Mapping Auditory Maturation from Neonates to Toddlers using Electrophysiological Responses of the Brainstem
Received: 21-Jul-2020 / Accepted Date: 20-Aug-2020 / Published Date: 27-Aug-2020 DOI: 10.4172/2161-119X.1000402
Abstract
Brainstem Evoked Response Audiometry (BERA) is the gold standard electrophysiological investigation for the assessment of synchrony of the auditory pathway. There can be variations in the synchrony based on many factors and age is an important parameter influencing it. From infants to toddlers, there is a temporal arborisation of the auditory brain due to neural plasticity and reorganisation over time. This study aimed to compile normative data in a group of healthy infants and toddlers, by analyzing the changes observed in BERA with regards to age, as an objective reflection of the status of maturation of the auditory pathway. Methods: A comparative cohort study in age-matched groups - group I being Neonates from birth till 7 days of age (n=37) and group II being infants and children from 6 months to 3 years of age (n=31). BERA was used to analyze the changes in wave patterns with respect to age and normative was statistically derived. Results: BERA proved to be an efficient electrophysiological tool to differentiate the auditory maturation between the two groups with statistical significance (p<0.05). Conclusion: BERA is a valid biomarker of the auditory pathway in children, with sequential changes noted in the wave patterns over age. This study highlights the importance of critical age for auditory maturation necessary to develop normal speech and language acquisition.
Keywords: Auditory pathway; Brainstem evoked response audiometry; Auditory synchrony; Neural plasticity; Neural reorganization
Introduction
Hearing is the first special sense which develops in-utero and begins to mature from the time of birth until about 3 years of age, which is deemed as the critical age for auditory maturation. Presence of a robust auditory network in response to complex noise exposure helps the sensory brain to develop normal speech and language skills.
Brainstem Evoked Response Audiometry (BERA) is a popular electrophysiological test with a series of five wave peaks arising from auditory nerve and brainstem structures. Normal responses arise in the first 10 milliseconds (ms) of the onset of a moderate-intensity click stimulus in otologically, audiologically and neurologically sound individuals [1]. This structural integrity and synchronous firing of auditory pathway from the spiral ganglia in the cochlear modiolus, onto the lateral lemniscus in the brainstem is evaluated by BERA. On analyzing the waves with regard to latency, amplitude and wave morphology, a fair idea about the abnormalities in this pathway is obtained [2]. A normal BERA graph has 5 peaks (I-V) when the sound stimulus is given at 50 dB above the hearing threshold. In the case of neonates, the BERA graph has three peaks (I, III & V) where waves II and IV are inconspicuous.
BERA is not significantly altered by the state of consciousness, drugs and a variety of environmental factors including other sensory input to the cortex. While BERA response thresholds only show a little age dependent effect, BERA latencies are age dependent especially in young infants. It is presumed that, there is varying time taken by cochlea to become electro-physiologically mature. Latency/ absolute latency of a wave is the time interval between the onset of the stimulus and the peak of the wave and is measured in milli seconds. The time interval between two different waves in the same ear and in the same BERA tracing in ms is known as ‘inter- wave latency or inter- peak latency’ (IPL). The time interval of the same wave between the two ears is known as ‘inter- aural latency’. In a healthy adult, for a stimulus at 75 dB Sound Pressure Level (SPL), absolute latency of wave I am 1.5 to 1.7 milliseconds. Wave III is usually present at or around 3.8 ms mark and wave V at 5.6 to 5.85 ms on the BERA graph. Waves II and IV are variably located and wave IV may sometimes even fuse with wave V. Among all BERA waves, the wave V is the most reliable, most robust and easily identifiable wave. The absolute latency of wave V is dependent upon the intensity of the sound stimulus. An increase in the intensity of the sound stimulus decreases the latency of wave V [2].
The amplitude of the wave is measured in micro-volts (μV) from the crest (peak) of one wave to its next trough. Since these amplitude studies are clinically not dependable and quite variable, the measurement commonly used is the relative amplitude ratio, usually between wave V and wave I. Wave morphology is the shape or configuration of the BERA graph and is judged visually to be good or poor by the audiologist.
