Case Report Open Access
Management of Siewert's Type III Gastroesophageal Junction Adenocarcinoma in a 52 Year Old Female
Adam L Bourgon1, Minia Hellan2 and Rebecca Tuttle2*
1Wright State University Department of Surgery, USA
2Wright State University Department of Surgery, Division of Surgical Oncology, USA
- *Corresponding Author:
- Rebecca Tuttle, MD
Wright State University, Department of Surgery
Division of Surgical Oncology, 3535 Southern Boulevard
Kettering, OH 45429, USA
Tel: 1 937 360-9229
E-mail: rebecca.tuttle@wright.edu
Received date: December 14, 2015 Accepted date: December 26, 2015 Published date: December 31, 2015
Citation: Bourgon AL, Hellan M, Tuttle R (2015) Management of Siewert's Type III Gastroesophageal Junction Adenocarcinoma in a 52 Year Old Female. J Gastrointest Dig Syst 5:369. doi:10.4172/2161-069X.1000369
Copyright: © 2015 Bourgon AL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
A 52 year old female presented with a Siewert's type III gastroesophageal junction (GEJ) adenocarcinoma treated with neoadjuvant chemotherapy, laparoscopic-robotic total gastrectomy, and adjuvant chemoradiation.
Keywords
Gastroesophageal junction; Laparoscopic gastrectomy
Abbreviations
GEJ: Gastroesophageal Junction; FISH: Fluorescence In Situ Hybridization; CT: Computed Tomography; PET: Positron Emission Tomography; EUS: Endoscopic Ultrasound
Case Description
A 52 year old female presented with a Siewert’s type III gastroesophageal junction (GEJ) adenocarcinoma treated with neoadjuvant chemotherapy, laparoscopic-robotic total gastrectomy, and adjuvant chemoradiation. Presenting symptoms were a 6 week history of worsening belching and abdominal bloating.
The patient was first evaluated by gastroenterology and underwent upper endoscopy with findings significant for a 1 cm nodule at the GEJ with thickening of the gastric cardia and associated erosions.
Biopsies were taken of the gastric body and antrum, and the nodule. Biopsies of the body and antrum returned as benign. Biopsy of the nodule returned as well-differentiated adenocarcinoma, HER2/neu was positive by fluorescence in situ hybridization (FISH). Computed tomography (CT) scans of the chest, abdomen, and pelvis revealed mild gastric wall thickening without evidence of metastatic disease.
Positron emission tomography (PET) scan identified two areas of hypermetabolic foci along the greater curvature of the stomach in addition to activity at the GEJ without evidence of metastatic disease (Figure 1A and B).
Endoscopic ultrasound (EUS) and diagnostic laparoscopy were performed. EUS revealed invasion into the adventitia of the cardia without evidence of surrounding adenopathy, staging T3N0. Diagnostic laparoscopy and port placement were completed concurrently.
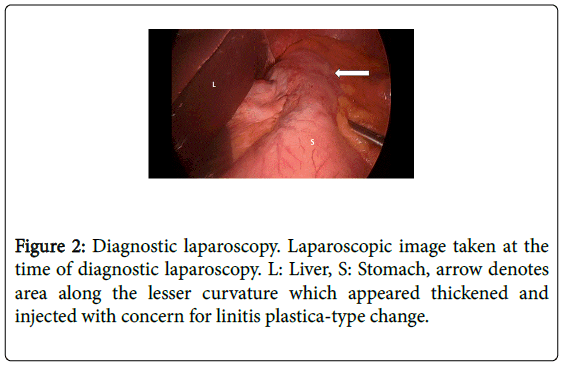
At the time of surgery, the stomach was found to be edematous at the cardia and fundus including the area of the lesser curvature with concern for linitis plastica extending into the distal esophagus (Figure 2). There was no evidence of peritoneal disease at the time of laparoscopy.
Additionally, genetic testing was performed as part of her initial work up and evaluation secondary to her family history of breast and rectal cancer with no clinically significant mutations identified.
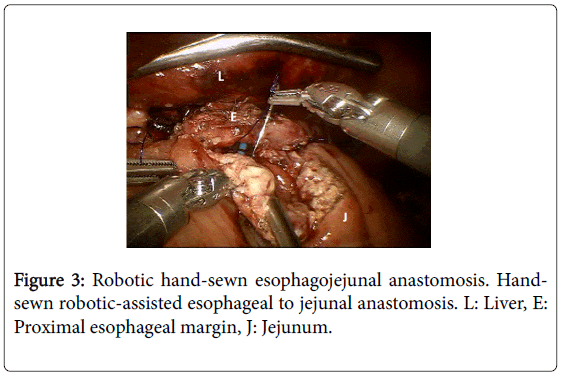
She received 6 cycles of neoadjuvant chemotherapy with mDCF and trastuzumab [1]. Four weeks after the completion of her neoadjuvant therapy, she underwent a laparoscopic, robotic-assisted total gastrectomy with roux-en-Y esophagojejunostomy, D2 lymphadenectomy and jejunostomy tube placement. The majority of the dissection was completed robotically and the anastomosis was hand-sewn using the robot (Figure 3). Pathology revealed grade 3, poorly-differentiated adenocarcinoma centered at the cardia and extending to the GEJ and fundus, surgical margins were negative, 6/16 lymph nodes were positive, and HER2/neu was negative. Final staging was ypT3N2, Stage IIIB. Final pathology did not reveal any evidence of chemotherapy response with all viable tumor noted. The postoperative course was complicated by a slow return to oral intake. Tube feeds were continued for 8 weeks postoperatively following which the jejunostomy tube was removed. Secondary to the poor pathologic response to neoadjuvant chemotherapy, it was decided to treat postoperatively with 5-fluorouracal-based chemoradiation (4500 cGy over 5 weeks) as per McDonald protocol, which she completed without further delay [2].
