Research Article Open Access
Marijuana Use Impacts Cognitive Interference: An fMRI Investigation in Young Adults Performing the Counting Stroop Task
Taylor Hatcharda1, Peter A Friedb2, Matthew J Hoganc3, Ian Cameron4 and Andra M Smitha1*1School of Psychology, University of Ottawa, 136 Jean Jacques Lussier, Ottawa, ON, Canada
2Department of Psychology Carleton University, 1125 Colonel By Drive, Ottawa, ON, Canada
3Ottawa Hospital Research Institute, 725 Parkdale Ave, Ottawa, ON, Canada
4Department of Diagnostic Imaging, The Ottawa Hospital, 1053 Carling Ave, Ottawa, ON, Canada
- Corresponding Author:
- Andra M Smitha
School of Psychology, University of Ottawa
136 Jean Jacques Lussier, Ottawa, ON, K1N 6N5, Canada
Tel: 613-562-5800
Ext : 2671
Fax: 613-562-5147
E-mail : asmith@uottawa.ca
Received date: August 06, 2014; Accepted date: October 13, 2014; Published date: October 16, 2014
Citation: Hatcharda T, Friedb PA, Hoganc MJ, Cameron I, Smitha AM (2014) Marijuana Use Impacts Cognitive Interference: An fMRI Investigation in Young Adults Performing the Counting Stroop Task. J Addict Res Ther 5:197. doi:10.4172/2155-6105.1000197
Copyright: © 2014 Hatcharda T, et al. This is an open-access article distributedunder the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
There is a growing body of evidence that marijuana use during adolescence, a critical period in neurocognitive development, may have lasting detrimental impact on executive functioning. The Ottawa Prenatal Prospective Study (OPPS) has followed participants over 20 years, from birth to young adulthood, and has collected data on potentially confounding lifestyle variables, such as prenatal drug exposure and current drug use. In the present study, we report the effects of heavy adolescent onset marijuana use on cognitive interference while performing a Counting Stroop task using fMRI in a sample of OPPS participants, while controlling for current nicotine use and prenatal marijuana exposure. Despite a lack of performance differences, the neural activity of young adults who use marijuana on a regular basis differed significantly compared to non-users while performing the task. This included increased activity in the right rolandic operculum, cerebellar tonsil, bilateral postcentral gyrus, cingulate gyrus, and right supplementary motor area. This recruitment of additional brain regions is suggestive of compensatory strategies among marijuana users in order to successfully complete the task, highlighting the impact of early marijuana use on neurocognitive development and altered brain function.
Keywords
Functional MRI; Marijuana; Executive functioning; Attention; Counting stroop; Cognitive interference
Abbreviations
ACC: Anterior Cingulate Cortex; BOLD: Blood Oxygen Level Dependent; dlPFC: Dorsolateral Prefrontal Cortex; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders; EPI: Echo Planar Imaging; fMRI: Functional Magnetic Resonance Imaging; OPPS: Ottawa Prenatal Prospective Study; SPM: Statistical Parametric Mapping
Introduction
Marijuana continues to be the most commonly used illicit drug of abuse in North America, with 15.6 typically being the average age of first use [1]. Exposure to potentially neurotoxic substances like marijuana before the age of 15, a critical period in neuro-maturation, considerably increases the risk of developing long-term neuropsychological deficits [2]. There is a growing body of evidence that the deleterious impact of marijuana use differs among adolescents and adults, with the developing brain of adolescents being particularly more vulnerable to the harmful effects of regular marijuana use [3]. Moreover, this vulnerability is further increased by the decreasing rates in perceived risk among adolescents, with the misperception that regular marijuana use carries little risk and has no persistent impact on neurocognitive functioning [4].
Despite the perception that marijuana use causes little harm, cognitive impairments associated with regular marijuana use, particularly when age of onset occurs during adolescence, is well documented in the literature [5-10]. Meier et al. [11] conducted a longitudinal study examining the association between persistent marijuana use among adolescent onset users and decline in neuropsychological functioning. Their results indicated that adolescent onset of marijuana use was associated with broad decline across domains, including verbal comprehension, processing speed, perceptual reasoning, and memory. In addition, more continual use was associated with greater decline. Among the adolescent onset users who eventually stopped using, the deficits caused by persistent use were not fully restored by cessation of use.