The premise of this research work was to analyze the maturation of the auditory pathway from infants to toddlers (who fall within the critical age of up to 3 years), using BERA which is known to be a sensitive, objective electrophysiological tool. The BERA parameters across the two age groups was to be used to develop normative data for the different stages of auditory maturation, which will help as valid reference for future cohorts of children.
Study Method
A comparative cohort study was conducted in the Audiology centre of Department of ENT, in a tertiary care hospital in Pune, Maharashtra, India between 2016 and 2018 (2 years). The aim of the study was to analyze the changes in BERA wave latencies, amplitudes and morphology in two groups of healthy young children: group I consisted of neonates (from birth to 7 days) and group II consisted of infants and children from 6 months to 3 years of age. The objectives of the study were to compile normative data of BERA in these two groups; to correlate the changes observed in BERA waves between these two groups with the maturation of auditory pathway, and; to estimate the efficiency of BERA as an objective tool to evaluate auditory maturation.
The inclusion criteriae were: neonates from birth till 7 days of life and children from 6 months to 3 years of age; voluntary informed consent from parents of the child for the study; normal otological examination findings; ‘Pass’ result by DPOAE (Distortion Product Oto-Acoustic Emission); children born of full term (more than 37 weeks of gestation) institutional deliveries, and; normal hearing of the child as stated by the mother. The exclusion Criteriae were: children with any external and middle ear diseases; cranio-facial anomalies; neurological deficits; family history of congenital deafness; history of maternal illness like gestational diabetes mellitus (GDM), pregnancy induced hypertension (PIH) or TORCH (Toxoplasmosis, Rubella, Cytomegalovirus and Herpes) infections during pregnancy; history of peri-natal complications like birth asphyxia, prolonged labour and meconium aspiration; post-natal insults like Neonatal Intensive Care Unit (NICU) admissions, administration of ototoxic medications, hyperbilirubinaemia, febrile illnesses which necessitated hospital admission, and; ‘Refer’ result in DPOAE test.
After getting ethical clearance from the institutional ethical committee in June 2016, assessment and selection of neonates for group I was done from the post-natal wards of the hospital. Infants and children falling into group II were selected from paediatric immunization clinic. Informed consent was obtained from the parents of the children for clinical examination, OAE screening, BERA study as well as for the administration of appropriate dose of oral sedative before BERA test. All the participants were clinically evaluated to rule out any external or middle ear pathology as well as any craniofacial anomalies. All children were tested using OAE screener and only those with ‘pass’ results were selected for BERA study. The equipment used for OAE screening was OtoRead, manufactured by Interacoustics, Denmark. As per the power calculation done for the study, a minimum of 30 sample size was required in each group to elicit a statistical significant difference. In this study, we had 37 neonates in group I and 31 children in group II (Figure 1).
The BERA test was done when the child was sleeping either naturally or after administration of sedative. The sedative was used only if the child did not go to natural sleep after being fed by the mother and waited for 2- 3 hours. The sedative used was Syrup Triclofos Sodium at a dose of 50 mg/kg body weight. The equipment used for BERA testing in this study was Brainstem Auditory Evoked Response equipment – ABR System EP15/EP25 manufactured by Interacoustics, Denmark. Both OAE and BERA testing were done for all participants by the same qualified audiologist blinded to the study, in a sound treated, air conditioned room with ambient room temperature (28 ± 1 0C) and lighting (Figure 2).
