Research Article Open Access
Management Of Unexpected Peritoneal Metastases With Primary Colorectal Cancer Using Second-Look Surgery With HIPEC
Paul H. Sugarbaker1* and Olivier Glehen2
1Center for Gastrointestinal Malignancies, MedStar Washington Cancer Institute, Washington, DC, USA
2Hospices Civils de Lyon and Universite Lyon, Centre Hospitalier Lyon Sud, Pierre Benite, France
- Corresponding Author:
- Sugarbaker PH
Medstar Washington Cancer Institute 106 Irving St., NW
Suite 3900, Washington, DC 20010 USA
Tel: (202) 877-3908
Fax: (202) 877-8602
E-mail: Paul.Sugarbaker@medstar.net
Received September 30, 2015; Accepted October 01, 2015; Published November 30, 2015
Citation: Sugarbaker PH, Glehen O (2015) Management Of Unexpected Peritoneal Metastases With Primary Colorectal Cancer Using Second-Look Surgery With HIPEC. Can surg 1:101.
Copyright: © 2015 Sugarbaker PH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Cancer Surgery
Abstract
Background: Peritoneal metastases (PM) will be unexpectedly present in approximately 10% of colorectal cancer patients having primary cancer resection. In the past this was considered to be an incurable condition with a terminal outcome. In patients determined to have peritoneal dissemination at the time of resection, the intervention was considered palliative. Recently, long term benefit from definitive treatment of PM with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) has become a reality. These treatments are now appropriate for primary appendiceal and colorectal cancer determined to have PM at the time of resection.
Methods: Modifications of the initial management of colorectal cancer patients found upon exploration to have PM are explored in this manuscript. In these patients, not only the primary cancer but also the PM must be optimally treated.
Results: The presentation of the primary colon or rectal cancer as asymptomatic, bleeding, obstructed or perforated is important in treatment planning. The surgical approach must facilitate subsequent interventions to definitely treat PM. Procedures performed on the primary cancer are designed to minimize tumor cell entrapment. These patients usually have short course of systemic chemotherapy prior to repeat intervention with HIPEC.
Conclusion: CRS and HIPEC must be integrated into the management of colorectal cancer patients who have PM identified unexpectedly at the time of primary cancer resection. Major resections in the absence of HIPEC should not occur in these patients in order to preserve an intact peritoneum as the first line of defense against PM and avoid tumor cell entrapment in subsequent CRS and HIPEC procedures.
Keywords
Peritoneal metastases; Peritoneal carcinomatosis; Colorectal cancer; HIPEC; Cytoreductive surgery; Perioperative chemotherapy; Second-look surgery
Introduction
Peritoneal metastases (PM) documented in a patient with primary colon or rectal cancer places the patient at extreme risk for local recurrence or progressive PM diagnosed in follow-up. In the past, peritoneal seeding diagnosed with the surgical exploration did not result in any major changes in the surgical approach to the disease process. Rather, conventional surgery was performed and then the patient was referred for palliative systemic chemotherapy. At this point in time potentially curative treatments for PM have evolved and become an important part of the standard of care of colorectal malignancy. In order to optimize the management of peritoneal metastases, a new approach to the primary cancer presenting with PM is required. The goal of manuscript is to describe a clinical pathway so that patients with PM diagnosed at the time of primary colorectal cancer resection receive treatment with curative intent.
Peritoneal metastases from colorectal cancer can be treated with curative intent
The gastrointestinal malignancies may develop PM as part of their natural history and this includes colorectal cancer. Varying levels of success depending on the clinical situation of the patient have been achieved when a combined treatment using cytoreductive surgery (CRS) plus hyperthermic perioperative chemotherapy (HIPEC) is used. It is important to establish for the reader that long-term survival should occur in a large proportion of patients if modern surgical management strategies are used at experienced treatment centers [1].
