Maneb and Mancozeb Increase Amyloid Îò Precursor Protein Expression and Activate PKR
Received: 24-Oct-2017 / Accepted Date: 16-Nov-2017 / Published Date: 26-Nov-2017
Abstract
Background: Oligomers of amyloid β (Aβ) in the brain correlate with synapse loss and dementia. The fungicide maneb (MB) and mancozeb (MZ) have been shown to activate the transcription factor NF-κB. Activation of NF-κB signaling pathway could initiate the transcription of β-amyloid precursor protein (AβPP) leading to increase Aβ level. Studies showed the cells treated with Aβ42 have the elevated level of phosphorylated double-stranded RNA dependent protein kinase (PKR) which is found to deteriorate the neurons in AD brains.
Objective: To reveal the effects of MB and MZ on AβPP and Aβ42 expressions and to elucidate the role of PKR in response to MB and MZ.
Methods: Western blot analysis and ELISA assay were conducted to investigate the expressions of AβPP and Aβ42 in PC12 cells treated with MB and MZ. The activation of PKR was determined by western blotting the level of phosphorylated PKR at Thr446 in SH-SY5Y cells treated with MB and MZ.
Results: MB and MZ increased AβPP and Aβ42 expressions in a dose-dependent manner. MB and MZ transiently activated PKR.
Conclusion: Studies showed that MB and MZ enhance parkinsonian toxin MPP+ cytotoxicity and trigger DNA damage. The results from this study further revealed that MB and MZ are associated with the increase of AβPP and Aβ42 expressions and elucidated that the PKR signaling pathway involves in MB and MZ induced cytotoxicity. This study provides the evidence about the relationship between fungicides and neurodegenerative diseases.
Keywords: Maneb; Mancozeb; AβPP; Aβ42; PKR
Introduction
Alzheimer’s disease (AD) is one of the most common neurodegenerative diseases affecting older people. The etiology of AD is multifactorial including genetic and environmental factors. The association between the exposure to environmental toxins with the pathogenesis of AD has been suggested [1,2]. Tartaglione et al. [3] indicated that exposure to environmental chemicals in the early stage of life can interfere with developmental programming which increases the susceptibility to develop neurodegenerative diseases at a later life stage. The brains of AD patients present extracellular deposits mainly composed of a set of hydrophobic peptides called amyloid β-peptides (Aβ) [4,5]. Aβ is generated from β-amyloid precursor protein (AβPP) by secretase cleavages [6]. Some neurotoxins have been shown to elevate β-amyloid precursor protein (AβPP) expression, increasing β-amyloid (Aβ) peptide levels [7,8]. Oligomers of Aβ in the brain correlate with synapse loss and dementia [9]. Studies have also suggested that Aβ42 is much more neurotoxic than Aβ40 and is responsible for forming the plaques that are associated with Alzheimer’s disease [10,11].
Increased evidences indicated that excessive or prolonged inflammatory reaction has a strong association with neurodegeneration [12,13]. The NF-κB (nuclear factor κB) transcription factor family which involves in cellular responses to various inflammatory stimuli is activated in AD brain [13,14]. NF-κB signaling pathway plays an unique role in neuronal survival and in increased vulnerability to neuronal cell death (13). Elevated activated NF-κΒ p65 increases Aβ production by enhancing endogenous beta site amyloid precursor protein cleaving enzyme-1 (BACE-1) transcription. Activation of NF- κB signaling pathway could also activate the transcription of AβPP [15]. The fungicide maneb (MB) and mancozeb (MZ) used in this study both contain manganese, which in high doses can be neurotoxic, and have been shown to activate the transcription factor NF-κB [16]. However, whether MB and MZ can increase AβPP and Aβ expressions is not clear.
Increased double-stranded RNA dependent protein kinase (PKR) expression is evidenced with aging and/or present in some neurodegenerative diseases [17-23]. Activated PKR is found to deteriorate the neurons in AD brains and also can regulate inflammatory responses of neurons [24]. PKR is one of several eukaryotic translation initiation factor 2α (eIF2α) kinases which are regulated by stresses. Phosphorylating eIF2α by eIF2α kinases will inhibit its function on protein translation. Studies showed that phosphorylation of PKR and eIF2α is increased in the cells treated with Aβ42. The molecular connection between PKR and mTOR also has been demonstrated in response to Aβ42 [21]. Dysregulation of PI3K/Akt/mTOR signaling pathway contributes to the pathogenesis of AD. Hugon et al. [23] indicated that PKR involves in Aβ synthesis via BACE 1. This study was also aimed to elucidate the involvement of PKR in response to MB and MZ in SHSY5Y cells.