If an appropriate tracing was not obtained, the child was subjected to the test again on a different day. Impedance electrode was kept below 5 K Ohms. The sound stimuli presented to the ear by insert ear phones were, clicks of 40 dB HL of negative polarity at a rate of 20.1/second, 2000 stimuli in total, in a frequency range of 300 – 3000 Hz (Hertz), with 15 ms analysis window. The following electrophysiological data from the BERA wave recordings were measured and analyzed;
Absolute latencies of waves I, III and V of both sides in ms
Inter- wave / inter- peak latencies (IPL) of wave I to III, III to V & I to V in ms
Inter-aural latency difference of wave V
Amplitude evaluation of waves I & V of R and L in micro volts (μV), & the inter-aural wave V amplitude difference (VR- VL)
Ratio of wave V to wave I (V/I) on both sides, & the inter-aural V/I ratio difference (V/I R- V/I L)
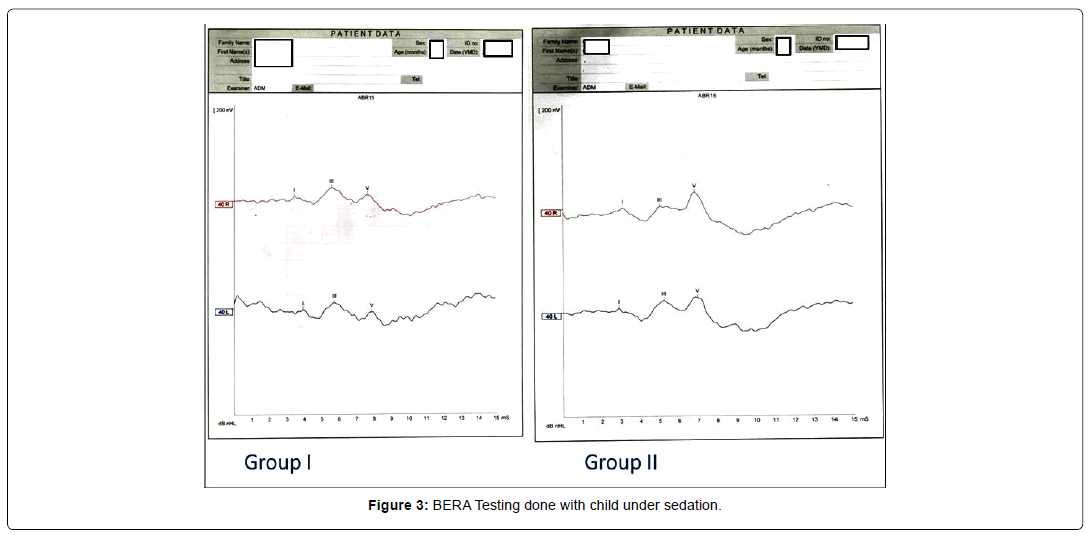
Wave morphology patterns based on 3 recognizable peaks - Good versus Poor (Figure 3).
Example of BERA tracing obtained in each group is given in Figure 3. All the measurements of latencies, amplitudes and morphology were interpreted from the BERA graph, by the same blinded audiologist. Morphology of the BERA tracing was assessed by evaluating the number of waves among I to V appeared in each recording. Good morphology was defined as presence of waves I, III and V. Poor morphology had less than three recognisable waves at 40 dB at the same auditory threshold. The data was collected on master chart, cross checking and data cleaning was done. Data analysis was performed using Microsoft excel and the software MINITAB-1513.
For statistical analysis of descriptive values, appropriate comparisons were made using paired t- test. Wherever the differences between the characters forming a pair was calculated in the first instance, one sample t- test was applied. For inter-group comparisons of amplitude and latency, Analysis of Variance (ANOVA) using General Linear Model (GLM) was used. Fisher exact test for proportions of error means, was used for the summative wave response analysis. For all tests, a P value < 0.05 implied a statistically significant difference.
Results
In group I, there were 21 females with a mean age of 4.38 days and 16 males with 4.25 days, forming an average age of 4.32 days for the group. In group II, 12 females had a mean age of 15.83 months and 19 males had 14.74 months, with the mean age for the group of 15.16 months. Analysis of wave-wise amplitude distribution between the groups is shown in (Table 1).
