Editorial Open Access
Manufacturing Process of Solar Cells Using Cadmium Oxide (CdO) and Rhodium (III) Oxide (Rh2O3) Nanoparticles
A Heidari*Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, USA
- Corresponding Author:
- A Heidari
Faculty of Chemistry, California South University
14731 Comet St. Irvine, CA 92604, USA
E-mail: Scholar.Researcher.Scientist@gmail.com
Received date: March 28, 2016; Accepted date: April 13, 2016; Published date: April 20, 2016
Citation: Heidari A (2016) Manufacturing Process of Solar Cells Using Cadmium Oxide (CdO) and Rhodium (III) Oxide (Rh2O3) Nanoparticles. J Biotechnol Biomater 6:e125. doi:10.4172/2155-952X.1000e125
Copyright: © 2016 Heidari A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Editorial
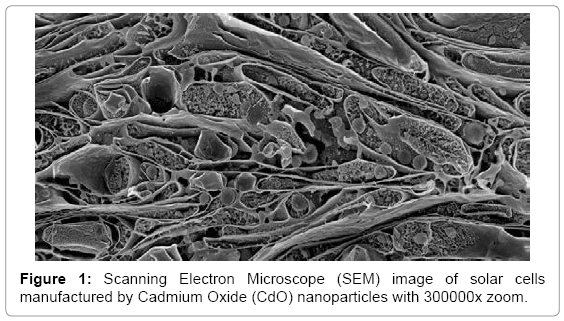
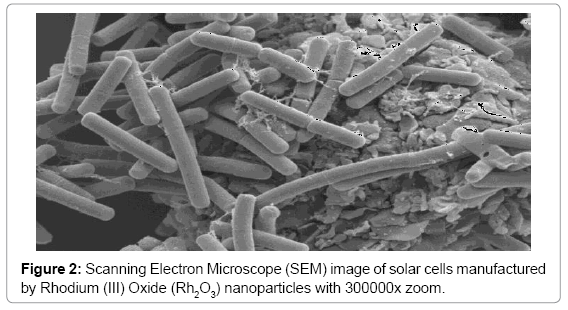
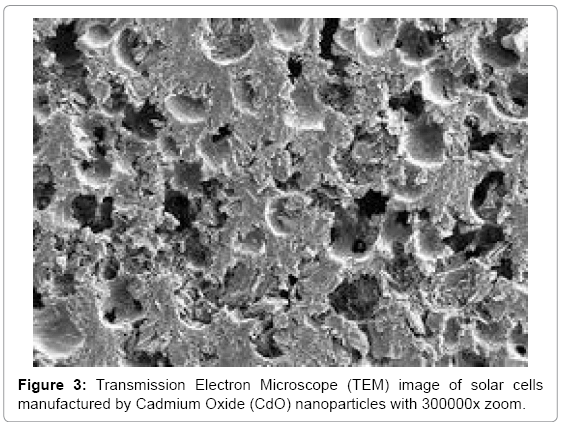
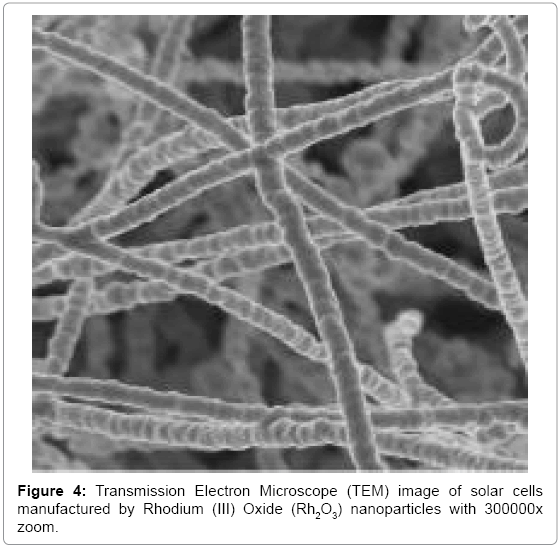
Effective conversion of sunlight to electricity has been final goal in organic and inorganic biophotochemistry. In recent years, solar cells manufactured by nanoparticles of Cadmium Oxide (CdO) and Rhodium (III) Oxide (Rh2O3) use extensively in order to converting sun light (Figures 1-4) [1-16]. Illuminating light equal to semiconductor band gap make produce electron and hole in them. If sunlight be able to supply required energy to produce electron and hole, sunlight can be converting directly to electrical energy. This process is being done easily in narrow band gap semiconductors. Bands’ gap of Cadmium Oxide (CdO) and Rhodium (III) Oxide (Rh2O3) nanoparticles are from IR to UV range. Since the only 5–10% of sunlight is in this range, in solar cells based on Cadmium Oxide (CdO) and Rhodium (III) Oxide (Rh2O3) nanoparticles, for making them sensitive to visible light, dyes are used as scavenger. In this editorial, the method of manufacturing electrodes which are synthesized by Mercurochrome have been studied and comparing of their efficiency due to their structure and morphology, have been done by Energy Dispersion Analysis (EDS), Scanning Electron Microscope (SEM), X–Ray Diffraction (XRD), Transmission Electron Microscope (TEM), Differential Thermal Analysis–Thermal Gravim Analysis (DTA–TGA), Energy–Dispersive X–Ray Spectroscopy (EDX), 1HNMR, 13CNMR, UV–Vis, Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR–FTIR) and FT–Raman spectroscopies and also ESI MS, PM5 and DFT studies.
