Figure 3, Coelastrella dominated in the summer of 2014, Chlorella and Acutodesmus were most common in the winter and spring of 2014, and then Pseudopediastrum became dominant in the summer of 2015. It seemed that the changes in CO2 allowed for the Pseudopediastrum to outcompete the Coelastrella.
Identification of dominant microalgae
The dominant microalgae were isolated and named as strains KNUA036, 037, 038, and 039. All the isolates were further identified by small subunit 18S ribosomal RNA (rRNA) and internal transcribed spacer (ITS) sequence data analyses (Table 1). For strain KNUA036, molecular characterization by 18S rRNA and ITS sequences indicated that the isolate belonged to the genus Micractinium, but the key compensatory base changes (CBS) in the ITS2 secondary structure confirmed that the isolate was a member of the genus Chlorella [15]. Due to its extremely simple morphology, no conclusion could be drawn by the morphological features of strain KNUA036. Strain KNUA037 was identified as a member of the genus Coelastrella by 18S rRNA and morphological characterization results. Even though its closet match inferred from the ITS sequence was Chlamydomonas moewusii, strain KNUA037 did not have any common features of the genus Chlamydomonas such as an eyespot and flagella. This may be due to the lack of sequence data in GenBank for Coelastrella ITS genes, so no identification could be made with this. It was found that strains KNUA038 and 039 belonged to the genera Acutodesmus and Pseudopediastrum, respectively. Their molecular and morphological identification results were in agreement. DNA sequences obtained in this study were deposited in the database of the National Center for Biotechnology Information (NCBI) under accession numbers KT883906-KT883913 (Table 1). Numerous studies have shown that Chlorella and Scenedesmus (some of them have recently been reclassified as Acutodesmus [16] are able to synthesize C14:0, C16:0, C18:1, C18:2, and C18:3 fatty acids, which can be used as biodiesel [17]. Therefore, strains KNUA036 and 038 could have potential to serve as a biodiesel feedstock. Not much is known about the possibility of deriving biofuels from Coelastrella and Pseudopediastrum. Further researches using the pure cultures of these dominant microalgae should be followed.
Microalgal productivity and biomass characterization
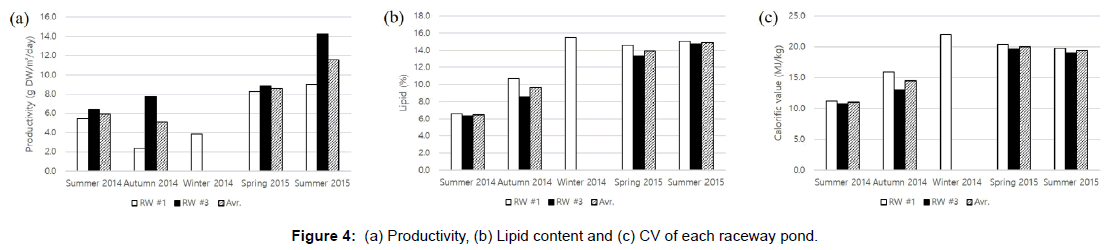
From June 2014 to August 2015, the microalgal biomass had an overall average productivity of approximately 7.0 g dry weight (DW)/ m2/day, a lipid content of 12.2%, and a CV of 17.5 MJ/kg (Table 4). Yearround cultivation, including during the winter season, was possible only in RW #1. The average biomass productivity of RW #3 was higher than that of RW #1. Nonetheless, the average lipid content and CV of RW #1 were slightly higher than those of RW #3 (Figure 4 and Table 4). This is probably due to the growth phases at the time of harvest [18]. In many cases, more actively growing cells were harvested in RW #3 compared to RW #1 to avoid biomass reduction and contamination since aging algal cultures tend to enter the death phase quickly. The highest monthly productivity was achieved in RW #3 in August 2015, but the highest monthly lipid content and CV were found in RW #1 in December 2014 and in RW #1 in February 2015, respectively. The long rainy weather in August 2014 led to substantial reductions in biomass productivity in both RWs. In addition, rotifers caused two sudden algal population crashes in RW #1 leading to the lowest monthly productivity measured of 1.1 g DW m2/day in September 2014. However, under favorable weather conditions in the spring and summer of 2015, the productivities of RW#1 and RW#3 were approximately 8.2-9.0 g DW/ m2/day and 14.2-15.1 g DW/m2/day, respectively. The average CV from June 2014 to September 2014 in both RWs was only 10.9 MJ/kg, which is lower than that of terrestrial energy crops (17.0-20.0 MJ/kg) [19-21]. Nevertheless, after the addition of extra CO2, the value (19.7 MJ/kg) became close to the CVs of the terrestrial biomass resources. Therefore, it can be concluded that when a higher concentration of CO2 is available, higher productivity, lipid content, and CV are attainable. Since industrial flue gas could serve as a CO2 source at no or a very little cost [22], the additional CO2 supply was not considered as an expense in this study. In addition, after extraction of C14 to C18 fatty acids, the resulting microalgae biomass could be used as energy pellets or organic fertilizer.