| Variables | Group I | Group II | ||
|---|---|---|---|---|
| N | Mean | N | Mean | |
| Amp I - R | 14 | 0.13857 | 13 | 0.1215 |
| Amp V - R | 37 | 0.17027 | 31 | 0.2529 |
| Amp V/I - R | 14 | 1.305 | 14 | 2.362 |
| Amp I - L | 12 | 0.1050 | 11 | 0.1145 |
| Amp V - L | 37 | 0.1870 | 31 | 0.2265 |
| Amp V/I - L | 12 | 2.058 | 11 | 2.269 |
| Amp (VR-VL) | 37 | -0.0168 | 31 | 0.0265 |
| Amp V/I Diff | 11 | -0.8572 | 9 | 0.3556 |
Table1: Wave-wise Distribution of Mean Amplitudes in BERA
In both right and left ears of group I & II, the V/I ratios were more than 1, with a higher V/I ration in group II, which indicated a higher amplitude for wave V in both groups and more maturation of the wave morphology in group II. Intra-group analysis of amplitudes of waves I, V and the V/I ratio difference between the ears, in both groups is shown in Table-2 along with their 95% CI and p-values (Table 2).
| Group I | ||||||
| Variables | N | Mean | SD | SE Mean | 95% CI | P value |
| Amp (IR–IL) | 11 | 0.0327 | 0.0508 | 0.0153 | (-0.0014, 0.0669) | 0.058 |
| Amp (VR-VL) | 37 | -0.0168 | 0.1028 | 0.0169 | (-0.0510, 0.0175) | 0.328 |
| Amp V/I Diff | 11 | -0.857 | 1.204 | 0.363 | (-1.6660, -0.048) | 0.040* |
| Group II | ||||||
| Variables | N | Mean | SD | SE Mean | 95% CI | P value |
| Amp (IR–IL) | 8 | 0.0075 | 0.0828 | 0.0293 | (-0.0617,0.0767) | 0.805 |
| Amp (VR-VL) | 31 | 0.0265 | 0.1329 | 0.0239 | (-0.0223, 0.0752) | 0.277 |
| Amp V/I Diff | 9 | 0.356 | 1.529 | 0.510 | (-0.820, 1.531) | 0.505 |
Table-2: Intra-group comparison of Wave Amplitudes between ears
Inter-group comparison of wave amplitudes between the ears is highlighted in (statistically significant values are indicated in bold and suffixed with an asterix) (Table 3).
| Variables | µ (GII) - µ (GI) | Fisher Exact Test | |
|---|---|---|---|
| F ratio | P value | ||
| Amp I - R | -0.0171 | 1.68 | 0.208 |
| Amp V - R | 0.0556 | 13.11 | 0.001* |
| Amp V/I (R) | 1.0570 | 14.72 | 0.001* |
| Amp I - L | 0.0095 | 0.19 | 0.669 |
| Amp V - L | 0.0395 | 1.90 | 0.173 |
| Amp V/I (L) | 0.2110 | 0.17 | 0.683 |
| Amp (VR-VL) | 0.0433 | 2.24 | 0.140 |
| AMP V/I Diff | 1.2128 | 3.52 | 0.079 |
Table 3: Inter-group comparison of Wave Amplitudes between ears
provides the descriptive statistics for analysis of wave latencies, generated in both groups (Table 4).
| Variables | Group I | Group II | ||
|---|---|---|---|---|
| N | Mean | N | Mean | |
| Lat I - R | 14 | 3.0071 | 13 | 2.992 |
| Lat I - L | 12 | 3.0710 | 11 | 2.923 |
| Lat III - R | 23 | 5.2717 | 17 | 4.962 |
| Lat III - L | 18 | 5.2528 | 15 | 5.003 |
| Lat V - R | 37 | 7.2986 | 31 | 6.973 |
| Lat V - L | 37 | 7.5162 | 31 | 7.053 |
| Lat (I-III) R | 14 | 2.1607 | 13 | 2.0731 |
| Lat (I-III) L | 12 | 2.1375 | 9 | 2.0220 |
| Lat (III-V) R | 23 | 2.0239 | 17 | 1.7030 |
| Lat (III-V) L | 18 | 2.0167 | 15 | 1.8167 |
| Lat (I-V) R | 14 | 4.2607 | 14 | 3.75 |
| Lat (I-V) L | 12 | 4.1460 | 11 | 3.855 |
| Lat V (R-L) | 37 | -0.2176 | 31 | -0.0810 |
Table 4: Wave-wise Distribution of Mean Latencies in BERA
Intra-group comparisons of wave latencies between the ears along with their 95%CI and p-values are shown in (Table 5).