Materials and Methods
Cell culture
Human neuroblastoma (SHSY5Y) and rat pheochromocytoma (PC12) cell lines were obtained from American Type Culture Collection (ATCC Inc). Both cell lines have been used for Alzheimer studies. Briefly, PC12 cells were grown in fresh media containing Dulbecco's Modified Eagle's Medium (DMEM), with 10% fetal bovine serum (FBS), 5% heat-inactivated donor horse serum, 50 μg/ml gentamicin in a humidified atmosphere of 37°C and 5% CO2. SHSY5Y cells were grown in fresh media containing DMEM/F12, 10% FBS and 50 μg/ml gentamicin in a humidified atmosphere of 37°C and 5% CO2. Cells were set up on a day before the chemical treatments to ensure that the cell density on the day of experiment was about 80% confluence.
Chemical treatment
Manganese-containing dithiocarbamates, maneb (MB) and mancozeb (MZ), were obtained from Sigma Aldrich. The various experimental concentrations of MB and MZ were freshly prepared 15 minutes prior to treatment. First, MB and MZ were dissolved in dimethylsulfoxide (DMSO) and then diluted to the experimental concentrations by 1x phosphate-buffered saline (PBS). The final DMSO concentration in the cells was less than 0.1%, which is below toxic dose. These tested concentrations of MB and MZ were chosen based on previous studies [16,24]. Cells were treated with their indicated chemicals and the control cells was treated with PBS for the indicated time periods at 37°C and 5% CO2 incubator.
Western blot analysis
After chemical exposures, cells were lysed with a mixture of Mammalian protein extraction reagent (M-PER) and Halt™ protease/ phosphatase inhibitor cocktail (Thermo Fisher). The concentration of protein samples was determined by using Bio-Rad DC (detergent compatible) protein assay reagents (Bio-Rad, Hercules, CA).
Thirty (30) μg of protein samples from each treatment condition were resolved via 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes. Membranes were blocked in PBS/0.05% Tween 20, containing 5% FBS, and were then probed overnight with primary antibody specific for AβPP (Sigma, A8717), Aβ42 (Sigma, A1650), β-actin (Sigma, A2228), PKR (Santa Cruz, SC707), p446 PKR (Santa Cruz, SC16565) at concentration of 1 μg/μl. The antibody was detected with corresponding horseradish peroxidase-linked secondary antibody. Blots were developed using Super Signal West Pico Chemiluminescent Substrate detection reagents from Pierce. Membranes were then stripped with Stripping buffer for 15 min at room temperature and re-probed with loading control antibody. Chemilluminescent signals were captured using Geliance 600 imaging system (Perkin Elmer, Shelton, CT) and analyzed by GeneTools software (Syngene, Frederick, MD).
ELISA for quantifying Aβ42
The level of Aβ42 was detected with Amyloid beta 42 ELISA Kit from Invitrogen following the manufacturer’s protocol. Briefly, the samples were added onto a 96-well plate, which is coated with a monoclonal antibody to N-terminus of Aβ42. The samples containing Aβ42 antigen were captured to the immobilized antibody coated on a 96-well plate. After washing, a rabbit monoclonal antibody specific for the C-terminus of Aβ42 was added. The bound rabbit antibody was then detected by the use of a horseradish peroxidase (HRP)-labeled anti-rabbit antibody. After washing, a substrate solution was added, which is acted upon by the bound HRP enzyme to produce a signal. The intensity of the signal is directly proportional to the concentration of Aβ42 in the samples.
Statistics
All experiments were performed at least in triplicate, and results are reported as means ± SEM. Statistical significance was determined using one way analysis of variance (ANOVA) followed by Dunnett’s posthoc test (p<0.05, with control at 100%) by using GraphPad PRISM® 6 software.