Nutrient consumption and CO2 fixation
The nutrient consumption was largely dependent on the biological (dominant species) and operational (culturing period) parameters. The overall average consumption of TN and TP for each run was approximately 13.1 mg/kg and 2.5 mg/kg, respectively (Table 5), suggesting that N/P-rich wastewater could be used as a growth medium [23,24]. It has been reported that 1.0 kg of dry algal biomass utilizes 1.83 kg CO2 [25]. In this study, a total of 2612.7 kg of dry microalgal biomass, which is equivalent to 4781.2 kg CO2, was produced. South Korea introduced carbon emission trading on January 12, 2015 and carbon is currently traded at the price of US$ 9.7 per ton of CO2 equivalent on the market. This opens up new opportunities for the microalgae-based industry to profit from carbon trading.
| Season | Month | Biomass production (kg) | CO2 utilized by biomass (kg)a |
|---|---|---|---|
| Summer 2014 | June | 183.4 | 335.6 |
| July | 193.3 | 353.7 | |
| August | 109.2 | 199.8 | |
| Sum | 485.9 | 889.1 | |
| Autumn 2014 | September | 115.6 | 211.6 |
| October | 170 | 311.1 | |
| November | 162.3 | 297 | |
| Sum | 447.9 | 819.6 | |
| Winter 2014-15 | December | 42.7 | 78.2 |
| January | 69.2 | 126.6 | |
| February | 54.2 | 99.2 | |
| Sum | 166.1 | 303.9 | |
| Spring 2015 | March | 180.5 | 330.3 |
| April | 221 | 404.5 | |
| May | 289.5 | 529.9 | |
| Sum | 691.1 | 1264.7 | |
| Summer 2015 | June | 329.2 | 602.4 |
| July | 279.9 | 512.2 | |
| August | 212.7 | 389.2 | |
| Sum | 821.8 | 1503.8 | |
| Total | 2612.7 | 4781.2 | |
aSlade and Bauen
Table 5: Microalgal biomass production and CO2 fixation (from June 2014 to August 2015).
Conclusions
This study demonstrated the potential of commercial-scale microalgal biomass production for biofuels under Korean geoclimatic conditions. Naturally occurring microalgae were allowed to dominate in the RW ponds to establish more reliable cultures. The species composition along with nutrient availability strongly affected the biomass productivity and characteristics. In this study, the CV was calculated to understand the potential of microalgal biomass as a biofuel feedstock and the overall microalgal biomass CV (17.5 MJ/kg) approached the CV found for terrestrial energy crops in other studies. Although more innovative work is still needed to enhance the biomass and lipid productivity, the present work showed that microalgae hold great promise as a potential biofuel source, more so than crop plants.
Acknowledgements
This work was supported by the Advanced Biomass R&D Center (ABC) of the Global Frontier Project funded by the Ministry of Science, ICT and Future Planning (2015M3A6A2065698), South Korea. This project was also supported by the Freshwater Microalgae-based Bioenergy Research and Development Project from Chilgok-gun, Gyeongsangbuk-do, South Korea. Hong JW and Kim OH contributed equally to this work.
References
- Huntley ME, Redalje DG (2007) CO2 mitigation and renewable oil from photosynthetic microbes: a new appraisal. Mitigation and Adaptation Strategies for Global Change 12: 573-608.
- Li Y, Horsman M, Wu N, Lan CQ, Dubois-Calero N (2008) Biofuels from microalgae. Biotechnology Progress 24: 815-820.
- Brennan L, Owende P (2010) Biofuels from microalgaea review of technologies for production, processing, and extractions of biofuels and co-products. Renewable and Sustainable Energy Reviews 14: 557-577.