Patients to be included in this clinical pathway
Many years and numerous publications have established that PM documented at the time of primary colorectal cancer resection place the patient at an extreme risk of disease progression on peritoneal surfaces diagnosed in follow up. In a review, Honore et al. estimated that 80% of patients would progress [2]. Equally problematic in terms of progression of peritoneal surface disease are ovarian metastases. Again, Honore et al. estimated an 80% of these patients would progress as PM [2]. A third group of patients documented to be at extreme risk of subsequent PM are patients with perforated primary colon or rectal cancer. Honore et al. estimated the incidence of PM in this group of patients as 60% [2]. These three groups of patients are the focus of this manuscript. Although they constitute a small proportion of patients with primary colon and rectal cancer, their outcome is extremely dismal in the absence of a comprehensive management plan. Modified surgical management of the primary cancer and second-look HIPEC are indicated for all of these patients unless comorbid conditions prevent future interventions.
Patients not to be included in this clinical pathway
There are patients with peritoneal metastases, ovarian metastases, or perforation who should have definitive surgical management of the primary colorectal cancer in the absence of the modifications suggested in this manuscript. Extreme old age and comorbid clinical features that mitigate against a second-look with HIPEC are within this group of patients. Also, patients with other sites of metastatic cancer that would rule out a potentially curative second-look with HIPEC should not have the modified primary cancer surgery. In order for this comprehensive management plan to be indicated, the patient must be a candidate for second-look with HIPEC.
Incidence of primary colon cancer with peritoneal metastases or perforation
According to the review by Honore et al., approximately 8% of patients will have PM some place within the abdomen or pelvis that can be documented at the time of primary colon cancer resection. Ovarian metastases will be found in approximately 8% of women; this would be 4% of the group which includes males and females. Perforation will be a part of the patients’ symptoms in 4% of patients. These patients taken together give a 16% incidence of PM in patients with primary colon cancer [2]. These 16% of patients may be asymptomatic or have obstruction, bleeding or perforation as a presenting symptom.
Incidence of primary rectal cancer with peritoneal metastases
Fewer sources of information regarding PM with primary rectal cancer exist. Peritoneal metastases with rectal cancer may occur less frequently because the mesorectal fat surrounds this organ and full thickness penetration of the rectal wall could not seed the peritoneal space with cancer cells with PM resulting. However, Shepherd et al. have rightly pointed out that the anterior aspect of the mid-rectum in women forms the posterior aspect of the cul-de-sac and is in immediate contact with the free peritoneal space. Also, the upper one-third of the rectum is covered by visceral peritoneum and full thickness invasion of the rectal wall at this anatomic site may result in peritoneal metastases [3]. A careful study of the incidence of peritoneal involvement in primary rectal cancer patients was published by Shepherd et al. [3]. A similar and confirmatory study was published by Mitchard et al. [4]. The two authors report a 24% and 22% involvement of the peritoneal surface by rectal cancer. Both of these publications strongly suggest that PM may occur with primary rectal cancer, women are at an increased risk and possible seeding of the pelvis by rectal cancer requires careful scrutiny at the time of primary rectal cancer resection.
Rectal cancer, because of the thicker muscular wall of the rectum, is unlikely to result in perforation as a presenting symptom. Also, the larger diameter lumen of the rectum makes obstruction less likely than with colon cancer. Emergency surgery, because of uncontrolled hemorrhage, may rarely occur. Most commonly, modification of primary rectal cancer surgery will be caused by PM associated with a middle rectal cancer in women or upper rectal cancer in both men and women.
Requirement for referral to an experienced treatment center
Cytoreductive surgery (CRS) plus HIPEC is a treatment recommended in the national guidelines of European nations (excluding Greece) [5]. Also, it is a standard of care at cancer centers in the United States. Treatments are to be administered at a limited number of experienced cancer centers. In contrast, the resection of colon and rectal cancer occurs at a great majority of hospitals throughout Europe and the United States. Because PM are detected unexpectedly a great majority of patients will have PM detected at institutions not familiar with CRS and HIPEC. Therefore, if a curative approach to this group of patients is contemplated, referral to an experienced PM treatment center is necessary. The recommendations for the surgical management of the primary colon or rectal cancer defined in this manuscript will optimize the results achieved by this referral.
