Management of Metastatic Bone Disease: An Update of the Role of Interventional Radiology
Received: 22-Apr-2018 / Accepted Date: 21-Sep-2018 / Published Date: 29-Sep-2018 DOI: 10.4172/2167-7964.1000299
Keywords: Bone lesions; Oligometastasis; Ablative therapy; Consolidative therapy; Minimally invasive technique; Palliative and curative intent
Introduction
Bone is the third most common site of metastases after liver and lungs for all kinds of tumours [1]. The primary tumours that most frequently metastasize to the bones include breast, prostate and lung cancer [2]. In the course of their disease, about 50% of patients with skeletal metastases will develop pain, which is the most common symptom among all the potential complications associated with bone metastasis, such as pathological fractures, spinal compression and hypercalcaemia. All these events result in a significant deterioration in quality of life as well as a reduction in survival [3].
External beam radiation is currently the standard of care for the management of painful bone metastases: this technique achieves pain relief through several biological effects, such as tumour burden and osteolysis reduction [4]. In addiction to radiotherapy, a systemic approach including chemotherapy, hormonal therapy, bisphosphonates and analgesics is also adopted in case of widespread metastatic disease.
However many patients do not obtain adequate benefits from the use of conventional therapies. In the last decades different minimally invasive image-guided techniques have been explored as new strategies for the management of patients with bone metastases, especially in those who are refractory to radiation therapy and systemic palliation.
Among these alternative strategies, which are mainly in the hands of interventional radiologists, image-guided percutaneous therapies can be distinguished into “ablative” or “consolidative”. Ablative techniques are based on the use of specific devices that induce tumour necrosis by dramatically increasing or decreasing intra-lesional temperature [5]. These include radio-frequency ablation (RFA), cryoablation (CA), microwave ablation (MWA) and high-intensity focused ultrasound (HIFU), and can be performed for either a curative or palliative intent. On the other hand, consolidative treatments like cementoplasty or osteoplasty achieve bone defect reinforcement through percutaneous injection of polymethylmethacrylate (PMMA) cement, in order to alleviate pain and prevent pathological fractures [6].
Despite the fact that these techniques are minimally invasive, several procedure-related adverse events can occur. According to the Society of Interventional Radiology (SIR) standards-of-practice classification, complications are distinguished into minor and major [7]. Major complications are events that lead to substantial morbidity and disability, and often require hospitalization; they include all those cases in which blood transfusions or interventional drainage procedures are needed. In literature a very low rate of major complications is reported, with the high percentage reported by Liberman et al. which find major complications in 3 of 43 treated patients. All other complications are considered minor, including asymptomatic minimal bleeding or fluid accumulation visible on CT, and post-ablation syndrome (fever, fatigue and malaise induced by the release of inflammatory factors from necrotic tissues).
In this review we present evidences supporting the role of interventional radiology in the management of skeletal metastases. We focused on the last updates in literature, reporting promising results in terms of both local tumour growth control and pain palliation. Study characteristics for each analyzed review for both curative and palliative treatment are reported in Tables 1 and 2 respectively.
| Curative Reference | Design | Tumor Histology | Site | Abiation Modality | Pts/Tumors | Tumors treated | LCR | OS | DFS | F-up | MC | mC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deschamps et al | R | Mixed | Bone | RFA, CA | 89/122 | 122 | 67%/1y | RFA:91%/1y,79%/2y;CA:90%/1y,63%/2y | 64%/1y | 22.8m(A) | 11 | NR |
| Wallance et al | R | Mixed | Bone | RFA+CP | NR/55 | 55 | 89%/3m,74%/6m,70%/1y | NR | NR | 34w(A) | 0 | NR |
| Mcmenomy et al | R | Mixed | Msk | CA | 40/52 | 19 | 68%/21m | 91%1y,84%/2y | 22%1y,7%/2y | 21m(A) | 2 | NR |
| Littrup et al | P(in press) | Mixed | Bone+st | CA | 126/251 | 34 | 97%/14.5m | NR | NR | 14.5(A) | 5 | NR |
| Aubry et al | P(in press) | NR | Bone+st | MWA | NR/16 | 16 | 76,9%/6m,63,6%/1y | NR | NR | NR | 0 | NR |
| Napoli et al | P | Mixed | Bone | HIFU | 18/18 | 18 | 89%/3m | NR | NR | 3m | 0 | NR |
Table 1. Curative treatment: presentation of study characteristics for each review, with summary of study design and final results.