Findings from other studies suggest that regular adolescent onset marijuana use is associated with a number of cognitive deficits, including processing speed, complex attention, memory, and executive functioning [8]. Within the context of executive functioning, one aspect particularly vulnerable to the impact of marijuana is cognitive inhibition, which refers to a set of abilities such as the capacity to time or delay a response, suppress an inappropriate response, and ignore distractions [12]. Early onset and prolonged marijuana exposure during adolescence has been shown to produce deficits in gathering and evaluating relevant information prior to decision-making [8,13]. This disinhibition typically manifests itself in risky and impulsive decision-making based on greater uncertainty and inefficient utilization of information, which worsens the earlier use of marijuana is initiated [14].
Gruber and Yurgelun-Todd [15] used functional magnetic resonance imaging (fMRI) to examine the impact of heavy marijuana consumption on cognitive inhibition using the Stroop task. The Stroop task is a measure of executive functioning that both challenges an individual’s ability to inhibit inappropriate responses and resist interference from distraction cues. Marijuana users made slightly more errors during the interference condition than controls. Despite this difference not reaching significance, there was a significant difference in neural activation pattern between groups. Heavy marijuana users showed significantly lower anterior cingulate activity and significantly higher mid cingulate activity compared to non-using controls. Similarly the users showed a bilateral pattern of dorsolateral prefrontal cortex activation that was not observed in non-users. The results of this study suggest that heavy marijuana use is related to significant changes in the magnitude and pattern of brain activity during this task of cognitive control and response inhibition.
In a follow-up study, Gruber et al. [16] examined how age of onset of heavy marijuana use impacts response inhibition and cognitive interference using the Multi-Source Interference Task in early onset users (<16 years old), late onset users (>16 years old), and healthy controls. Although there were no major performance differences between the three groups, early onset marijuana users showed increased activation in the mid-right cingulum, where as late onset users showed increased activation in the mid-left cingulum. Early onset users also showed increased activation in a focal region of the mid anterior cingulate cortex (ACC) more similar to healthy controls while late onset users showed increased activity in a more anterior area of that region. Again, despite no significant performance differences, early onset users showed faster reaction times and committed more errors than late onset users or healthy controls. These findings suggest that early exposure to marijuana is associated with poorer inhibitory control. The differences in brain activity observed in this study may also reflect neural compensation among early onset marijuana users who began using during a critical period in neurocognitive development.
Despite these significant results, societal perception of the risks of marijuana use has yet to be challenged. Further research is required to solidify the empirical evidence of the damaging effects of this drug on the young brain. The present study used fMRI to examine the impact of regular marijuana use on cognitive interference in a sample of participants from the Ottawa Prenatal Prospective Study (OPPS) while performing the Counting Stroop. The OPPS is a longitudinal project that has been following a cohort of individuals over the past 25 years, from the time they were in utero, which has provided data on potentially confounding lifestyle and drug exposure variables. This unique sample allows for the examination of the neurobehavioral effects of current marijuana use on executive functioning, while controlling for adolescent drug use, prenatal drug exposures, and other lifestyle variables such as socioeconomic status and mental health status.
Originally conducted by Bush et al. [17], the Counting Stroop is a variant of the original Stroop task that measures cognitive interference in fMRI. It was designed to minimize head movement in the scanner. In the task, participants are presented with a group of stimulus words and asked to report the number of words listed using buttons on a response pad. The stimulus words are either congruent (e.g. animal names) or incongruent (e.g. number words) with counting. In a sample of healthy controls, Bush et al. [17] found increased neural activity in the anterior cingulate, middle frontal gyrus, left precentral gyrus, left premotor cortex, inferior temporal gyrus, and superior parietal lobule during the incongruent minus congruent contrast.
It was hypothesized, for the present study, that there would be no performance differences between young adult marijuana users and non-users while performing the Counting Stroop, as the task was designed to ensure participants were able to perform the task to ensure measurement of the processing of interest. However, we anticipated that current marijuana users would need to engage in compensatory strategies to complete the task compared to the nonuser group, which would manifest itself as increased brain activation. In particular, we anticipated increased neural activity in areas of the brain related to cognitive functioning, such as decision-making, information and visual processing, as well as motor control, including the dorsolateral prefrontal cortex (DLPFC), anterior cingulate and premotor areas.
