Management of Otogenic Brain Abscess: A Case Report and Review of the Literature
Received: 30-Oct-2024 / Manuscript No. ocr-24-150598 / Editor assigned: 02-Nov-2024 / PreQC No. ocr-24-150598 (PQ) / Reviewed: 18-Nov-2024 / QC No. ocr-24-150598 / Revised: 22-Nov-2024 / Manuscript No. ocr-24-150598 (R) / Published Date: 30-Nov-2024 QI No. / ocr-24-150598
Abstract
Otogenic brain abscess (OBA) is a rare but serious complication of otitis media, particularly when associated with mastoiditis. We report a case of an otogenic brain abscess of a 56-year-old female who was transferred to our medical institution with clinical symptoms of otorrhea, otalgia and headache. Pre-operative CT and MRI Scan revealed right-sided suppurative otitis media, right-sided subdural abscess, mastoiditis and pansinusitis. Laboratory results showed CRP over 300mg/L before antibiotic therapy. Septicemia protocol was initiated upon admission, and Oto-rhino-laryngological and Neurosurgical consultations were requested. Emergent right-sided mastoidectomy including intracerebral abscess punction was performed whereby pus from the right temporal part of the brain was drained out. A few days later, due to the deterioration of the patient’s condition, a right-sided intracerebral abscess resection was performed. A histopathological examination later confirmed an abscess cavity/wall. The current follow-up examination revealed no recurrent/residual intracerebral abscess. This review aims to summarize the current understanding of the management of otogenic brain abscesses. The diagnosis of otogenic brain abscess (OBA) requires a high index of suspicion, thorough clinical evaluation, and neuroimaging studies, such as computed tomography (CT) and magnetic resonance imaging (MRI). Prompt and accurate diagnosis is crucial for timely intervention and improved outcomes. Multidisciplinary consultation/team (MDT) is essential to the success of OBA diagnosis and management. Despite advancements in diagnostics and therapeutics, otogenic brain abscesses remain associated with significant morbidity and mortality. Therefore, early recognition, aggressive treatment, and close monitoring are essential for optimizing patient outcomes.
Keywords
brain abscess; Otogenic; Intracerebral; Surgical drainage; Mastoiditis.
Introduction
Otogenic brain abscess (OBA) is a serious medical condition characterized by the formation of a collection of pus within the brain, typically resulting from an infection originating from the middle ear or mastoid bone that affects brain parenchyma and can lead to brain hernia. This condition poses significant health risks and requires prompt medical intervention.
Common symptoms of OBA are not specific but may include nausea, fever, vomiting, headache, weakness, numbness, and other neurological deficits. OBA is rare due to the increasing attention paid to middle-ear diseases with early application of antibiotics therapy and advanced imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI). Inappropriate use of antibiotics can alter or hide the characteristics of the disease which can make diagnosis difficult [1].
Treatment of OBA usually involves a multidisciplinary approach including sometimes neurosurgical interventions like aspiration of abscess or burr-hole drainage. Prompt diagnosis and appropriate management are essential for optimizing outcomes and reducing the risk of long-term neurological sequelae or mortality associated with otogenic brain abscess [2]. Early recognition of symptoms, timely medical intervention, and close follow-up care are crucial in the management of this potentially life-threatening condition.
The present study reports a case of a female patient with OBA. The neuro-radiological, histo-pathological findings and multi-disciplinary management are discussed in detail.
Case report
A 56-year-old female patient was transferred to our hospital (B.A.Z. County Hospital and University Teaching Hospital, Miskolc, Hungary) in September 2023, with clinical symptoms of right-sided otorrhea, otalgia and severe headache on presentation. The pain comes and goes and sometimes gets worse. A throbbing headache felt all over the right side of the head radiating to the right ear region. For the past few months, the patient felt unbalanced walking, spinning, and never getting better. Complaints were accompanied by no fever and vomiting. She complained of ear pain accompanied by decreased hearing and a thick yellowish-like discharge from the right ear. She was initially examined at the Emergency department of another medical institution where laboratory test and Contrast-enhanced Head CT scan were performed revealing CRP over 300mg/L, leukocytosis, otogenic infection, extradural and subdural abscess.
She had a past medical history of hypertension and hysterectomy due to cervix cancer. However, from the family history, it should be noted that there was no significant medical history. Physical examination revealed left-sided peripheral facial paresis, mild dysarthria with no diplopia, mydriasis, low visual acuity and other neurological deficits. She was oriented, and alert with slower psychomotor movements.