It is possible at some time in the future that the expertise and equipment required for CRS and HIPEC will be generally available at a majority of hospitals in the USA and Europe. Simultaneous use of CRS and HIPEC as part of the primary cancer treatment has been associated with favorable results [6-9]. It is also the focus of two active clinical trials [10,11].
Extent of disease as a prognostic indicator in colorectal cancer with peritoneal metastases
Extent of disease with colorectal cancer PM has also been shown to have a profound effect on survival with CRS and HIPEC. Extent of disease in patients with PM is estimated most accurately with the peritoneal cancer index (PCI). Elias and colleagues in the Association of French Surgeons Monograph on peritoneal carcinomatosis presented survival data on 496 patients with PM from colorectal cancer [1]. At 5 years, patients with a PCI between 0 and 6 showed a 50% survival. Patients with PCI between 7 and 12 and PCI between 13 and 19 had a 30% 5-year survival, but patients with PCI greater than 19 had only 10% survival at 5 years. Clearly, the extent of PM indicating an early intervention in the natural history of this process results in a superior treatment outcome.
Glehen and colleagues performed a retrospective multi-institutional study of 506 patients with PM from colorectal cancer. A limited extent of PM had a median survival of 34.8 months and extended PM a survival of 14.4 months (p ≤ 0.0001). These differences remained significant in a multivariate analysis (p ≤ 0.001) [12].
Favorable prognosis with early peritoneal metastases
Patients unexpectedly identified with PM at the time of colon or rectal cancer resection may have an unusually favorable outcome when treated with CRS and HIPEC. In these patients a low PCI is expected, or as low as can be achieved in an individual patient. Timely definitive management with a low PCI is not the only advantage of a clinical pathway that utilizes second-look HIPEC. The procedure should be designed to minimize the disruption of peritoneal surfaces to maintain as much as possible the first line of defense against peritoneal metastases [13]. This requires a limited dissection which accompanies the management of the primary cancer to keep tumor cell entrapment to a minimum. Modifications of the management of the primary cancer occurring with simultaneous PM will optimize the long-term survival of these patients.
Rationale for modifications of the surgical approach to primary colorectal cancers presenting with peritoneal metastases
With some exceptions, the intact peritoneal surface is an excellent “first line of defense” in carcinomatosis [13]. In low grade malignancies such as pseudomyxoma peritonei or nuclear grade I and II mesothelioma, very large volumes of malignancy may occur throughout the abdomen and pelvis without invasion beneath the peritoneum. Also, high grade malignancy with tumor nodules of small size does not invade beneath the peritoneum. Often, parietal peritonectomy procedures can be used to completely eradicate a low volume of high grade PM. The adequacy of the peritoneum as a first line of defense against PM may fail at three different anatomic sites. Lymphoid aggregates which resorb peritoneal fluid are abundant at the junction of small bowel and small bowel mesentery. Cancer cells that are trapped at this site may progress as invasive cancer nodules. Also, the lymphatic lacunae present on the undersurface of the hemidiaphragm and especially abundant on the membranous portion of the diaphragm may accumulate cancer cells that progress to invade into this structures. Finally, large amounts of fluid resorption from the peritoneal space in and around the terminal ileum, ileocecal valve, appendix and ascending colon may accumulate tumor cells at this site which over time invade into the intestinal surfaces [14].