| Group I | |||||
| Variables | N | Means | Mean-Diff | 95% CI | P value |
| Lat I R,L | 11 | 2.982, 3.014 | -0.0318 | (-0.2020,0.1383) | 0.686 |
| Lat III R,L | 17 | 5.321, 5.244 | 0.077 | (-0.0074, 0.1603) | 0.071 |
| Lat (I-III) R,L | 11 | 2.182, 2.141 | 0.041 | (-0.1129,0.1947) | 0.567 |
| Lat (III-V) R,L | 17 | 2.0412,2.0176 | 0.0236 | (-0.1107,0.1578) | 0.715 |
| Lat (I-V) R,L | 11 | 4.218, 4.209 | 0.009 | (-0.1666,0.1848) | 0.911 |
| Group II | |||||
| Variables | N | Means | Mean-Diff | 95% CI | P value |
| Lat I R,L | 9 | 3.000, 2.589 | 0.411 | (0.465, 1.287) | 0.311 |
| Lat III R,L | 10 | 4.840, 4.875 | -0.035 | (-0.1896,0.1196) | 0.621 |
| Lat (I-III) R,L | 6 | 2.050, 2.000 | 0.050 | (-0.295,0.395) | 0.725 |
| Lat (III-V) R,L | 10 | 1.730, 1.750 | -0.020 | (-0.1729,0.1329) | 0.774 |
| Lat (I-V) R,L | 8 | 3.819, 3.800 | 0.019 | (-0.262, 0.300) | 0.879 |
Table 5: Intra-group comparison of Wave Latencies between ears
In both the groups, there was no statistically significant difference between the R and L ears for wave latencies. ANOVA based Fisher Exact test was used for inter-aural and inter-group analysis of wave latencies. These results are summarized in Table-6 (statistically significant values are indicated in bold and suffixed with an asterix) (Table 6).
| Variables | µ (GII) - µ (GI) | Fisher Exact Test | |
|---|---|---|---|
| F ratio | P value | ||
| Lat I R | -0.0151 | 0.05 | 0.832 |
| Lat I L | -0.0940 | 2.03 | 0.170 |
| Lat III R | -0.3107 | 6.43 | 0.016* |
| Lat III L | -0.2498 | 3.63 | 0.067 |
| Lat V R | -0.3256 | 6.77 | 0.011* |
| Lat V L | -0.4632 | 10.77 | 0.002* |
| Lat(I-III) R | -0.0876 | 0.75 | 0.397 |
| Lat (I-III) L | -0.1155 | 0.74 | 0.403 |
| Lat (III-V) R | -0.3299 | 11.45 | 0.002* |
| Lat (III-V) L | -0.2000 | 3.81 | 0.061 |
| Lat (I-V) R | -0.3127 | 13.78 | 0.001* |
| Lat (I-V) L | -0.2910 | 3.51 | 0.077 |
| Lat V(R- L) | 0.1366 | 1.09 | 0.301 |
Table 6: Inter-group and Inter-aural comparison of Wave Latencies
With respect to the differences in mean latency between group I and group II, for all waves, group II had shorter latency values than group I (p<0.05). This suggests robust arborisation of the auditory network in older children with more sound exposure, leading to quicker firing of the neural synapses and faster relay of the signals to the higher auditory centres. With regard to the inter–aural latency difference, in group I, 73% of the neonates had inter-aural latency difference for wave V values ≤ 0.4 ms and 27% have values>0.4 ms. In group II, 67.74% of children have values ≤ 0.4 ms and 32.26% have values>0.4 ms. Overall, the mean wave V latency was 7.298 ms for group I and 6.97 ms for group II, the latency difference of which was statistically significant at p<0.05 (p=0.011). This reflects on a variable rate of maturation happening on both the sides, due to the phenomenon of cerebral dominance and neural plasticity. Depending on the sensory dominant brain, such trivial differences between the sides can occur and should not be considered pathological for this age group and with the sound stimulus given being 40 dB HL.