Pre-operative contrast-enhanced head CT scan was performed at another medical institution which revealed right-sided fronto-parietal 3mm thickening hypodensity subdural fluid accumulation, filled up right-sided external and internal acoustic meatus. The radiologist suggested a head MRI examination to clarify the findings.
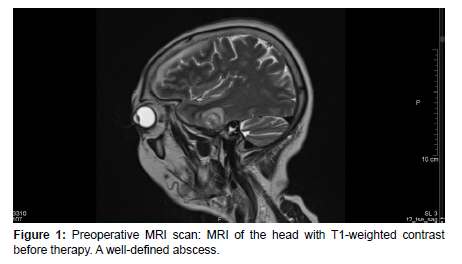
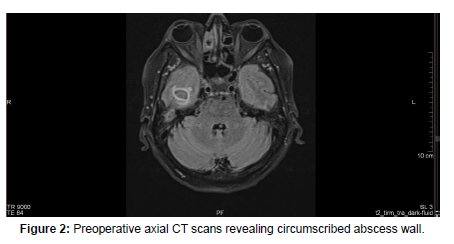
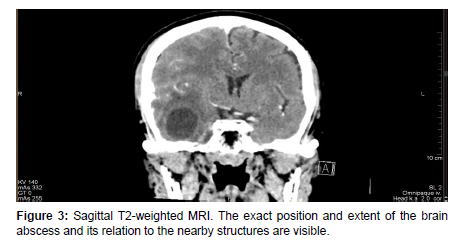
On arrival at our institution, she was admitted to the Neurology department where neurosurgical, and ENT consultation was requested. A laboratory test, hemoculture, lumbar punction and Head MRI scan were performed which revealed WBC: 11.09G/l, CRP: 280.59mg/l, positive streptococcus AG, Otitis and cerebral abscess [Figure1-4]. Septicemia protocol was initiated in which the patient was given parenterally Ceftriaxone 2x2gr, Standacillin 4x3gr, Clexane 0.4ml Sc, Solu-Medrol 1x40mg, Mannisol-A2x250ml and Salsol 500ml.
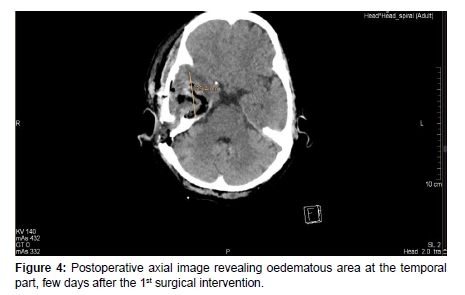
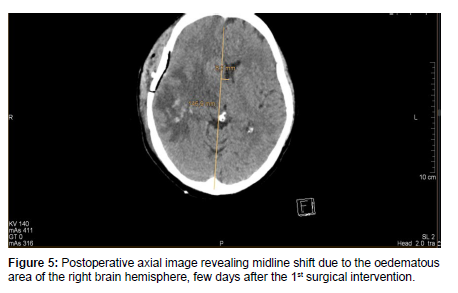
After a few days, the patient’s condition did not improve, therefore she was transferred to our department for further management. On admission, in continuation with antibiotic therapy, after an anesthesiologic and pre-operative investigations was done, an urgent right-sided mastoidectomy including intracerebral abscess punction was performed in conjunction with the Neurosurgical team, clearing away the infected cavity, bone and tissue. Due to the slow recovery of the patient’s conditions and on the 8th day postoperatively, a control contrast-enhanced Head CT scan was performed revealing a progressive right-sided temporal intracerebral region abscess formation of 20x26x32mm in size, a 4 mm right-sided dislocation from the midline and a total regression in subdural empyema formation [Figure 5,6].
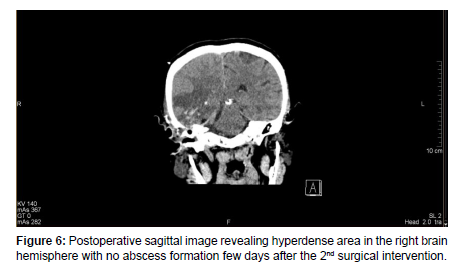
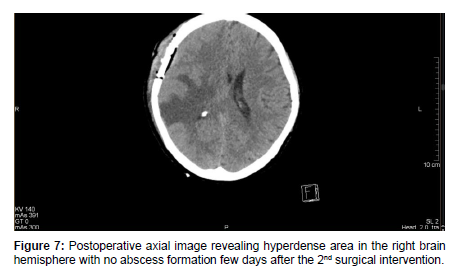
Therefore, a control Neuro-surgical consultation was requested, which recommended an urgent surgical procedure. After an anesthesiologic and pre-operative investigation was done, a right-sided temporal craniotomy with intracerebral abscess resection was performed by the Neurosurgical team. Histo-pathological sample was sent to the Pathology department which confirmed an abscess cavity with no malignancy. A microbiological sample was sent for analysis which revealed an anaerobic microbe of Propionbacterium, resistant to Metronidazole. The patient’s condition gradually improved post-operatively. After a few days of post-op management, a control Head MRI revealed right-sided mild edematous swelling with no abscess formation [Figure 7].