Ten months postoperatively the patient developed difficulty with oral intake and was admitted to the hospital several times for presumed partial small bowel obstruction. Ultimately, parenteral nutrition was initiated. CT scanning of the abdomen and pelvis revealed dilated small bowel loops in the epigastrium, bilateral hydronephrosis and ascites. Diagnostic laparoscopy was performed and revealed diffuse peritoneal disease. Biopsy confirmed metastatic adenocarcinoma. Jejunostomy tube was replaced. After multidisciplinary discussion, the patient elected to enroll in Hospice and succumbed to her disease shortly thereafter.
Discussion
The described case represented an unfortunate circumstance of locally advanced gastric cancer progressing to metastatic disease despite best medical therapy. It highlights the complex presentation of gastric cancer patients and the importance of multidisciplinary discussion in their management.
The classification of GEJ cancers was first described by Siewert in 2000 [3]. The classification system allows for uniform description of these tumors in addition to guiding recommended surgical therapies. Classically, type I tumors are recommended for esophagectomy and type III tumors are recommended for total gastrectomy [4]. Type II tumors can be treated with either surgical approach with the goal to obtain adequate lymphadenectomy and negative surgical margins. In their original series, postoperative mortality was higher for the esophagectomy group suggesting a preference for gastrectomy when technically feasible [3].
There remains some debate in the published literature regarding the management of Siewert’s type III GEJ tumors. Type III tumors are known to be larger at presentation and more infiltrative when compared to type II tumors [5,6]. The median 5 year survival for type III tumors is 51-63% [7-9]. In a 2007 series by Barbour et al. 52% of patients with type III tumors were treated with gastrectomy with limited esophagectomy while the remaining type III patients were treated with extended esophagectomy.[10] The median esophageal margin length was 2.5 cm for type III tumors compared to 3.5 cm for type 1 and 4.0 for type II. Margin length of >3.8 cm was found to be an independent predictor of overall survival, similar to the number of positive nodes, AJCC T classification, and poorly differentiated tumors. Margins were not predictive of survival in patients with greater than 6 positive lymph nodes.[10] Similar studies have supported wide margin resection [11]. However, in a 2015 series from the US Gastric Cancer Collaborative, the importance of gastric cancer resection margins was re-examined. Proximal margin length was not found to be predictive of increased local recurrence or overall survival. Further, while R1 resection was associated with increased nodal disease burden, it was not found to be associated with increased local recurrence or decreased overall survival. The recommendations of this series were to limit wide margin resection, particularly if it required extended esophageal resection [12].
Kneuertz et al. evaluated the type of surgery for Siewert’s type II and III tumors in relation to overall survival [7]. The majority of patients in this study presented with locally advanced disease and were treated neoadjuvantly with chemoradiation. Seventy-five percent of patients with type II tumors were treated with esophagectomy, while 88% of patients with type III tumors were treated with gastrectomy. The type of surgery was not found to be predictive of R0 resection rates or overall survival. The only factor found to be associated with improved overall survival was extended lymphadenectomy. Similar results were previously reported by Barbour et al. in 2007 with adequate staging, as defined by >/= 15 lymph nodes, in T2 or greater tumors to be an independent prognostic factor of improved survival [13].
Siewert’s type II/III tumors have been shown to have a better response to neoadjuvant chemoradiation with increased rates of complete pathologic response. However, while there was no individual differences between the classifications, when Siewert’s type I tumors are compared to II/III tumors, type II/III tumors are found to have an overall increased rate of local recurrence [14]. Patterns of recurrence have been shown to differ between the groups with disseminated recurrence being the most common for Siewert’s type III lesions [15].
In this case we utilized a minimally invasive approach with both laparoscopic and robotic resection. Several studies have demonstrated the safety and oncologic efficacy of minimally invasive resection for advanced gastric cancer [16-18]. In a recent meta-analysis comparing open verses minimally invasive laparoscopic total gastrectomy, minimally invasive total gastrectomy was found to have equivalent lymph node yield and mortality with shorter time to first flatus, decreased hospital stay, and fewer complications [16]. The data for robotic gastric cancer resections is still evolving. However, it has been shown to be equivalent to laparoscopic resections with increased operative time but lower intraoperative blood loss and improved lymph node retrieval [19].