The most common disruption of the peritoneal barrier to cancer cells occurs as a result of surgical dissection. Any surgical dissection which creates a raw surface within the peritoneal space or resection of the intestine that leaves a resection site will locally disrupt the first line of defense. Recurrences of cancer at the vaginal cuff post-hysterectomy, recurrence of cancer within suture lines, recurrence of cancer within the abdominal incision or within laparoscopy port sites confirm that surgical trauma is a process that localizes cancer progression within the abdomen and pelvis. A new principal of surgery for patients with primary gastrointestinal cancer with peritoneal seeding or a high risk for subsequent local-regional recurrence should be respect for the peritoneum as a first line of defense. Resections and intestinal reconstructions performed in the presence of PM should only be tolerated in patients having palliative cancer surgery. In all other circumstances tumor cell entrapment can and should be avoided.
A report of similar strategy for these patients with PM at primary colorectal cancer resection was presented by Elias and colleagues from Villejuif, France [15]. This was a highly selected group of patients who had biopsy-proven PM, ovarian PM, or perforation confirmed at the time of primary colorectal cancer resection. The second-look surgery was performed within 1 year after the first surgery and after the completion of systemic adjuvant chemotherapy. The patients were asymptomatic with a completely negative work-up. The authors detected additional PM in 63% of patients who had synchronous PM, 75% of patients with ovarian metastases, and 33% of patients with a perforated primary tumor. Patients with macroscopic PM were treated with cytoreductive surgery plus HIPEC with no mortality, a low morbidity, and a 2-year disease-free survival rate exceeding 50%. Patients without macroscopic PM received carcinomatosis prevention surgery with or without HIPEC. It is interesting to note that, in this subgroup with no macroscopic PM, 17% who received HIPEC showed recurrence versus 43% showed recurrence who did not receive HIPEC.
Modification of primary colon cancer surgery in patients with peritoneal metastases
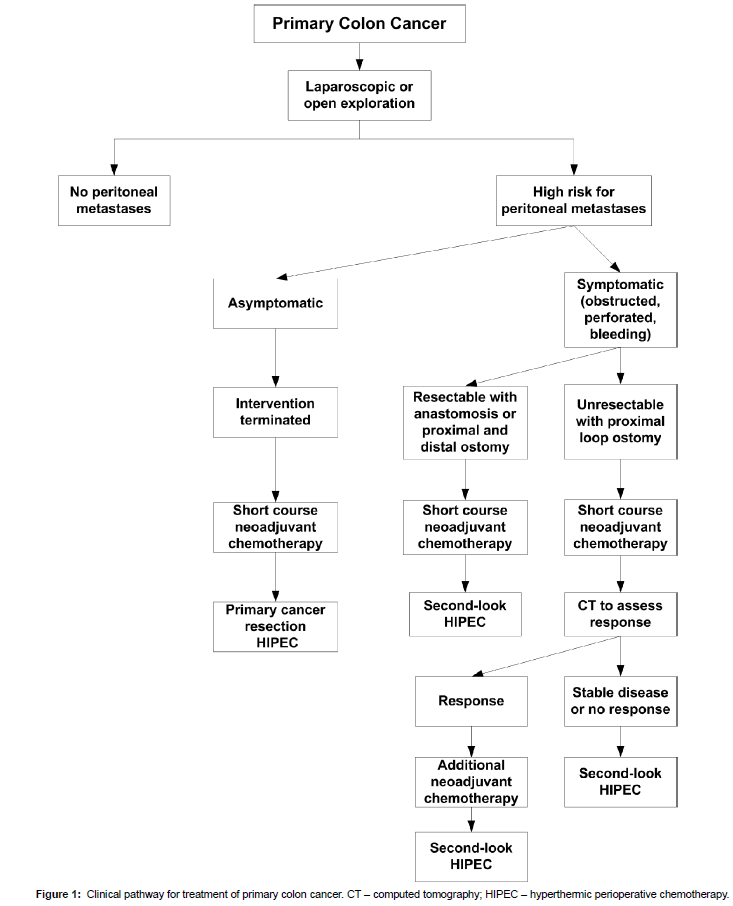
A discussion regarding the optimal surgical intervention in a patient with primary colon cancer must be made at the time of a thorough abdominal and pelvic exploration. In order to rule out peritoneal metastases, the undersurface of right and left hemidiaphragm must be explored. The left paracolic sulcus, the entire pelvis and then the right paracolic sulcus must be visualized. The omentum must be examined and the small and large bowel visualized. The peritoneal surfaces surrounding the portion of the colon that contains the primary cancer is at high risk for PM. It must be carefully inspected. A cytological study is indicated (Figure 1).