Results and Discussion
MB and MZ increase AβPP and Aβ42 oligomer expressions in PC12 cells
Alzheimer’s disease (AD), like many other neurodegenerative diseases, has a complex mechanism and is not fully understood. The major component of amyloid plaques found in AD patients’ brains is amyloid β-peptides (Aβ) which is the cleavage product of β-amyloid precursor protein (AβPP). Elevating AβPP expression and Aβ peptide levels can been triggered by neurotoxins, such as diethyldithiocarbamate [1,8]. Maneb (MB) and mancozeb (MZ) which are also dithiocarbamate compounds have been shown to cause DNA damage and enhance parkinsonian toxin MPP+ toxicity [16,25]. Manganese which MB and MZ both contain is known neurotoxic in high doses [26]. Tong et al. [27] indicated that excessive Mn exposure increases Aβ by inhibiting its degradation. However, the association of MB and MZ with AD is not so clear.
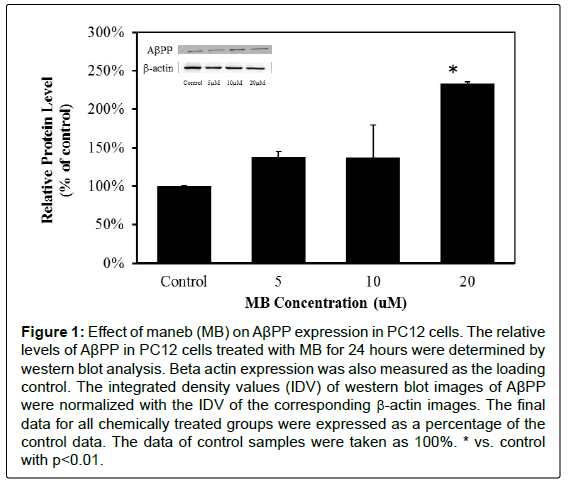
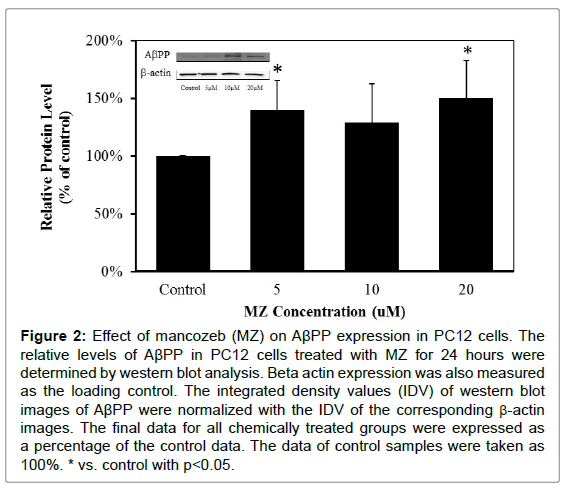
In order to determine whether MB and MZ can increase AβPP and Aβ42 expressions, PC12 cells were treated with MB and MZ at different concentrations (5-20 μM) for 24 hours and the protein samples were then analyzed by western blot analysis. The western blot results showed that cells treated with MB for 24 hours have significantly increased AβPP expression by 133% in the cells treated with 20 μM MB (Figure 1). The cells treated with MZ for 24 hours have significantly elevated AβPP expression levels by 39% in the cells treated with 5 μM MZ and 50% in the cells treated with 20 μM MZ (Figure 2).
Figure 1: Effect of maneb (MB) on AβPP expression in PC12 cells. The relative levels of AβPP in PC12 cells treated with MB for 24 hours were determined by western blot analysis. Beta actin expression was also measured as the loading control. The integrated density values (IDV) of western blot images of AβPP were normalized with the IDV of the corresponding β-actin images. The final data for all chemically treated groups were expressed as a percentage of the control data. The data of control samples were taken as 100%. * vs. control with p<0.01.
Figure 2: Effect of mancozeb (MZ) on AβPP expression in PC12 cells. The relative levels of AβPP in PC12 cells treated with MZ for 24 hours were determined by western blot analysis. Beta actin expression was also measured as the loading control. The integrated density values (IDV) of western blot images of AβPP were normalized with the IDV of the corresponding β-actin images. The final data for all chemically treated groups were expressed as a percentage of the control data. The data of control samples were taken as 100%. * vs. control with p<0.05.