Materials and Methods
Participants
Twenty-four participants were recruited from the OPPS and signed informed consent prior to inclusion in the study. The Ottawa Hospital research ethics board approved this study. Participants were between 19 and 21-years-old, right handed, had English as their first language, and did not meet diagnostic criteria for an Axis I diagnosis from the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). In addition, all participants were from middle class homes and no parents of the participants were reported to have a history of an Axis I diagnosis. All participants also met fMRI compatibility criteria, including no claustrophobia, no metal implants, no pacemaker, no recent surgery, and suitable vision for viewing stimuli.
Regular marijuana use was defined as smoking more than 1 marijuana cigarette per week for at least three years. The resulting sample consisted of ten marijuana users (six males, four females, mean age 20) and fourteen non-using controls (nine males, five females, mean age of 20). The users reported smoking an average of 11.48 marijuana joints per week (ranging from 2 – 37.5 joints per week) for an average of 4.55 years. This level of marijuana use would approximate a lifetime average of 2697 joints smoked, which is considered very heavy exposure [18]. Participants in the non-users group reported never using marijuana regularly. Although three of the fourteen non-users reported sporadic use, it was no more than one to four times over the past year.
No participants had used illicit drugs on a regular basis or within a month before fMRI testing, which included no use of amphetamines, crack, cocaine, heroin, mushrooms, hashish, lysergic acid, steroids, solvents, and tranquilizers. Seven of the ten marijuana users reported regular use of nicotine, while no participants in the non-users group reported smoking cigarettes on a regular basis. Therefore, nicotine use has been controlled for in all statistical analyses.
fMRI
The task was presented to participants on a screen located at the foot of the patient table, which participants viewed through a mirror mounted on the head coil, and all lighting in the scanning room was turned off. Participants responded using a 4-button MRI-compatible fibre optic response pad (Lightwave Medical, Vancouver, British Columbia, Canada). Stimuli were presented and responses were recorded using Visual and Auditory Presentation Package.
Counting Stroop Task
The Counting Stroop task used in the current study was a block design that adhered to the standard format used in the original article by Bush et al. [17]. The design consisted of two block types: congruent and incongruent blocks. During the congruent trials, stimulus words were the names of common animals (i.e. dog, cat, bird, or mouse), whereas during incongruent trials, the stimulus words were number words (i.e. one, two, three, or four). Participants completed sixteen 30-sec blocks, which consisted of eight congruent word blocks and eight incongruent word blocks. All blocks were counterbalanced and the task took 8 minutes in total to complete. Each block contained 20 trials, with an interstimulus internal of 1.5 sec, which resulted in a total of 160 trials.
Participants were instructed to respond as quickly and accurately as possible and to do their best. During each trial, participants were presented with 1 to 4 identical words printed in white on a black background, which presented horizontally one above another. They were instructed to indicate the number of words observed for each group using the appropriate button on the response pad (index finger for one word, middle finger for two words, etc). Stimulus words were common words from each of the two semantic categories and were balanced for word length.
Procedures
Prior to imaging, participants were also required to view the task outside the scanner and perform one block of each condition to ensure accurate performance. The marijuana users were not asked to abstain from smoking marijuana days prior to the scanning session but were asked to not smoke for 2 hours prior. Each participant was also present at the scanner for approximately 2 hours prior to scanning and thus acute marijuana effects were ruled out. In order to assess current drug use, participants were asked to provide a urine sample upon arrival at the MRI clinic. Samples were screened for cannabis, amphetamines, opiates, cocaine, creatinine and cotinine. Participants were also asked to complete a self-report drug questionnaire, which examined use of alcohol, marijuana, nicotine, mushrooms, amphetamines, crack, cocaine, tranquilizers, heroin, lysergic acid diethylamide, solvents and steroids.
Imaging parameters
All imaging was performed using a 1.5 Tesla Siemens Magnetom Symphony MR scanner. Participants were asked to lay supine, with their head secured in a custom head holder while a conventional T1-weighted spin echo localizer was acquired and used to prescribe a subsequent 3D FLASH (TR/TE 11.2/21 ms, flip angle 60°, field of view (FOV) 26×26 cm2, 256×256 matrix, slice thickness 1.5 mm) volume acquisition. Whole brain blood oxygen level dependent (BOLD) fMRI was performed using a T2*-weighted echo planar pulse sequence (TR/TE 3000/40 ms, flip angle 90°, FOV 24×24 cm2, 64×64 matrix, slice thickness 5 mm, 27 axial slices, bandwidth 62.5 kHz).