She was later discharged home against medical advice even though further in-patient follow-up was recommended. Therefore, she was placed on ambulatory monitoring with an oral antibiotic prescription. After several weeks, control Head CT revealed a significant improvement in the brain edematous swelling with no abscess formation. Clinically she demonstrated a mild headache with no other important symptoms.
Further post-operative follow-up clinical and MRI examination is planned for 5 to 6 months later. Informed consent was obtained from the patient for this study and publication of accompanying images and videos. Our study was performed according to the Declaration of Helsinki.
Discussion
An otogenic brain abscess is an accumulation of pus in the cerebrum or cerebellum that develops into encephalitis. Otogenic brain abscess is caused by pyogenic microorganisms originating from the inflammatory process in the middle ear cavity. Acute and chronic otitis media both cause autogenic brain abscesses. Possible pathways of propagation of brain abscess consist of direct extension and hematogenous pathways. Cholesteatoma can not only erode bone but also often form bacterial biofilms, rendering antibiotics useless [3].
Among these, the most common cause is a direct extension of infection through the osteitis bone. Autogenic brain abscess usually presents as a solitary abscess rather than in multiple forms. The infection then develops in the temporal lobe 2-4 times more often than in the cerebellum. However, there are many different reports about the location of the abscess. After the development of antibiotics, the incidence of auto-genic brain abscesses decreased statistically. Although rare, the mortality rate is high enough to consider the possibility of intracranial complications in the treatment of otitis media.
The most common symptoms across all studies were headache, altered mental status, papilledema, and meningeal irritation. In the post-CT era, the most common presenting symptoms were fever (with a similar proportion to the pre-CT era), headache and nausea [4,5,6]. A temporal bone and brain CT scan or MRI should be considered in any patient with a history of chronic ear disease presenting with new onset fever, headache, and nausea, although it should be emphasized that only a minority of patients with OBA will present with any one of these symptoms. In rare cases, lateralization may occur on motor examination. Vertigo symptoms will be more dominant in the vestibulocerebellum structures (flocculus, para flocculus, tonsils, and nodules). In addition, gaze-evoked nystagmus and saccadic movements are often found on physical examination, and symptoms of head-shaking nystagmus appear (2%-15%). and central-positional nystagmus (12%-28%) [7].
Investigations in patients with brain abscesses can provide a general picture of infection, such as erythrocyte sedimentation rate, blood leukocytosis or elevated CRP as was seen in this patient. In the case report, complete blood count showed leukocytosis 14,980 /uL, neutrophils 89.4%, C-reactive protein (CRP) 227 mg/L, and erythrocyte sedimentation rate (ESR) 30 mm/hour, suggesting an infection. Brain imaging should be repeated to see the response to treatment in 1-2 weeks. If antibiotic therapy is adequate, the abscess will resolve with a gradual decrease in abscess size [8].
Smaller abscesses may eventually heal with no residual abnormalities on imaging [9]. CT scan is the preferred radiological investigation as it provides useful details on bony erosion of the mastoid and can be used to identify the source of the abscess and the best courses of action [10]. MRI examination provides higher sensitivity and specificity in diagnosing brain abscesses. Brain abscesses are typically discovered on a brain CT scan in the watershed regions between vascular territories that connect the grey and white. These abscesses have smooth, uniform, thin-walled capsules with areas of ring enhancement surrounding hypodense centers [11]. Patients with symptoms for less than one week respond better to medical treatment than those who persist for more than one week. In certain circumstances, brain abscesses can be treated without surgery. Small abscesses (< 2.5 cm) and cerebritis may respond to antibiotics alone. Patients treated with medical therapy alone improved clinically before the CT scan showed improvement. CT scan and MRI will show a decrease in lesion size and oedema and a reduction in the number of lesions. Improvements in CT scans generally show complete resolution in 1-11 months, although radiological abnormalities may persist for months after successful therapy [12]. The early stages of cerebritis can be managed in a shorter time, about 4-6 weeks. However, patients with encapsulated abscesses, tissue necrosis, uncontrolled abscess growth, multiple abscesses, lesions at vital sites, and immunocompromise require 6-8 weeks. It takes a long time for brain tissue to repair and close the abscess space. Initial antibiotics are administered intravenously, followed by 2-6 months of oral therapy. Penetration of antibiotics is poor across the blood-brain barrier, so the choice of antibiotics is limited, and maximum doses are often required [13]. In this case report, the patient was in the encapsulation stage, so the patient was considered for long-term use of antibiotics. Empirical antibiotic therapy should be based on the most likely underlying etiologic agent, source of primary infection and pathogenesis. Parenteral antibiotics are active against pathogens, penetrate the abscess fluid and the site of the underlying disease in adequate concentrations and are bactericidal.