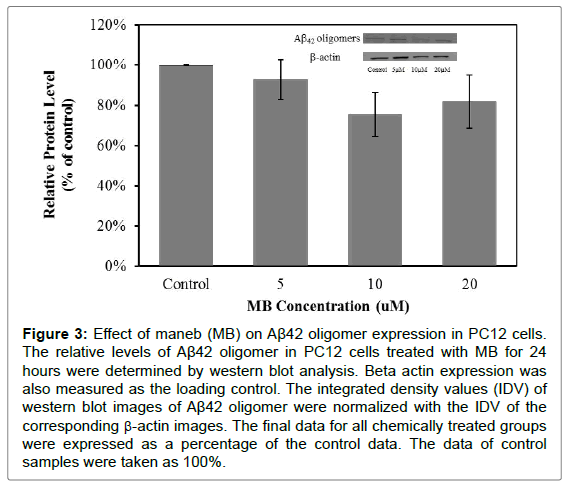
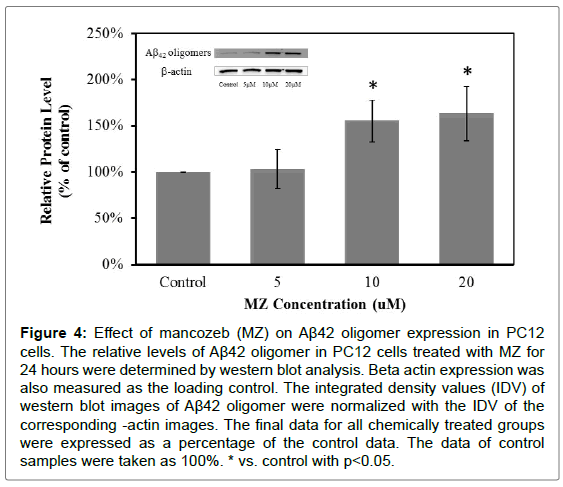
The expression of Aβ42 was also monitored by western blot analysis. The Aβ42 expression detected in this experiment was of an oligomer. The results showed that cells treated with MB for 24 hours did not have the significant changes in Aβ42 oligomer expression (Figure 3). But cells treated with MZ for 24 hours have significantly increased Aβ42 oligomer expression levels, 55% increase in the cells treated with 10 μM MZ and 63% increase in the cells treated with 20 μM MZ (Figure 4).
Figure 3: Effect of maneb (MB) on Aβ42 oligomer expression in PC12 cells. The relative levels of Aβ42 oligomer in PC12 cells treated with MB for 24 hours were determined by western blot analysis. Beta actin expression was also measured as the loading control. The integrated density values (IDV) of western blot images of Aβ42 oligomer were normalized with the IDV of the corresponding β-actin images. The final data for all chemically treated groups were expressed as a percentage of the control data. The data of control samples were taken as 100%.
Figure 4: Effect of mancozeb (MZ) on Aβ42 oligomer expression in PC12 cells. The relative levels of Aβ42 oligomer in PC12 cells treated with MZ for 24 hours were determined by western blot analysis. Beta actin expression was also measured as the loading control. The integrated density values (IDV) of western blot images of Aβ42 oligomer were normalized with the IDV of the corresponding -actin images. The final data for all chemically treated groups were expressed as a percentage of the control data. The data of control samples were taken as 100%. * vs. control with p<0.05.
These results showed that MB and MZ enhanced AβPP expression in a dose-dependent manner (Figures 1 and 2). Interestingly, the increased level of Aβ42 oligomers was only observed in cells treated with MZ in a dose-dependent manner (Figure 4). The level of Aβ42 oligomers in cells treated with MB did not change in a dose-dependent manner (Figure 3). Since Aβ is the cleavage product of AβPP, the smaller increases of AβPP expression in the cells treated with MZ might be due to the cleavage of AβPP. Therefore, the cells treated with MZ have the higher increases of the Aβ42 oligomers levels. On the other hand, the level of Aβ42 oligomers in MB treated cells might be increased after longer chemical exposure.