Cognitive performance parameters and analyses
Reaction time and errors of commission (i.e. any inaccurate responses) for each response were recorded. Mean reaction times were calculated for both the ‘Animal’ and the ‘Number’ conditions for all accurate responses occurring within 900 ms of stimulus presentation.
Image post-processing
The functional brain images were realigned to correct for motion by employing the procedure of Friston et al. [19], using Statistical Parametric Mapping (SPM8) software. The motion correction did not exceed 1 mm for any participant. Images were spatially normalized to match the echo planar imaging (EPI) template provided in SPM8. Images were then smoothed with an 8 mm full-width at half-maximum Gaussian filter.
Whole brain analyses with SPM8
Fixed effects analyses were performed on all participants individually and then statistical parametric maps were obtained for the two groups: current marijuana users and non-users. Contrast images were calculated for these analyses for the contrast of interest: ‘Numbers’ minus ‘Animals’. These images were subsequently used for second-level random effects analyses, which allowed for a comparison between groups.
Multiple independent samples t-tests were conducted at a set threshold of puncorr = 0.001, with a cluster-wise correction at pFWE = 0.05. This allowed for assessment of group differences in neural activity during the Counting Stroop between current marijuana users and non-users, while manipulating the co-variates that were included in the analysis, cotinine (i.e. current nicotine use) and prenatal marijuana.
Although both current nicotine and alcohol were significantly different between users and non-users, both substances were correlated with each other. Moreover, current nicotine use was more significantly different between the groups and more highly correlated with marijuana than current alcohol, and as such, only cotinine was used in the final analyses. Both prenatal marijuana and prenatal nicotine were not significantly different between users and non-users. While non-significant, prenatal marijuana exposure has been shown to impact response inhibition [20] and therefore was included as a covariate, as these exposures could potentially impact the results of current marijuana use on neural functioning.
In an attempt to control for acute marijuana effects, additional analyses were performed with only those participants who had not smoked on the day of testing and then again with all participants. In addition, analyses were also performed with and without the one nonuser who had smoked marijuana 3 days prior to testing. All participants were included in the reported results, as these variations on the analyses had no impact on the results.
Results
Drug questionnaire and urine sample data
All participants in the users group reported smoking marijuana within 1 week of fMRI testing. Over the 7 days prior to testing, the users reported their average number of joints smoked per day was 4.2, 4.55, 3.15, 2.75, 2.9, 4.6, and 4.35, with an average of 2.5 joints smoked on the day of testing. Four of the ten participants smoked marijuana on the day of testing, with two of the four having smoked a joint in the morning while the other two smoked throughout the day, which was consistent with their regular pattern of use. There was an average of 460 µg/L cannabis found in the urine sample of the users group, ranging from 16 to 1325 µg/L. In the non-users group, one participant had smoked marijuana 3 days prior to testing and had 45 µg/L in his urine though no other exposure was reported for the months prior to testing. Cannabis was not found in the urine sample of any other non-users.
The urine samples of the marijuana users group also showed an average of 888 µg/L for cotinine (seven out of ten were cigarette smokers), while the non-using group had an average of only 9.8 µg/L (which may be attributable to second hand smoke exposure). This significant difference warranted including cotinine as a covariate in the imaging analyses. There was no reported alcohol consumption from participants in either group on the day of testing, though one participant in the marijuana users group reported consuming 15 alcohol drinks on the day prior to testing. No other participants reported consuming more than seven drinks over the 2 days prior to the scanning sessions, eliminating the likelihood of the testing session results being attributable to the acute effects of alcohol consumption. Table 1 provides a complete description of drug exposure based on groups.