References
- Heidari A, Brown C (2015) Study of composition and morphology of cadmium oxide (CdO) nanoparticles for eliminating cancer cells, JNanomed Res2: 00042.
- Heidari A, Brown C (2015) Study of surface morphological, phytochemical and structural characteristics of rhodium (III) oxide (Rh2O3) nanoparticles.Int JPharmacolPhytochemEthnomed 1: 15-19.
- Zhang S, Yu Z, Li P, Li B, Isikgor FH, et al. (2016) Poly(3,4-ethylenedioxythiophene):polystyrene sulfonate films with low conductivity and low acidity through a treatment of their solutions with probe ultrasonication and their application as hole transport layer in polymer solar cells and perovskite solar cells. Organic Electronics 32: 149-156.
- Dissanayake MAKL, Divarathna KKDWMN, Dissanayake CB, Senadeera GKR, Ekanayake PMPC, et al. (2016) An innovative TiO2 nanoparticle/nanofibre/nanoparticle, three layer composite photoanode for efficiency enhancement in dye-sensitized solar cells. Journal of Photochemistry and Photobiology A: Chemistry322-323: 110-118.
- Shen W, Tang J, Wang D, Yang R, Chen W, et al. (2016) Enhanced efficiency of polymer solar cells by structure-differentiated silver nano-dopants in solution-processed tungsten oxide layer. Materials Science and Engineering: B 206: 61-68.
- Rattanawan R, Promarak V, Sudyoadsuk T, Namuangruk S, Kungwan N, et al. (2016) Theoretical design of coumarin derivatives incorporating auxiliary acceptor with D-π-A-π-A configuration for dye-sensitized solar cells. Journal of Photochemistry and Photobiology A: Chemistry 322-323: 16-26.
- Tunuguntla V, Chen W, Newman TD, Chen CY, Hsieh MC, et al. (2016) Enhancement of charge collection at shorter wavelengths from alternative CdS deposition conditions for high efficiency CZTSSe solar cells. Solar Energy Materials and Solar Cells 149: 49-54.
- Joseph KLV, Anthonysamy A, Easwaramoorthi R, Shinde DV, Ganapathy V, et al. (2016)Cyanoacetic acid tethered thiophene for well-matched LUMO level in Ru(II)-terpyridine dye sensitized solar cells. Dyes and Pigments 126: 270-278.
- Iwan A, Palewicz M, Tazbir I, Boharewicz B, Pietruszka R, et al. (2016) Influence of ZnO:Al, MoO3 and PEDOT:PSS on efficiency in standard and inverted polymer solar cells based on polyazomethine and poly(3-hexylthiophene).ElectrochimicaActa 191: 784-794.
- Celic N, Pavlica E, Borovšak M, Strle J, Buh J, et al. (2016) Factors determining large observed increases in power conversion efficiency of P3HT:PCBM solar cells embedded with Mo6S9−xIx nanowires. Synthetic Metals 212: 105-112.
- Moon BJ, Lee KS, Shim J, Park S, Kim SH, et al. (2016) Enhanced photovoltaic performance of inverted polymer solar cells utilizing versatile chemically functionalized ZnO@graphene quantum dot monolayer. Nano Energy 20: 221-232.
- Wei S, Shao Y, Shi X, Lu X, Li K, et al. (2016)Heteroleptic Cu(I) complexes integrating functionalized chromophores for dye-sensitized solar cells: An in-depth analysis of electronic structure, spectrum, excitation, and intramolecular electron transfer. Organic Electronics29: 142-150.
- Zhang J, Vlachopoulos N, Jouini M, Johansson MB, Zhang X, et al. (2016)Efficient solid-state dye sensitized solar cells: The influence of dye molecular structures for the in-situ photoelectrochemically polymerized PEDOT as hole transporting material. Nano Energy19: 455-470.
- Dawidowski W, Sciana B, Zborowska-Lindert I, Mikolášek M, Bielak K, et al. (2016)The influence of top electrode of InGaAsN/GaAs solar cell on their electrical parameters extracted from illuminated I–V characteristics. Solid-State Electronics 120: 13-18.
- Insignares-Cuello C, Oliva F, Neuschitzer M, Fontané X, Broussillou C, et al. (2015) Advanced characterization of electrodeposition-based high efficiency solar cells: Non-destructive Raman scattering quantitative assessment of the anion chemical composition in Cu(In,Ga)(S,Se)2 absorbers. Solar Energy Materials and Solar Cells 143: 212-217.
- Krajangsang T, Inthisang S, Dousse A, Moollakorn A, Hongsingthong A, et al. (2016) Band gap profiles of intrinsic amorphous silicon germanium films and their application to amorphous silicon germanium heterojunction solar cells. Optical Materials 51: 245-249.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 14249
- [From(publication date):
June-2016 - Apr 26, 2025] - Breakdown by view type
- HTML page views : 13140
- PDF downloads : 1109