MB and MZ increase Aβ42 monomer expression in PC12 cells
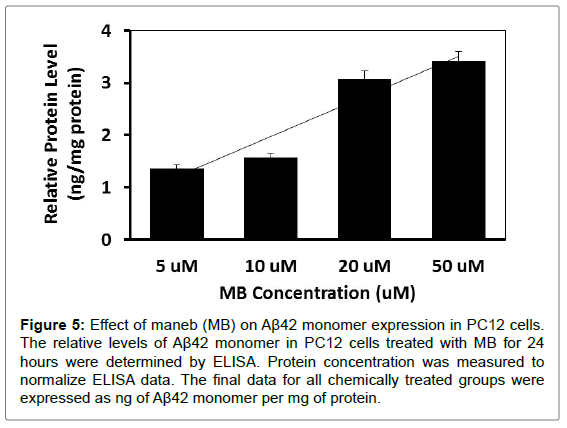
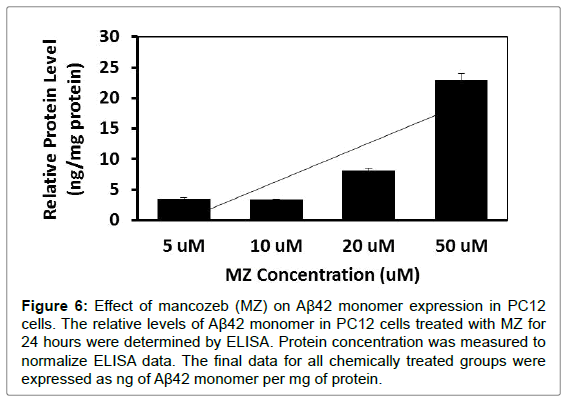
To further investigate whether MB and MZ can increase Aβ42 monomer expression, PC12 cells were treated with MB and MZ at different concentrations (5-50 μM) for 24 hours. The level of Aβ42 monomer expression was determined through ELISA analysis. The results showed that cells treated with MB for 24 hours increased Aβ42 monomer expression from 1.4 ng/mg protein (cells treated with 5 μM) to 3.4 ng/mg protein (cells treated with 50 μM) (Figure 5). When cells were treated with MZ for 24 hours, Aβ42 monomer expression increased from 3.5 ng/mg protein (cells treated with 5 μM) to 22.9 ng/ mg protein (cells treated with 50 μM) (Figure 6).
Figure 5: Effect of maneb (MB) on Aβ42 monomer expression in PC12 cells. The relative levels of Aβ42 monomer in PC12 cells treated with MB for 24 hours were determined by ELISA. Protein concentration was measured to normalize ELISA data. The final data for all chemically treated groups were expressed as ng of Aβ42 monomer per mg of protein.
Figure 6: Effect of mancozeb (MZ) on Aβ42 monomer expression in PC12 cells. The relative levels of Aβ42 monomer in PC12 cells treated with MZ for 24 hours were determined by ELISA. Protein concentration was measured to normalize ELISA data. The final data for all chemically treated groups were expressed as ng of Aβ42 monomer per mg of protein.
ELISA assay results from this study showed the increases of Aβ42 monomer expressions in cells treated with MB and MZ in a dosedependent manner (Figure 5 and 6). MB triggered less of an increase in Aβ42 monomer expression as compared with MZ. Studies indicated that Aβ oligomers are neurotoxic and can impair synaptic plasticity and Aβ monomer has neuroprotective properties [28-32]. These results along with previous cytotoxicity results of MB and MZ, reinforce that MZ is much more toxic compared to MB [16,26,33]. These results also were consonant with western blot analysis data of Aβ42 oligomer expressions that MZ triggered larger increases in Aβ42 oligomer expressions as compared with MB.
MB and MZ transiently activate PKR in SH-SY5Y cells
PKR constitutively expressed in mammalian cells is one of serine-threonine kinases and can phosphorylate eIF2α to block protein synthesis leading to cell death in response to cellular stresses. Numerous studies suggested that PKR is dysregulated in cellular and animal models of neurodegenerations and in the brains of AD patients [21-23,34,35]. Morel et al. [21,22] suggested that PKR activation could be an early indicator of the neuronal death in AD.