Conclusion
In conclusion, the current literature would support that the type of surgery selected is not an independent predictor of outcome. But rather, the adequacy of oncologic resection focusing on R0 resection and, more importantly, adequate lymphadenectomy appear to improve patient outcome. As this case demonstrates, despite best surgical management including negative-margin resection and extended lymphadenectomy, gastroesophageal junction adenocarcinoma remains an aggressive disease with increased rates of local and distant recurrence.
References
- Keskin S, Yaldaz I, Sen F, Aydogan F, Kilic L, et al. (2013) Modified DCF (mDCF) regimen seems to be as effective as original DCF in advanced gastric cancer (AGC). ClinTranslOncol 15: 403-408.
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, et al. (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345: 725-730.
- RüdigerSiewert J, Feith M, Werner M, Stein HJ (2000) Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 232: 353-361.
- Goto H, Tokunaga M, Miki Y, Makuuchi R, Sugisawa N, et al. (2014) The optimal extent of lymph node dissection for adenocarcinoma of the esophagogastric junction differs between Siewert type II and Siewert type III patients. Gastric Cancer .
- Zhang WH, Chen XZ, Liu K, Anil K, Yang K, et al. (2014) Comparison of the clinicopathological characteristics and the survival outcomes between the Siewert type II/III adenocarcinomas. Med Oncol 31: 116.
- Curtis NJ, Noble F, Bailey IS, Kelly JJ, Byrne JP, et al. (2014) The relevance of the Siewert classification in the era of multimodal therapy for adenocarcinoma of the gastro-oesophageal junction. J SurgOncol 109: 202-207.
- Kneuertz PJ, Hofstetter WL, Chiang YJ, Das P, Blum M, et al. (2015) Long-Term Survival in Patients with Gastroesophageal Junction Cancer Treated with Preoperative Therapy: Do Thoracic and Abdominal Approaches Differ? Ann SurgOncol .
- Hosokawa Y, Kinoshita T, Konishi M (2012) Clinicopathological features and prognostic factors of adenocarcinoma of the esophagogastric junction according to Siewert classification: experiences at a single institution in Japan. Ann SurgOncol 19: 677-683.
- Fang WL, Wu CW, Chen JH, Lo SS, Hsieh MC, et al. (2009) Esophagogastric junction adenocarcinoma according to Siewert classification in Taiwan. Ann SurgOncol 16: 3237-3244.
- Barbour AP, Rizk NP, Gonen M, Tang L, Bains MS, et al. (2007) Adenocarcinoma of the gastroesophageal junction: influence of esophageal resection margin and operative approach on outcome. Ann Surg 246: 1-8.
- Mine S, Sano T, Hiki N, Yamada K, Kosuga T, et al. (2013) Proximal margin length with transhiatalgastrectomy for Siewert type II and III adenocarcinomas of the oesophagogastric junction. Br J Surg 100: 1050-1054.
- Postlewait LM, Squires MH, Kooby DA (2015) The importance of the proximal resection margin distance for proximal gastric adenocarcinoma: A multi-institutional study of the US Gastric Cancer Collaborative. J SurgOncol 112: 203-207.
- Barbour AP, Rizk NP, Gonen M, Tang L, Bains MS, et al. (2007) Lymphadenectomy for adenocarcinoma of the gastroesophageal junction (GEJ): impact of adequate staging on outcome. Ann SurgOncol 14: 306-316.
- Moureau-Zabotto L, Teissier E, Cowen D, Azria D, Ellis S, et al. (2015) Impact of the Siewert Classification on the Outcome of Patients Treated by Preoperative Chemoradiotherapy for a Nonmetastatic Adenocarcinoma of the Oesophagogastric Junction. Gastroenterol Res Pract 2015: 404203.
- Hosokawa Y, Kinoshita T, Konishi M, Takahashi S, Gotohda N, et al. (2014) Recurrence patterns of esophagogastric junction adenocarcinoma according to Siewert's classification after radical resection. Anticancer Res 34: 4391-4397.
- Straatman J, van der Wielen N, Cuesta MA, de Lange-de Klerk ES, Jansma EP, et al. (2015) Minimally Invasive Versus Open Total Gastrectomy for Gastric Cancer: A Systematic Review and Meta-analysis of Short-Term Outcomes and Completeness of Resection: Surgical Techniques in Gastric Cancer. World J Surg.
- Tuttle R, Hochwald SN, Kukar M, Ben-David K (2015) Total laparoscopic resection for advanced gastric cancer is safe and feasible in the Western population. SurgEndosc .
- Kelly KJ, Selby L, Chou JF, Dukleska K, Capanu M, et al. (2015) Laparoscopic Versus Open Gastrectomy for Gastric Adenocarcinoma in the West: A Case-Control Study. Ann SurgOncol 22: 3590-3596.
- Shen W, Xi H, Wei B, Cui J, Bian S, et al. (2015) Robotic versus laparoscopic gastrectomy for gastric cancer: comparison of short-term surgical outcomes. SurgEndosc .
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 16939
- [From(publication date):
December-2015 - Jul 01, 2025] - Breakdown by view type
- HTML page views : 15904
- PDF downloads : 1035