In an asymptomatic patient, if peritoneal metastases are confirmed by cryostat study of a peritoneal or omental nodule, the surgical intervention should be terminated. The primary cancer should not be resected in order to preserve the intact peritoneal surfaces and minimize tumor cell entrapment. The patient should be started on neoadjuvant chemotherapy for approximately two months. While on chemotherapy an evaluation by the peritoneal surface oncology team and presentation at a multidisciplinary team meeting should occur. As soon as the patient is ready physically and psychologically a CRS and HIPEC procedure should be performed.
In symptomatic patients the definitive CRS and HIPEC is usually more significantly delayed. The primary colon cancer must, in most patients, be resected. Resection of the cancerous mass will eliminate possible complications from the primary cancer during the neoadjuvant chemotherapy.
If the primary cancer is so advanced that it cannot be resected, a diverting loop ileostomy or colostomy should be performed. The patient receives 4 to 6 cycles of cancer chemotherapy in an attempt to shrink the primary cancer. Frequent CT monitoring of the primary cancer must occur. If shrinkage of the primary cancer occurs the neoadjuvant chemotherapy should continue to achieve a maximal response. In the absence of a response (stable disease) or with progression secondlook CRS and HIPEC should proceed. With the exposure obtained with CRS and a preperitoneal approach to the large primary cancer, complete resection should be possible with maximal safety.
Modification of primary rectal cancer surgery in patients with peritoneal metastases
The data documented on rectal cancer patients with peritoneal metastases diagnosed in follow up shows a guarded prognosis in these patients even though there is a complete cytoreduction. Da Silva and Sugarbaker showed a median survival of 17 months following CRS and perioperative chemotherapy for rectal cancer with perioperative chemotherapy for rectal cancer with peritoneal metastases as compared to 34 months with colon cancer. There were no five-year survivors in the rectal cancer group as compared to 30 in the colon cancer group [16]. Verwaal showed in a multivariate analysis of 102 patients that peritoneal metastases treated with CRS and HIPEC had a poorer prognosis (p=0.069) [17].
One of the mechanisms for failure for rectal cancer patients with PM was suggested to be tumor cell entrapment with the pelvic dissection. There would be an imperfect peritonectomy of the after rectal resection by abdomino-perineal resection or low anterior resection.
The modifications of primary rectal cancer resection in the presence of peritoneal metastases are designed to avoid tumor cell entrapment within the pelvis. If upon abdominal and pelvic exploration prior to resection, peritoneal metastases are documented, the intervention is terminated. With rectal cancer, symptoms of obstruction, perforation or uncontrolled bleeding are unusual. The patient receives a short course of neoadjuvant chemotherapy and an evaluation by an experienced peritoneal metastases treatment center. The rectal cancer resection, cytoreductive surgery, and HIPEC are done as a unified treatment.
Extent of colon resection for symptomatic colon cancers
In some patients, the primary colon malignancy will present as an urgent clinical situation. The most common clinical presentations are obstruction, perforation, and bleeding. In patients with these symptoms a resection of the primary cancer may help to minimize adverse events occurring during subsequent chemotherapy treatments.
However, these resections should not be performed as wide resections that include the relevant lymph nodes down to the superior mesenteric vessels on the right colon or the origin of the inferior mesenteric artery on the left. A limited resection of the colon with 5-10 cm on either side of the cancer is adequate. The marginal vessels should be included with the specimen but a definitive lymph node dissection is contraindicated.