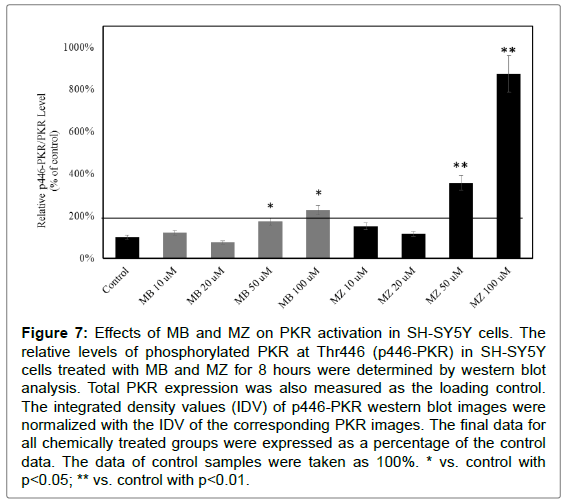
Studies suggested that PKR signaling pathway is activated in response to Aβ42. To determine the involvement of PKR in response to MB and MZ, SH-SY5Y cells were treated with MB and MZ at 10-100 μM for 8 and 16 hours and the protein samples were then analyzed by western blot analysis. The results showed that 8 hours of MB and MZ exposures increased the level of phosphorylated PKR at T446 (p446 PKR) in a dose-dependent manner (Figure 7). MZ triggered stronger PKR activation as compared to MB.
Figure 7: Effects of MB and MZ on PKR activation in SH-SY5Y cells. The relative levels of phosphorylated PKR at Thr446 (p446-PKR) in SH-SY5Y cells treated with MB and MZ for 8 hours were determined by western blot analysis. Total PKR expression was also measured as the loading control. The integrated density values (IDV) of p446-PKR western blot images were normalized with the IDV of the corresponding PKR images. The final data for all chemically treated groups were expressed as a percentage of the control data. The data of control samples were taken as 100%. * vs. control with p<0.05; ** vs. control with p<0.01.
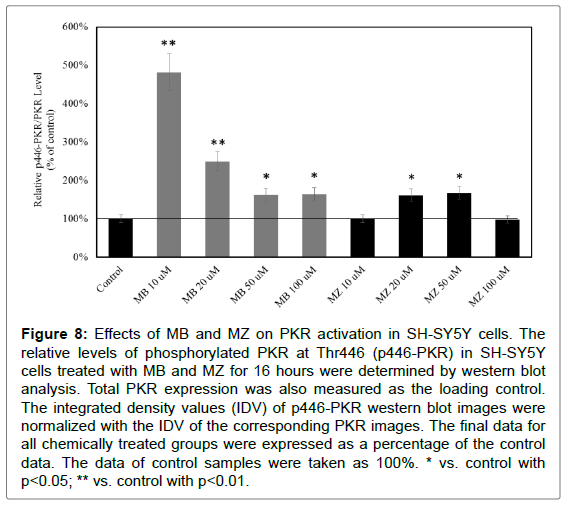
The level of phosphorylated PKR (p446 PKR) still increased after 16 hours of MB and MZ exposures (Figure 8). For cells treated with 10 and 20 μM of MB for 16 hours, the increases were higher as compared with cells treated with 10 and 20 μM of MB for 8 hours. The increases after 16 hours of 50 and 100 μM MZ exposures were much less compared to those treated for 8 hours. This indicated that the activation of PKR was transient.
Figure 8: Effects of MB and MZ on PKR activation in SH-SY5Y cells. The relative levels of phosphorylated PKR at Thr446 (p446-PKR) in SH-SY5Y cells treated with MB and MZ for 16 hours were determined by western blot analysis. Total PKR expression was also measured as the loading control. The integrated density values (IDV) of p446-PKR western blot images were normalized with the IDV of the corresponding PKR images. The final data for all chemically treated groups were expressed as a percentage of the control data. The data of control samples were taken as 100%. * vs. control with p<0.05; ** vs. control with p<0.01.
Marchal et al. indicated that PKR is able to phosphorylate p53 tumor suppressor, which activates RTP801 [36]. RTP801 activation can hinder mTOR activity leading to cell death [36]. In 2014, Cheng et al. revealed that MB and MZ can up-regulate RTP801 [26]. This study further elucidated that PKR could be the up-stream signaling pathway of RTP801 in response to MB and MZ.