On analysis of wave morphology, in a number of children, either wave I, III or both were not generated in both groups at 40 dB HL. Wave I on right side was formed in 37.84% in group I, and in 41.94% in group II. Wave I on left side was formed in 32.43% of children in group I and 35.48% in group II. Wave III on the right side was formed in 62.16% in group I and 54.84% in group II. Similarly wave III was identified on the left side in 48.65% in group I and in 48.39% in group II. However, robust wave V was generated in 100% of cases for both the groups, which suggests normal auditory thresholds for all the members of the cohorts. Overall, 40.54% in group I and 51.61% in group II had good morphology with well differentiated three waves on BERA, at the standard auditory threshold of 40 dB. This reflects on a significant improvement in group II, suggesting wave morphology improves with auditory maturation over age.
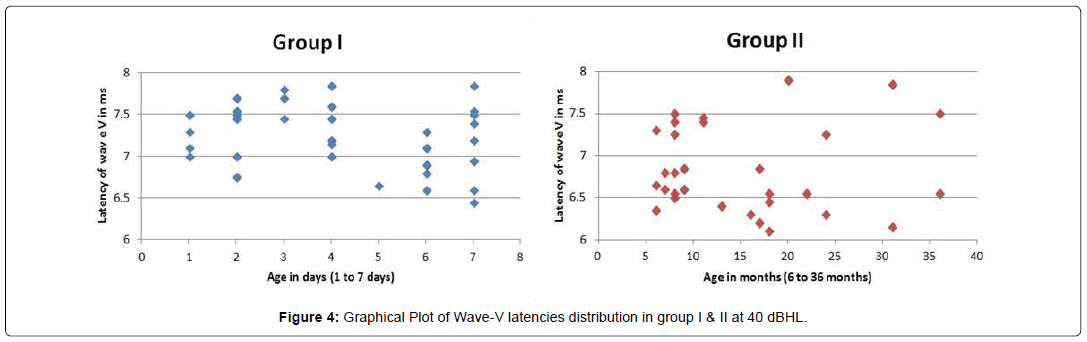
A graphical plot of the auditory maturation, as depicted by the wave V latency with regards to age, is shown in Fig-4. It was observed that latencies were shorter and were grouped closer together in group II as compared to group I. This infers that older children have better synchrony of neural signals due to longer exposure to sounds, which sensitize the auditory brain to adapt and relay faster.
BERA proved to be a sensitive and effective tool to study the auditory maturation in children with the wave patterns being more distinct in group II as compared to group I, heralding the completion of auditory maturation in the older children. Among the younger children of group I, the presence of wave forms was signalling normal hearing and indicating ongoing auditory arborisation and synchrony at different levels akin to their age. The normative data compiled from the two groups of children were fed into the institutional audiology database, to be used as a reference tool for judging the auditory maturation of future cohorts of children.
Discussion
The central auditory system starts its development intra uterine and its maturation continues after birth. In humans, the auditory pathway from the cochlear nerve to the inferior colliculus undergoes myelination between the fetal weeks of 26 and 29. By 29 weeks, myelination in all auditory pathways occur and myelination density increases until 1 year of age. As the brainstem in the central nervous system mature, there is continuous decrease in absolute wave latencies and an improved BERA wave morphology. Such decrease in the latencies relate to the progressive myelinisation of central nervous structures, increased axon diameter, improved synchronism of the neural activity, effective structural connections and improved synaptic function [3,4]. Maturation of auditory pathway up to the brainstem progresses in the caudal to cranial direction. The peripheral pathway (wave I) matures earlier and the cranial portion (wave III, V) matures later [5].