| Drug exposure | Users n = 10 Mean (SE) |
Non-Users n = 14Mean (SE) | Results (ANOVA) |
|---|---|---|---|
| Current nicotine (cigarettes/day) |
7.75 (1.29) | 0.00 (1.09) | F (1,22) = 20.91, p<0.001 |
| Currentalcohol (AA/day) | 4.77 (1.02) | 2.00 (0.86) | F (1,22) = 4.48, p<0.05 |
| Prenatal marijuana (joints/week) | 8.82 (3.4) | 1.12 (2.87) | F (1,22) = 2.99, p<0.10 |
| Prenatal nicotine (cigarettes/day) | 10.41 (3.15) | 3.09 (2.66) | F (1,22) = 3.14, p<0.09 |
| Prenatalalcohol (AA/day) |
0.13 (0.10) | 0.28 (0.08) | F (1,22) = 1.41, p<0.25 |
Table 1:Drug exposurebased on groups.
Results from the self-report drug questionnaire were compared to the urine samples in order to validate participant responses. The Pearson correlation for levels of marijuana use was 0.97 (p<0.001) and 0.91 (p<0.001) for levels of nicotine (cotinine/creatine). As such, this high concordance rate between participant responses and urinalysis validated the use of the self-report drug questionnaire results for current use and drug history.
Performance data
There were no significant performance differences between current marijuana users and non-users on reaction time, errors of commission, or the Stroop effect (i.e. Incongruent – Congruent trials) based on ANCOVA results using current nicotine and prenatal marijuana as covariates (Table 2). Additional analyses were performed using other drug exposures as covariates, including current alcohol, prenatal nicotine, and prenatal alcohol, as well as the analysis without covariates, and the results between current marijuana users and non-users remained non-significant. As such, reaction time and errors were not used as covariates in the imaging data analysis.
| Performance measure | Users n = 10 Mean (SE) |
Non-Users n = 14 Mean (SE) |
Results (ANCOVA) |
|---|---|---|---|
| Errors of commission (Animals) |
5.6 (1.166) | 4.786 (1.415) | F(1,20) = 0.28, p<0.603 |
| Errors of commission (Numbers) |
11.5 (2.651) | 9.5 (2.2) | F(1,20) = 1.4, p<0.251 |
| Reaction time (Animals) |
0.733 (0.02) | 0.674 (0.026) | F(1,20) = 0.573, p<0.458 |
| Reaction time (Numbers) |
0.795 (0.022) | 0.718 (0.025) | F(1,20) = 1.264, p<0.274 |
| StroopEffect (Incongruent – Congruent) |
0.0622 (0.013) | 0.043 (0.014) | F(1,20) = 0.477, p<0.498 |
Table 2: Performance data for the Stroop conditions based on marijuana use.
Whole brain analysis
In order to ensure that the Counting Stroop task was activating the appropriate areas, within group, first level fixed effects analyses were performed. Results showed that both users and non-users displayed an expected pattern of activity for the incongruent minus congruent contrasts, which included increased activation of the DLPFC, superior temporal gyrus, superior parietal lobule, and premotor and primary motor cortices. Despite the similar pattern of activity observed in anticipated areas for the Stroop effect, there remained significant differences between users and non-users when performing the random effects group comparison.
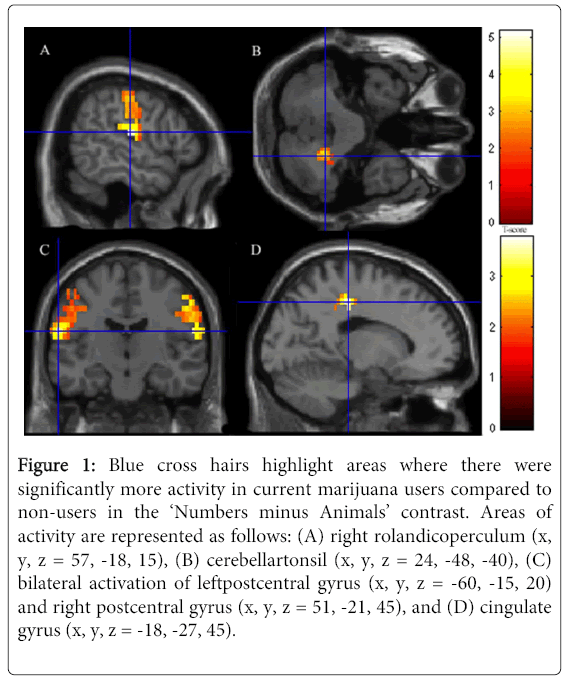
The most robust results are presented at threshold pcrit<0.001 uncorrected with pFWE = 0.05 cluster-wise correction. Compared to non-users, marijuana users showed significantly increased neural activity in the right rolandic operculum (x, y, z = 57, -18, 15), the right cerebellar tonsil (x, y, z = 24, -48, -40), and the right postcentral gyrus (x, y, z = 51, -21, 45). These regions made up a cluster of 1670 activated voxels. Marijuana users also showed significantly more activation than non-users in another large cluster of 854 voxels, including the cingulate gyrus (x, y, z = -18, -27, 45), the left postcentral gyrus (x, y, z = -60, -15, 20), and the right supplementary motor area (x, y, z = 9, -18, 50) (Figure 1 A-D). All results were significant after controlling for multiple comparisons at p = 0.05 and controlling for current nicotine use and prenatal marijuana exposure. These drugs were used as covariates to ensure the observed results were as representative of marijuana alone, though we acknowledge that completely removing the co-occurring impact of these variables is not entirely possible.