Conclusion
Studies showed that MB and MZ enhance MPP+ cytotoxicity and trigger DNA damage. RTP801 activation via NF-κB in response to MB and MZ was evidenced. The results from this study further revealed that MB and MZ were associated with the increases of AβPP and Aβ42 expressions and elucidated that the PKR signaling pathway involved in MB and MZ induced cytotoxicity. The activated PKR could lead to the activation of RTP801 and cell death. This study provides the evidence about the relationship between fungicides and neurodegenerative diseases.
Grant
This work was supported by PSC CUNY grant and the PRISM program at John Jay. Special thanks to John Jay College of Criminal Justice Start-up Fund. PRISM is the Program for Research Initiatives for Science Majors at John Jay College and funded by the Title V, HSISTEM and MSEIP programs within the U.S. Department of Education; the PAESMEM program through the National Science Foundation; and New York State’s Graduate Research and Teaching Initiative.
References
- Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B (2015) Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci 9: 124.
- Yan D, Zhang Y, Liu L, Yan H (2016) Pesticide exposure and risk of Alzheimer’s disease: a systematic review and meta-analysis. Sci Rep 6: 32222.
- Tartaglione AM, Venerosi A, Calamandrei G (2016) Early-life toxic insults and onset of sporadic neurodegenerative diseases-an overview of experimental studies. Curr Top Behav Neurosci 29: 231-264.
- Glenner GG, Wong CW (1984) Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloidfibril protein. Biochem Biophys Res Commun 122: 1131-1135.
- Glenner GG, Wong CW (1984) Alzheimer’s disease: initial report of the purification and characterization of a novel cerebro-vascular amyloid protein. Biochem Biophys Res Commun 120: 885-890.
- Li Y, Zhou W, Tong Y, He G, Song W (2006) Control of APP processing and Ab generation level by BACE1 enzymatic activity and transcription. FASEB J 20: 285-292.
- Huang H, Bihaqi SW, Cui L, Zawia NH (2011) In vitro Pb exposure disturbs the balance between Aβ production and elimination: The role of AβPP and neprilysin. Neurotoxicology 32: 300-306.
- Cheng SY, Trombetta LD (2004) The Induction of amyloid precursor protein and α-synuclein in rat hippocampal astrocytes by diethyldithiocarbamate and copper with or without glutathione. Toxicology Letters 146: 139-149.
- Shankar GM, Walsh DM (2009) Alzheimer's disease: synaptic dysfunction and Aβ. Mol Neurodegener 4: 48.
- Hatami A, Albay R 3rd, Monjazeb S, Milton S, Glabe C (2014) Monoclonal antibodies against Aβ42 fibrils distinguish multiple aggregation state polymorphisms in vitro and in Alzheimer disease brain. J Biol Chem 289: 32131-32143.
- Sun X, Chen WD, Wang YD (2015) b-Amyloid: the key peptide in the pathogenesis of Alzheimer’s disease. Front Pharmacol 6: 221.
- Lukiw WJ (2012) NF-kappaB-regulated, proinflammatory miRNAs in Alzheimer’s disease. Alzheimer’s Research Therapy 4: 47.
- Srinivasan M, Bayon B, Chopra N, Lahiri DK (2016) Novel nuclear factor kappa B targeting peptide suppresses β-amyloid induced inflammatory and apoptotic responses in neuronal cells. PLoS ONE 11: e0160314.
- Hwang CJ, Park MH, Choi MK, Choi JS, Oh KW et al. (2016) Acceleration of amyloidogenesis and memory impairment by estrogen deficiency through NF-kB dependent beta-secretase activation in presenilin 2 mutant mice. Brain Behavior Immunity 53: 113-122.
- Marwarha G, Raza S, Prasanthi JRP, Ghribi O (2013) Gadd153 and NF-κB crosstalk regulates 27-hydroxycholesterol-induced increase in BACE1 and β-amyloid production in human neuroblastoma SH-SY5Y cells. PLOS ONE 8: e70773.
- Williams CA, Lin Y, Maynard A, Cheng SY (2013) Involvement of NF kappa B in potentiated effect of Mn-containing dithiocarbamates on MPP+ induced cell death. Cell Mol Neurobiol 33: 815-823.
- Ladiges W, Morton J, Blakely C, Gale M (2000) Tissue specific expression of PKR protein kinase in aging B6D2F1 mice. Mech Ageing Dev 114: 123-132.