The combination of penicillin or a third-generation cephalosporin (cefotaxime or ceftriaxone) plus metronidazole is effective empiric therapy in most cases [14]. Community-acquired brain abscesses in adults, a combination of intravenous cefotaxime 8-12 g/day or ceftriaxone 4 g/day, and intravenous metronidazole 1.5 g/day is recommended in the majority of cases [15]. Aerobic bacteria that can cause auto-genic brain abscesses include Staphylococcus, Proteus sp, and Streptococcus pneumonia, while anaerobic bacteria Pepto coccus, Pepto streptococcus, and Bacteroides sp. Streptococcus pneumoniae is susceptible to empiric antibiotics [16, 17].
Neurosurgical treatment of the abscess is the first line for lesions 2.5 cm or larger. The specific method of abscess treatment (burr hole, stereotactic drainage, or open craniotomy) is less important than infection control and the radiographic character of the lesion [18, 19, 20]. It has been proposed that abscesses less than 2.5cm can be managed conservatively with antibiotics though this is controversial [21]. Removal of abscess via excision is the first-line surgical technique for superficial brain abscesses (as is often the case with an otogenic origin) encapsulated abscesses, or abscesses of the cerebellum, Otherwise, the abscess is stereo tactically aspirated under image guidance (generally CT) via burrhole. If needle aspiration fails to decrease the abscess size, excision is performed as observed in this case. Cultures should be taken at the time of aspiration for further investigation and management. Abscess evacuation surgery without proper antibiotic coverage is usually less meaningful in handling [22].
Coordination between otology and neurosurgical teams is key in treating otogenic abscesses where source control must be obtained via mastoidectomy, in a single staged or delayed fashion depending on availability and patient stability. In cases of clinical deterioration or neurological alteration, the brain abscesses should be evacuated urgently by the Neurosurgical team and the mastoidectomy can follow as source control. However, a combined surgical approach between the two teams is optimal when allowed by the clinical scenario. Intravenous antibiotic coverage is narrowed based on culture data and continued for 4-8 weeks and follow-up CT scans are used to ensure that abscess has resolved. Ratnaike et al. have shown that the state of consciousness is an important indication of the poor prognosis of brain abscesses [23].
Long-term antibiotic treatment can be an alternative if surgery cannot be performed. It should be accompanied by close monitoring of the side effects of antibiotics. Initial empiric antibiotic therapy should be based on the expected etiologic agent, possible predisposing conditions, source of primary infection and suspected pathogenesis. The antibiotic selected must be able to penetrate the abscess fluid and the site of primary infection in adequate concentrations and be effective with improvement in the patient’s general medical condition. Surgery is still considered if there is no good clinical response during medical therapy.
Conclusion
In summary, otogenic brain abscess represents a serious otitis media complication requiring prompt diagnosis, aggressive treatment, and close monitoring. Furthermore, while advancements in diagnostics and therapeutics have improved outcomes, otogenic brain abscesses remain associated with significant morbidity and mortality. Complications such as cranial nerve palsies, meningitis, and intracranial hypertension may occur and require vigilant management. In light of these challenges, ongoing research is warranted to explore novel diagnostic modalities, treatment strategies, and preventive measures for otogenic brain abscesses. OBA should still be considered by all relevant specialities, especially the emergency department, infection department, clinical pharmacy laboratory, otolaryngology, neurosurgery and imaging department, as multidisciplinary consultation (MDT) is critical for the success of the first diagnosis of OBA. The diagnosis and treatment team develops personalized treatment plans by integrating MDT treatment opinions and combining patients' actual condition, making the diagnosis and treatment of OBA accurate and timely.