Figure 1: Blue cross hairs highlight areas where there were significantly more activity in current marijuana users compared to non-users in the ‘Numbers minus Animals’ contrast. Areas of activity are represented as follows: (A) right rolandicoperculum (x, y, z = 57, -18, 15), (B) cerebellartonsil (x, y, z = 24, -48, -40), (C) bilateral activation of leftpostcentral gyrus (x, y, z = -60, -15, 20) and right postcentral gyrus (x, y, z = 51, -21, 45), and (D) cingulate gyrus (x, y, z = -18, -27, 45).
Discussion
The current study investigated the fMRI BOLD response to a Counting Stroop task of cognitive interference, contrasting young adults who consumed marijuana on a regular basis with individuals who were not regular users. During the first level fixed effects analysis, patterns of brain activity in users and non-users were comparable to previous results by the original Counting Stroop study by Bush et al. [17] in the incongruent minus congruent contrast. This included increased BOLD response in the DLPFC, superior temporal gyrus, superior parietal lobule, and premotor and primary motor cortices, providing support that the Stroop effect was being measured.
Although there were no performance differences, significant differences emerged in the BOLD response with current marijuana users displaying significantly greater and more extensive activation than non-users when challenged by the cognitive conflict processing of the task. Compared to non-users, regular marijuana users revealed an extensive pattern of activation during the numbers minus animals contrast in the right rolandic operculum, cerebellar tonsil, bilateral postcentral gyrus, cingulate gyrus, and right supplementary motor area. Recruitment of these additional brain regions observed in marijuana users and typically not associated with Stroop performance suggest that the brain may be engaging in compensatory strategies in order to complete the task. This may possibly be attributable to underlying neurocognitive deficits. This explanation has been reported in other fMRI studies of adolescent marijuana users performing spatial working memory and cognitive control tasks. Again, suggested to be related to decrements in attentional and visual processing following chronic marijuana exposure [21,22]. Importantly, these differences remained significant even after controlling for previous drug exposure, including current nicotine use and prenatal marijuana exposure. Unlike previous research, this allows for more conclusive inferences to be made on the exclusive contribution of marijuana on cognitive interference.
The most significant difference observed between marijuana users and non-users was increased activation of the right rolandic operculum, which occupies the lower portion of the post-central gyrus, and activation of these areas is typically associated with processing sensory input [23]. The current results showed activation of a large number of voxels in both the right rolandic operculum and bilaterally in the postcentral gyrus in current marijuana users. This may be reflective of a form of compensatory strategy when processing sensory information due to altered brain function, such as reading the congruent or incongruent word being presented and deciding what button to press on the response pad.
Marijuana users also displayed significantly more activity in the cerebellum and the cingulate gyrus, areas of the brain related to motor control and cognitive/attentional motor processing, respectively. Previous research by Lopez-Larson et al. [24] used fMRI to evaluate the impact of adolescent marijuana use on cortico-cerebellar circuits while performing a bilateral finger-tapping task. Unlike the results of the current study, the authors found that, compared to healthy controls, heavy adolescent onset marijuana use was associated with decreased activation of the cingulate and cerebellum. However, this discrepancy in results may be attributable to a number of factors, including the fact that the task used in their study was primarily motor based whereas the task in the current study is looking at aspects of executive functioning, lack of control for concurrent substance use, and a noteworthy difference in the amount of lifetime average marijuana use (i.e. 1500 versus 2697 joints smoked).