This limited resection causes the definitive colon resection including the relevant lymph nodes to be performed as part of the second-look HIPEC procedure. This plan minimizes the problems with unresectable cancer cells trapped in the retroperitoneal dissection. A minimal tumor cell entrapment constitutes the rationale for this comprehensive and curative approach to peritoneal metastases that occur with a primary colorectal cancer.
Rationale for a short course of neoadjuvant chemotherapy
Neoadjuvant systemic chemotherapy will produce the shrinkage of peritoneal cancer nodules in approximately 50% of patients [18]. Other patients experience the toxic side effects of these drugs with disease progression occurring. Radiologic tests such as abdominal and pelvic CT or MRI are extremely inaccurate as a monitor of disease progression or response of small PM. In order to prevent many months of undetected disease progression the neoadjuvant chemotherapy is limited to 3 to 5 two-week cycles. The regimen most commonly used is a combination of 5-fluorouracil and oxaliplatin often referred to as FOLFOX.
Other clinical features that may require a second-look with HIPEC
In this manuscript the clinical features of colorectal cancer which indicated the need for second-look with CRS and HIPEC were peritoneal metastases, ovarian metastases, and perforation through the primary cancer. There may be other indications for second-look HIPEC that are derived from a histopathologic study of the resected primary cancer. A positive margin of resection would be an adequate cause to recommend second-look HIPEC after neoadjuvant chemotherapy. The likelihood of progressive disease in a patient with an R-1 resection approaches 100%. Another adequate cause would be a T4 cancer, especially a mucinous T4 lesion. Hompes found the incidence of PM in patients whose histopathology showed a T4 cancer to be 55% [19]. A positive cytology may also be considered an adequate cause for secondlook HIPEC. Patients with positive cytology prior to or after primary colorectal cancer resection have an incidence of peritoneal metastases of 50% (Table 1) [9].
|
Table 1: Patients with primary colorectal cancer identified to be at high risk for localregional recurrence and/or peritoneal metastases. These patients should have the primary colorectal cancer modified so that they are candidates for proactive second-look surgery with HIPEC.
Second-look surgery with HIPEC
The second-look surgery with HIPEC follows the same principles and technology as the treatment of peritoneal metastases from colorectal cancer in patients diagnosed in follow up. There are five different peritonectomy procedures that may be indicated. Also, visceral resections are required to remove invasive peritoneal implants on the visceral peritoneal surfaces [20].
Currently, there are two frequently used HIPEC regimens in this group of patients. The bidirectional chemotherapy treatment was introduced by Elias which involves intravenous 5-fluorouracil and intraperitoneal oxaliplatin [21]. Another regimen frequently used involves intravenous 5-fluorouracil and intraperitoneal mitomycin C plus doxorubicin [20]. The oxaliplatin regimen uses 30 minutes of hyperthermia and the mitomycin C/doxorubicin regimen uses 90 minutes of hyperthermia. Either an open or a closed HIPEC technology can be used.
References
- Glehen O, Elias D, Gilly F-N (2008)CarcinosesPéritonéalesD'Origine Digestive et Primitive: Rapport du 110ème Congrès de L'AssociationFrançaise de Chirurgie—Monographie de L'Associationfrançaise de Chirurgie. Arnette: Rueil-Malmaison, France 101-151.
- Honore C, Goere D, Souadka A, Dumont F, Elias D (2013) Definition of patients presenting a high risk of developing peritoneal carcinomatosis after curative surgery for colorectal cancer: A systematic review. Ann SurgOncol;183-192.
- Shepherd NA, Baxter KJ, Love SB (1995) Influence of local peritoneal involvement on pelvic recurrence and prognosis in rectal cancer. J ClinPathol849-855.
- Mitchard JR, Love SB, Baxter KJ, Shepherd NA (2010) How important is peritoneal involvement in rectal cancer? A prospective study of 331 cases. Histopathology 671-679.
- O’Dwyer S, Verwaal VJ, Sugarbaker PH (2015) Evolution of treatments for peritoneal metastases from colorectal cancer. J ClinOncol2122.