Although the first phase of development is independent of external neuro-sensory stimulation, the second phase is only effective from the auditory inputs that will direct and organize the process of synapses in the neural network. This is due to a phenomenon called ‘neural plasticity’, which is the ability of the CNS to adapt and modify in response to changes in environmental stimuli. This neural plasticity in response to auditory stimulation is important, as there is a critical age for auditory maturation. This is supported by the fact that there is a very fast maturation of auditory system of deaf children if proper auditory stimulation is given before 3 yrs of age. After this period, the development is different from that of normally hearing children, as the synaptic connections occur abnormally resulting in abnormal neuronal arborisation, functional disintegration and immaturity of cortical auditory areas [6]. In addition, cerebral dominance, cognitive and behavioural influences of the auditory brain are dependent on this critical age for maturation.
BERA is an excellent tool for identifying the auditory synchrony indicating the arborisation and synaptic maturation as indicted by its waves denoting various locations of the peripheral auditory pathway and the brainstem. Absence of BERA wave indicates lack of myelination and relay, suggestive of auditory signal deprivation. Age is one of the important factors in the clinical interpretation of BERA waves. There are changes in the values of absolute wave latencies and inter-peak or inter-wave latencies known among different age groups. Changes in BERA waves serve as guide for assessing the maturation of auditory pathway. Latency changes are reliable indicators for auditory maturity. Wave V is taken as the most important wave as it is the most robust among the BERA waves. The amplitude and morphology studies give us supporting evidence for maturation of BERA waves with age [3].
In this study, a sample of 68 children, were divided into two groups - 37 (of birth to 7 days of age) and 31 (of 6 months to 36 months of age). Considering the shortest average latency among the two ears for each wave per group, it was found that a mean latency of 3.007, 5.25 and 7.298 ms for wave I,III and V respectively occurred for group I and 2.92, 4.96 and 6.97 ms respectively occurred for group II, at the standard setting of 40 dB HL stimulation.
Age and sample size matched studies using BERA have been done in a similar way in literature. Guilhoto et al, performed BERA in 47 normal newborns on their second day of life with 80dBHL and found the latencies in ms as: 1.79 (wave I), 4.54 (wave III) and 6.75 (wave V) [7]. Sleifer et al, studied 51 full term children and measured the BERA at stimulus intensity of 80dBHL, at 4, 12 and 20 months of age. They found the mean latency of wave V progressively deceasing to be 6.90, 6.60 and 6.11 ms respectively, with progressive age [4]. Spitzer et al, studied 71 normally hearing preschool children with a stimulus intensity of 73 dB HL on BERA and they found the wave V at 3 and 5 years to be 5.76 and 5.57 ms [8].
In an extensive study Sharma et al, tested 80 children (from new born to 12 years of age) after dividing them into eight groups of 10 children each. BERA stimuli was given at 30 dB HL and wave V recorded in BERA. The mean latency from new born up to 6 months of age was 8.16ms; similarly from 7 to 12 months was 7.67ms, from 13 to 24 months was 7.56ms, from 25 to 36 months was 6.58 ms, from 37-48 months was 6.15ms, from 49 to 60 months was 5.99ms, from 61 to 84 months was 5.74ms and from 85 to 144 months was 5.65ms respectively. They found that latency of wave V decreased rapidly till 3 years of age and after that wave V maturation became slower and was finally became of adult value by 12 years of age. This study indicated that faster auditory maturation happens earlier in life [9]. However, other studies have shown adult like auditory neural maturity can happen upto 5 years of age [10]. According to another study, by the age of 3 years, wave I latency had reached the adult value and wave V latency reached adult value approximately at 5 years of age [11].
Inter-aural latency difference ideally should not be more than 0.2 ms and if this difference is more than 0.4 milliseconds, the existence of some lesion in the neural pathway is to be suspected on the side having higher latency [2]. In this study, on an average in both the groups, only around 70% (73% in group I and 67.74% in group II) children showed inter-aural latency difference values less than or equal to 0.4ms, suggesting a variable rate of maturation on both the sides, which should not be considered pathological, unless suspected otherwise by radio-imaging. These findings are in agreement with the results of a 2010 study, where they found that the inter-aural latency difference for wave V ≤ 0.4 ms were observed in 86% of term and 80% of premature infants at 80 dB HL, 83% and 77% at 60 dB HL, 75% and 80% at 40 dB HL & 89% and 83% at 20 dB HL [12].