Previous studies with the OPPS sample examining the impact of young adult marijuana use on executive functioning are consistent with the compensatory recruitment of additional brain areas observed in the current study in order to complete the task [9,10]. Although task performance was similar between users and non-users on a Go/No-Go task of response inhibition and a visual-spatial working memory 2-Back task, a number of functional differences were observed. During the Go/No-Go task, marijuana use was positively associated with activation of the right thalamus and middle frontal gyrus, as well as a dose-dependent relationship with marijuana and activation of the inferior parietal lobe and precuneus. These four brain regions are associated with the neural network responsible for response inhibition, suggesting that alterations in this network may be necessary to compensate for neural changes caused by adolescent onset marijuana use. Similarly, in the 2-Back task, marijuana users showed more activity than controls in the inferior and middle frontal gyri, two regions typically associated with working memory, as well as the right superior temporal gyrus, an area related to auditory processing. The recruitment of this additional area is again suggestive that marijuana use alters neural functioning and that the recruitment of blood flow to additional areas may help to compensate for the underlying changes that result from marijuana exposure.
Possible limitations of the study related to sample size and the task design itself should be considered. For example, given the relatively small sample size in each group replication of these findings would be essential to establish firmer conclusions. Based on the low quality of the structural scans acquired during the scanning session, we were unable to assess differences in brain volume between groups. This would have been beneficial, as it could have elucidated whether the differences in brain activation observed between marijuana users and non-users were related to structural differences. Task design also should be considered. The block design does not allow for assessing event related activation. Despite the benefits of event related design, the block design was thought to be more appropriate for this type of processing. It may have been beneficial to include rest blocks between the congruent and incongruent blocks to eliminate possible carryover effects by allowing the hemodynamic response to equilibrate.
In addition to measuring cognitive interference, the Counting Stroop also contains a working memory component, requiring participants to map the number of words in each trial to the finger representing each number on the response pad. Moreover, as the two experimental conditions differ in semantic content of the words (i.e. numbers or animals) the subtraction of congruent from incongruent trials includes the effects of cognitive interference as well as semantic meaning of the words. Consequently, the differences observed in brain activation between groups may be attributable, at least in part, to differences in semantic processing in addition to attentional and/or interference processing. Both conditions have the same requirements so this is controlled for but needs to be mentioned. Lastly, future research should consider conducting a more difficult version of the Counting Stroop task than that of the current study. This could potentially challenge the neural circuitry involved to a point where compensation was not sufficient and deficits would be observed, both in performance and in BOLD activity, between marijuana users and non-users.
Conclusions
The present study provides support for a significant, possibly detrimental, impact of regular marijuana use in young adulthood based on significant differences in brain activity during a task that required self-regulatory control to inhibit responses. The increased neural activity observed in the recruitment of additional brain regions to complete the task is reflective of a form of neural compensation. This may be attributable to altered brain circuits resulting from a disruption in normal neuromaturation due to marijuana use during a critical period in neurocognitive development. Moreover, although there were no performance deficits, it is possible that the compensatory strategies observed through alterations in brain activity may not be sufficient if further levels of difficulty were added. Thus, these findings highlight the vulnerability of the developing brain to the impact of marijuana on inhibitory control, a cognitive process important in suppression of actions and interference from irrelevant stimuli for success in establishing and reaching appropriate goals in adulthood.
Acknowledgements
This manuscript is dedicated to the memory of Barbara Watkinson, a beloved colleague and inspiring person. The authors would like to thank the MRI technologists at the Ottawa Hospital. This research was funded by a NIDA supplemental grant, a Canadian Institutes for Health Research Post-doctoral fellowship to A.M. Smith and a research grant from the Ontario Research and Development Challenge Fund. The authors do not have any conflicts of interest to report.
References
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE (2014) Monitoring the Future national results on drug use: 1975-2013: Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan.
- Fontes MA, Bolla K, Cunha PJ, Almeida PP, Jungerman F, et al. (2011) Cannabis use beforeage 15 and subsequentexecutivefunctioning. Br J Psychiatry 198: 442-447.