- Hannon M, Gimpel J, Tran M, Rasala B, Mayfield S (2010) Biofuels from algae: challenges and potential. Biofuels 1: 763-784.
- Wang H, Zhang W, Chen L, Wang J, Liu T (2013) The contamination and control of biological pollutants in mass cultivation of microalgae. Bioresource Technology 128: 745-750.
- Carney LT, Lane TW (2014) Parasites in algae mass culture. Frontiers in Microbiology 5.
- Mutanda T, Ramesh D, Karthikeyan S, Kumari S, Anandraj A, et al. (2011) Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresource Technology 102: 57-70.
- Odlare M, Nehrenheim E, Ribé V, Thorin E, Gavare M, et al. (2011) Cultivation of algae with indigenous species potentials for regional biofuel production. Applied Energy 88: 3280-3285.
- Rawat I, Kumar RR, Mutanda T, Bux F (2013) Biodiesel from microalgae: a critical evaluation from laboratory to large scale production. Applied Energy 103: 444-467.
- Innis MA, Gelfand DH, Sninsky JJ, White TJ (1990) PCR Protocols: A Guide to Methods and Applications. Academic Press, San Diego.
- Mishra SK, Suh WI, Farooq W, Moon M, Shrivastav A, et al. (2014) Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresource Technology 155: 330-333.
- Given PH, Weldon D, Zoeller JH (1986) Calculation of calorific values of coals from ultimate analyses: theoretical basis and geochemical implications. Fuel 65: 849-854.
- Smith VH, Sturm BS, Billings SA (2010) The ecology of algal biodiesel production. Trends in Ecology and Evolution 25: 301-309.
- Kazamia E, Aldridge DC, Smith AG (2012) Synthetic ecology a way forward for sustainable algal biofuel production?. Journal of Biotechnology 162: 163-169.
- Hoshina R, Iwataki M, Imamura N (2010) Chlorella variabilis and Micractiniumreisseri sp. nov. (Chlorellaceae, Trebouxiophyceae): Redescription of the endosymbiotic green algae of Paramecium bursariaPhycologicalResearch 58: 188-201.
- Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renewable and Sustainable Energy Reviews. 14: 217-232.
- Krienitz L, Bock C (2012) Present state of the systematics of planktonic coccoid green algae of inland waters. Hydrobiologia 98: 295-326.
- Ryckebosch E, Bruneel C, Muylaert K, Foubert I (2012) Microalgae as an alternative source of omega-3 long chain polyunsaturated fatty acids. Lipid Technology 24: 128-130.
- Demirbas (1997) A Calculation of higher heating values of biomass Fuels. 76: 431-434.
- Ross AB, Jones JM, Kubacki ML, Bridgeman T (2008) Classification of macroalgae as fuel and its thermochemical behavior. Bioresource Technology 99: 6494-6504.
- Naik S, Goud VV, Rout PK, Jacobson K, Dalai AK (2010) Characterization of Canadian biomass for alternative renewable biofuel. Renewable Energy 35: 1624-1631.
- Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends in Biotechnology 26: 126-131.
- Park JBK, Craggs RJ (2011) Nutrient removal in wastewater treatment high rate algal ponds with carbon dioxide addition. Water Science and Technology 63: 1758-1764.
- Boelee NC, Temmink H, Janssen M, Buisman CJ, Wijffels RH (2012) Scenario analysis of nutrient removal from municipal wastewater by microalgal biofilms. Water 4: 460-473.
- Slade R, Bauen A (2013) Micro-algae cultivation for biofuels cost, energy balance, environmental impacts and future prospects. Biomass and Bioenergy 53: 29-38.
Relevant Topics
Recommended Journals
- Oil & Gas Research Journal
- Renewable Energy and Applications Journal
- Oceanography Journal
- Industrial Pollution Control Journal
- Coastal Zone Management Journal
- Climatology & Weather Forecasting Journal
- Geoinformatics & Geostatistics Journal
- Engineering and Technology Journal
- Petroleum & Environmental Biotechnology Journal
- Polymer Sciences Journal
Article Tools
Article Usage
- Total views: 14213
- [From(publication date):
April-2016 - Jul 02, 2025] - Breakdown by view type
- HTML page views : 12939
- PDF downloads : 1274