- Peel AL, Rao RV, Cottrell BA, Hayden MR, Ellerby LM, et al. (2001) Double-stranded RNA-dependent protein kinase, PKR, binds preferentially to Huntington’s disease (HD) transcripts and is activated in HD tissue. Hum Mol Genet 10: 1531-1538.
- Chang RC, Wong AK, Ng HK, Hugon J (2002) Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer’s disease. Neuroreport 13: 2429-2432.
- Bando Y, Onuki R, Katayama T, Manabe T, Kudo T, et al. (2005) Double-strand RNA dependent proteinkinase (PKR) is involved in the extrastriatal degeneration in Parkinson’s disease and Huntington’s disease. Neurochem Int 46: 11-18.
- Morel M, Couturier J, Pontcharraud R, Gil R, Fauconneau B, et al. (2009) Evidence of molecular links between PKR and mTOR signalling pathways in Abeta neurotoxicity: role of p53, Redd1 and TSC2. Neurobiol Dis 36: 151-161.
- Morel M, Couturier J, Lafay-Chebassier C, Paccalin M, Page G (2009) PKR, the double stranded RNA-dependent protein kinase as a critical target in Alzheimer's disease. J Cell Mol Med 13: 1476-1488.
- Hugon J, Mouton-Liger F, Dumurgier J, Paquet C (2017) PKR involvement in Alzheimer's disease. Alzheimers Res Ther 9: 83.
- Carret-Rebillat AS, Pace C, Gourmaud S, Ravasi L, Montagne-Stora S, et al. (2015) Neuroinflammation and Aβ accumulation linked to systemic inflammation are decreased by genetic PKR down-regulation. Sci Rep 5: 8489.
- Cheng SY, Oh S, Velasco M, Ta C, Montalvo J, et al. (2014) RTP801 regulates maneb- and mancozeb-induced cytotoxicity via NF-ĸB. J Biochem Mol Toxicol 28: 302-311.
- Dobson AW, Erikson KM, Aschner M (2004) Manganese neurotoxicity. Annals New York Academy Sci 1012: 115-128.
- Tong Y, Yang H, Tian X, Wang H, Zhou T, et al. (2014) High manganese, a risk for Alzheimer’s disease: high manganese induces amyloid-β related cognitive impairment. J Alzheimers Dis 42: 865-878.
- Walsh DM, Selkoe DJ (2007) A beta oligomers – a decade of discovery. J Neurochem 101: 1172-1184.
- Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, et al. (2006) A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440: 352-357.
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, et al. (2008) Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14: 837-842.
- Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, et al. (2009) Beta-amyloid monomers are neuroprotective. J Neurosci 29: 10582-10587.
- Nag S, Sarkar B, Bandyopadhyay A, Sahoo B, Sreenivasan VKA, et al. (2011) Nature of the amyloid-b monomer and the monomer-oligomer equilibrium. J Biol Chem 286: 13827-13833.
- Hoffman L, Hardej D (2012) Ethylene bisdithiocarbamate pesticides cause cytotoxity in transformed and normal human colon cells. Environmental Tox Pharm 34: 556-573.
- Chang RC, Suen KC, Ma CH, Elyaman W, Ng HK, et al. (2002) Involvement of double-stranded RNA-dependent protein kinase and phosphorylationof eukaryotic initiation factor-2alpha in neuronal degeneration. J Neurochem 83: 1215-1225.
- Suen KC, Yu MS, So KF, Chang RC, Hugon J (2003) Upstream signaling pathways leading to the activation of double-stranded RNA-dependent serine/threonine protein kinase in beta-amyloid peptide neurotoxicity. J Biol Chem 278: 49819-49827.
- Marchal JA, Lopez GJ, Peran M, Comino A, Delgado JR, et al. (2014) The impact of PKR activation: from neurodegeneration to cancer. The FASEB J 28: 1965-1974.
Citation: Cheng SY, Lopez Y, Montes J (2017) Maneb and Mancozeb Increase Amyloid β Precursor Protein Expression and Activate PKR. J Cell Sci Apo 1: 110.
Copyright: © 2017 Cheng SY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 5498
- [From(publication date): 0-2017 - Apr 25, 2025]
- Breakdown by view type
- HTML page views: 4500
- PDF downloads: 998