- Pestieau SR, Sugarbaker PH (2000) Treatment of primary colon cancer with peritoneal carcinomatosis: A comparison of concomitant versus delayed management. Dis Colon Rectum 1341-1348.
- Tentes AAK, Spiliotis ID, Korakianitis OS, Vaxevanidou A, Kyziridis D (2011) Adjuvant perioperative intraperitoneal chemotherapy in locally advanced colorectal carcinoma: Preliminary results. ISRN Surgery
- Tentes AA (2013) Preliminary results with the use of hyperthermic intraoperative intraperitoneal chemotherapy or systemic chemotherapy in high-risk colorectal cancer patients, Translational Gastrointestinal Cancer.
- Noura S, Ohue M, Shingai T, Kano S, Ohigashi H, et al. (2011) Effects of intraperitoneal chemotherapy with mitomycin C on the prevention of peritoneal recurrence in colorectal cancer patients with positive peritoneal lavage cytology findings. Ann SurgOncol396–404.
- Tanis PJ (2015) Adjuvant HIPEC in High Risk Colon Cancer (COLOPEC). Bethesda MD: National Library of Medicine (US).
- Sammartino P (2015)SocietaItaliana di ChirurgiaOncologica (SICO). PROMENADE Trial (PROactive Management of Endoperitoneal spread in colonic cancer).
- Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA et al. (2004)Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J ClinOncol3284-3292.
- Sugarbaker PH (2007) Peritoneum as the first line of defense in carcinomatosis. J SurgOncol;93-96.
- Carmignani P, Sugarbaker TA, Bromley CM, Sugarbaker PH (2003)Intraperitoneal cancer dissemination: Mechanisms of the patterns of spread. Cancer Metastasis Rev;465-472.
- Elias D, Goéré D, Di Pietrantonio D, Boige V, Malka D et al. (2008) Results of systematic second-look surgery in patients at high risk of developing colorectal peritoneal carcinomatosis. Ann Surg;445-450.
- Da Silva RG, Sugarbaker PH (2006)Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am CollSurg;878-886.
- Verwaal VJ, van Tinteren H, van Ruth S, Zoetmulder FA (2004) Predicting the survival of patients with peritoneal carcinomatosis of colorectal origin treated by aggressive cytoreduction and hyperthermicintraperitoneal chemotherapy. Br J Surg;739-746.
- Passot G, You B, Boschetti G, Fontaine J, Isaac S et al, (2014) Pathological response to neoadjuvant chemotherapy: A new prognosis tool for the curative management of peritoneal colorectal carcinomatosis. Ann SurgOncol;2608-2614.
- Hompes D, Tiek J, Wolthuis A, et al, (2012) HIPEC in T4a colon cancer: a defendable treatment to improve oncologic outcome? Ann Oncol; 3123-3129.
- Sugarbaker PH (2012) An overview of peritonectomy, visceral resections, and perioperative chemotherapy for peritoneal surface malignancy. Cytoreductive Surgery & Perioperative Chemotherapy for Peritoneal Surface Malignancy. Textbook and Video Atlas. Cine-Med Publishing: Woodbury, CT. 1-30.
- Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F et al. (2008) Complete cytoreductive surgery plus intraperitonealchemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J ClinOncol;681-685.
Relevant Topics
- Breast Cancer Surgery
- Colon Cancer Surgery
- Dermatologic Surgery
- Kidney Cancer Surgery
- Leukemia Surgery
- Lung Cancer Surgery
- Lymphoma Surgery
- Oesophageal Cancer Surgery
- Pancreatic Cancer Surgery
- Prostate Cancer Surgery
- Radiation Therapy
- Skin Cancer Surgery
- Stomach Cancer Surgery
- Throat Cancer Surgery
- Thyroid Cancer Surgery
Recommended Journals
Article Tools
Article Usage
- Total views: 14825
- [From(publication date):
September-2016 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 13931
- PDF downloads : 894