- Monti PM, Miranda R Jr, Nixon K, Sher KJ, Swartzwelder HS, et al. (2005) Adolescence: booze, brains, and behavior. Alcohol Clin ExpRes 29: 207-220.
- Johnston LD, Malley PM, Bach- man JG, and Schulenberg JE (2011) Marijuana use Continues to Rise Among U.S. Teens, WhileAlcohol use Hits HistoricLows. Ann Arbor, MI: University of Michigan News Service.
- Tapert SF, Granholm E, Leedy NG, Brown SA (2002) Substance use and withdrawal: neuropsychologicalfunctioning over 8 years in youth. J Int Neuropsychol Soc 8: 873-883.
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R (2013) Effects of cannabis on neurocognitive functioning: Recentadvances, neurodevelopmental influences, and sexdifferences. NeuropsychologyReview 23: 117-137.
- Jacobus J, Goldenberg D, Wierenga CE, Tolentino NJ, Liu TT, et al. (2012) Alteredcerebralblood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology (Berl) 222: 675-684.
- Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S (2013) Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry 4: 53.
- Smith AM, Longo CA, Fried PA, Hogan MJ, Cameron I (2010) Effects of marijuana on visuospatialworking memory: an fMRIstudy in youngadults. Psychopharmacology (Berl) 210: 429-438.
- Smith AM, Lopez Zunini RA, Anderson CD, Longo CA, Cameron I et al. (2011) Impact of marijuana on response inhibition: An fMRIstudy in youngadults. Journal of Behavioural and Brain Science 1: 124-133.
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, et al. (2012) Persistent cannabis users show neuropsychologicaldeclinefromchildhood to midlife. Proc NatlAcadSci U S A 109: E2657-2664.
- Lezak MD, Howieson DB, Loring DW (2004)Neuropsychologicalassessment (4th ed.). New York: Oxford University Press.
- Lisdahl KM, Price JS (2012) Increased marijuana use and genderpredictpoorer cognitive functioning in adolescents and emergingadults. Journal of the International Neuropsychological Society 18: 678-688.
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J et al. (2012) Reflection impulsivity in adolescent cannabis users: acomparisonwithalcohol-using and non-substance using adolescents. Psychopharmacology 219: 575-586.
- Gruber SA, Yurgelun-Todd DA (2005) Neuroimaging of marijuana smokers during inhibitory processing: A Pilot Investigation. Brain Res Cogn Brain Res 23: 107-118.
- Gruber SA, Dahlgren MK, Sagar KA, Gönenc A, Killgore WD (2012) Age of onset of marijuana use impacts inhibitory processing. Neurosci Lett 511: 89-94.
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, et al. (1998) The counting Stroop: an interference task specialized for functional neuroimaging--validation study with functional MRI. Hum Brain Mapp 6: 270-282.
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF (2009) ltered white matter microstructure in adolescent substance users. Psychiatry Research: Neuroimaging 173: 228-237.
- Friston KJ, Ashburner J, Poline JB, Frith CD, Heather JD, Frackowiak RSJ (1995) Spatial realignment and normalization of images. Human Brain Mapping 3: 165-189.
- Smith AM, Fried PA, Hogan MJ, Cameron I (2004) Effects of prenatal marijuana on response inhibition: an fMRIstudy of youngadults. Neurotoxicol Teratol 26: 533-542.
- Harding IH, Solowij N, Harrison BJ, Takagi M, Lorenzetti V et al. (2012) Functionalconnectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacology 37: 1923-1933.
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, et al. (2005) fMRIresponse to spatial working memory in adolescents withcomorbid marijuana and alcohol use disorders. Drug Alcohol Depend 79: 201-210.
- Willeumier K, Taylor DV, Amen DG (2011) Decreasedcerebralblood flow in the limbic and prefrontal cortex using SPECT imaging in a cohort of completed suicides. Transl Psychiatry 1: e28.
- Lopez-Larson MP, Rogowska J, Bogorodzki P, Bueler CE, McGlade EC, et al. (2012) Cortico-cerebellarabnormalities in adolescents withheavy marijuana use. Psychiatry Res 202: 224-232.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 15760
- [From(publication date):
December-2014 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 11300
- PDF downloads : 4460