Financial disclosure: None.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Declarations: All authors have read the final version of the manuscript with full agreement of submission. This paper has not been submitted to elsewhere simultaneously and it is not under consideration in other journals or books. We declare that it is an original report.
Acknowledgements: None.
Patient consent: Written consent was obtained from the patient for publication of this case and any accompanying images.
References
- Kwak MK, Chung JH, Lee SH, Park CW (2014) A Case of Otogenic Brain Abscess Causing Loss of Consciousness. Korean J Audiol 18:76-79.
- Li X, Du H, Song Z, Wang H (2022) Polymicrobial Anaerobic Meningitis Detected by Next-Generation Sequencing: Case Report and Review of the Literature Front.
- Ziheng Z, Cong Wu, Haiou X, Xinru Y (2023) Clinical analysis, diagnosis, and treatment strategies for Otogenic brain abscesses.
- Kao PT, Tseng HK, Liu CP, Su SC, Lee CM, et al. (2003) Brain abscess: clinical analysis of 53 cases. J Microbiol Immunol Infect 36:129-136.
- Keet PC (1990) Cranial intradural abscess management of 641 patients during the 35 years from 1952 to 1986. Br J Neurosurg 4:273-278.
- Lakshmi V, Rao RR, Dinakar I (1993) Bacteriology of brain abscess—observations on 50 cases. J Med Microbiol 38:187-190.
- Murthy PSN, Sukumar R, Hazarika P, Rao AD (1991) Otogenic brain abscess in childhood. Int J Pediatr Otorhinolaryngol 22:9-17.
- Newlands WJ (1965) Otogenic brain abscess: a study of eighty cases. J Laryngol Otol 79: 130-140.
- Morgan H, Wood MW, Murphey F (1973) Experience with 88 consecutive cases of brain abscess. J Neurosurg 38:698-704.
- Smith JA, Danner CJ (2006) Complications of chronic otitis media and cholesteatoma. Otolaryngol Clin North Am. 2006, 39:1237-55.
- Lu CH, Chang WN, Lui CC (2006) Strategies for the management of bacterial brain abscess. J Clin Neurosci. 13:979-85.
- Yang SY (1981) Brain abscess: a review of 400 cases. J Neurosurg 55:794-799.
- Brook I (2017) Microbiology and treatment of brain abscess. J Clin Neurosci 2017; 38:8-12.
- Lavin JM, Rusher T, Shah RK (2016) Complications of pediatric otitis media. Otolaryngol Head Neck Surg 154:366-370.
- Cantiera M, Tattevin P, Sonneville R (2019) Brain abscess in immunocompetent adult patients. Rev Neurol (Paris) 175: 469-74.
- Cho SH, Park MK, Lee JD, Hwang SC (2012) Otogenic brain abscess presenting with gait ataxia. Korean J Audiol 1:314.
- Chen M, Low DCY, Low SYY, Muzumdar D, Seow WT, et al. (2018) Management of brain abscesses: where are we now? Child’s Nerv Syst 10: 187180.
- Hafidh M, Keogh I, Walsh R, Walsh M (2006) Otogenic intracranial complications. a 7-year retrospective review. Am J Otolaryngol 27:390-395.
- Sharma B, Gupta S, Khosla V (2000) Current concepts in the management of pyogenic brain abscess. Neurol India 2000; 48:105-111.
- Corsini Campioli C, Castillo Almeida NE, O’Horo JC (2021) Bacterial brain abscess: an outline for diagnosis and management. Am J Med 10: 1210-1217.
- Harbaugh R, Shaffrey CI, Couldwell WT, Berger MS (2015) Neurosurgery Knowledge Update: A Comprehensive Review. New York: Thieme.
- Duarte MJ, Kozin ED, Barshak MB (2018) Otogenic brain abscesses: a systematic review. Laryngoscope Investig Otolaryngol 3:198-208.
- Ratnaike TE, Das S, Gregson BA, Mendelow AD (2011) A review of brain abscess surgical treatment–78 years: aspiration versus excision. World Neurosurg 5:431-436.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Ifeoluwa A, Amika OP, Béla D, Tamás K (2024) Management of Otogenic Brain Abscess: A Case Report and Review of the Literature. Otolaryngol (Sunnyvale) 14: 592.
Copyright: © 2024 Ifeoluwa A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1102
- [From(publication date): 0-0 - Jul 02, 2025]
- Breakdown by view type
- HTML page views: 876
- PDF downloads: 226