Congenital and acquired factors which adversely influence auditory maturity like prematurity, kernicterus, birth asphyxia, hyperbilirubinemia, consanguinity, congenital hearing loss, TORCH infections, ototoxic medications etc were excluded from this study. Thereby this work highlights the actual temporal relation of auditory maturation among healthy children at birth, during their infancy and through their toddler ages. Inference from this study has helped develop a database of age-matched normative values for the various parameters of BERA, as an objective reflector of auditory maturation.
Conclusion
This study has focused on normal children who have achieved age-matched auditory maturation. BERA was used as an objective biomarker of the maturation of their auditory pathway as evident from the changes in the BERA wave morphology, latencies and amplitudes, in comparison to their age. This data has implications in evaluation of children with various forms of hearing loss and also for comparison with children having bilateral profound hearing loss, who have received interventions like cochlear implantation or auditory brainstem implantation, using which they continue to acquire auditory maturation. Future direction will be to do such a comparative analysis of auditory maturation among children with hearing loss (wearing hearing aids and/or implants), using the current normative data as reference.
References
- Jewett DL, Romano MN, Williston JS. (1970) Human auditory evoked potentials: possible brain stem components detected on the scalp. Science 167: 1517–1518.
- Biswas A. (2009) BERA chapter in Clinical Audio-vestibulometry for Otologists and Neurotologists. Mumbai: Bhalani publishing house 4: 100-132.
- Tafti FM, Gharib K, Teimuri H. (2007) Study of Age Effect on Brainstem Auditory Evoked Potential Waveforms. J.Med.Sci 7: 1362-1365.
- Sleifer P, Costa SS, Cóser PL, Goldani MZ, Dornelles C, et al. (2007) Auditory brainstem response in premature and full-term children. Int J Pediatr Otorhinolaryngol 71: 1449–1456.
- Morgan DE, Zimmerman MC, Dubno JR. (1987) Auditory brain stem evoked response characteristics in the full-term newborn infant. Ann Oto Rhinol Laryn 96: 142–151.
- Silva LAF, Couto MIV, Tsuji RK, Bento RF, Carvalho ACM, et al. (2015) Auditory cortical maturation in a child with cochlear implant: Analysis of electrophysiological and behavioural measures. Case Reports in Otolaryngo l 1-6.
- Guilhoto LM, Quintal VS, Costa MT. (2003) Brainstem auditory evoked response in normal term neonates. Arquivos de neuro-psiquiatria 61: 906–908.
- Spitzer E, Schwoch TW, Carr KW, Skoe E, Kraus N. (2015) Continued maturation of the click-evoked auditory brainstem response in pre-schoolers. J Am Acad Audiol 26: 30–35.
- Sharma AM, Bist SS, Kumar S. (2016) Age-Related Maturation of Wave V Latency of Auditory Brainstem Response in Children. J Audiol Otol 20: 97–101.
- Johnson KL, Nicol T, Zecker SG, Kraus N. (2008) Developmental plasticity in the human auditory brainstem. J Neurosci 28: 4000–4007.
- Khatoon M, Nighute S, Singh R, Awari A, Ishaque M. (2012) Maturation of Brainstem Auditory Evoked Potential from full term infants & children to young adult Int.J.Biomed.Res 3: 439-443.
- Casali RL, Santos MFC. (2010) Auditory Brainstem Evoked Response: response patterns of full-term and premature infants. Braz J Otorhinolaryngol 76: 729-738.
Citation: Mathews S, Nandhan R, Sabarigirish K, Das AK, Prasad BK (2020) Mapping Auditory Maturation from Neonates to Toddlers using Electrophysiological Responses of the Brainstem. Otolaryngol (Sunnyvale) 10:402. DOI: 10.4172/2161-119X.1000402
Copyright: © 2020 Mathews S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3392
- [From(publication date): 0-2020 - Apr 18, 2025]
- Breakdown by view type
- HTML page views: 2531
- PDF downloads: 861