| Palliative Reference | Design | Tumor histology | Site | Ablation modality | Pts/Tumors | Tumors treated | Pain reduction rate | Mean reduction in Maximum Pain Score | Opioid reduction rate | F-up | MC | mC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Goetz et al | P | Mixed | Bone | RFA | 43/43 | 43 | 95%(41) | 4.9/1y-6.5/2y | NR(MED red 53,6 mg/die-1y) | 16w | 3 | NR |
| Dupuy et al | P | Mixed | Bone | RFA | 55/55 | 55 | NR | 26.9/1m-14.2/3m(out of 100) | NR | 3m | 3 | NR |
| Wallace et al | R | Mixed | Bone(Spine) | RFA+CP | 72/110 | 110 | 78%(45/58) | 4.1/1w-5.1/4w | 31% | 4w | 0 | 4 |
| Callstrom et al | PM | Mixed | Bone | CA | 61/69 | 69 | 69%(42) | 5.7/6m | 83%(MED red 84 mg/die-6m) | 6m | 1 | NR |
| Tomasian et al | R | Mixed | Bone(Spine) | CA | 14/31 | 31 | 100%(14) | 5.0/3m | NR(MED red 280 mg/die-3m) | 10m | 0 | 2 |
| McArthur et al | R | Mixed | Bone | CA | 16/16 | 16 | 100%/3m(16) | 5.8/3m | NR | 3m | 0 | NR |
| Kastler et al | R | Mixed | Bone | MWA | 17/20 | 20 | 100%(20) | 5.2/3m-5.1/6m | 65% | 6m | 0 | NR |
| Pusceddu et al | R | Mixed | Bone | MWA+CP | 35/37 | 37 | 100%/1m(35) | 6.1/1m- 6.2/6m | NR | 1y | 0 | 7 |
| Wei et al | R | NSCLC | Bone(extraspinal) | MWA+CP | 26/33 | 33 | 96%(25/26) | 6.5/3m-6.2/6m | NR(MED red 38,5 mg/die-6m) | 6m | 2 | 6 |
| Gainfelice et al | R | Mixed | Bone(extraspinal) | HIFU | 11~/12 | 12 | 100%(11) | 4.7/1m-5.5/3m | 100% | 3m | 0 | NR |
| Hurwitz et al | PRT | Mixed | Bone | HIFU | 147/147 | 112(35 control) | 64%(72) vs. placebo 20%(7) | 3.6/3m(vs. placebo 0.7/3m) | 44% vs.placebo 14% | 3m | 3 | NR |
| Napoli et al | P | Mixed | Bone | HIFU | 18/18 | 18 | 89%(16) | 6.1/3m | NR | 3m | 0 | NR |
| Anselmetti et al | PM | NR | Bone(Spine) | CP | 4547/644 | 644 | 88%(of all parients) | 5.9/48h-5.4/12m | NR | 12m | 0 | ##### |
| Berenson et al | PRT | Mixed | Bone(Spine) | CP | 134/247 | 138(109 control) | NR | NR | NR | 1m | 2 | NR |
| Cazzato et al | SR | Mixed | Bone(extraspinal) | CP | 196/223 | 223 | 95.60% | 5.5/6.3m | NR | 6.3m | 5 | 16 |
Table 2. palliative treatment: presentation of study characteristics for each review, with summary of study design and final results
Curative Treatment
Patients with distant metastases are considered non-completely curable. However recently published data suggest that a small percentage of oncologic patients with a low number of metastases at the time of diagnosis, treated with a curative purpose, may remain disease-free for years without developing additional metastatic lesions. The oligometastatic disease has increasingly gained support within the oncology community, which has defined a distinct clinical entity [8], such that in patients with oligometastatic disease, local treatments can still be performed in order to completely destroy the tumoral tissue with a curative intent.
In regards to bone metastases, surgical resection is less common than hepatic or pulmonary metastasectomy, due to its high morbidity levels, while stereotactic body radiotherapy (SBRT) is considered less invasive and seems to be a promising and valid alternative according to literature evidence [9]. In their prospective study, Milano et al analyzed the long-term overall survival (OS) and cancer control outcomes of 121 patients with five or fewer clinically detectable metastases, showing good long-term survival (> 4 years) outcomes after SBRT for limited metastases, particularly in patients with breast cancer (1 of 2) rather than patients with cancer froma other primary sites (1 of 6). Furthermore, the variables of “bone metastases” and “one vs. more than one metastasis” were associated with a fourfold and threefold reduced hazard of death.
Similarly, as mentioned above, curative treatment can be achieved with several percutaneous thermal ablation techniques [10]. Unfortunately the evidence supporting the application of these methods originates from single-center experiences, and is therefore limited due to the lack of randomized controlled trials [6]. This work reports that the therapeutic option should be reserved to selected patients presenting limited bone disease (<3 potentially treatable bone metastases, each sized <3 cm) especially if they are young, affected by slow-evolving disease or without extra-bone disease.
Although there are no well-defined inclusion criteria, an aggressive loco-regional approach should be reserved to patients with limited bone disease: in most studies it is considered limited disease when there is a number of 1 to 5 metastases localized in non-critical body structures. Importantly, other characteristics influencing the prognosis such as age, tumour histology and performance status should also be evaluated during patient selection. Curative image-guided ablation is therefore often reserved to young patients, affected by slow-evolving disease and/or without extra-bone metastases, and especially if they are not surgical candidates [11].
It is also important to point out that according to quality improvement guidelines for bone tumour management, ablation of malignant skeletal lesions should be established through a multidisciplinary evaluation. A curative approach should be attempted in slow-growing tumours with metastases measuring < 3 cm in < 3 proven locations [12].
RFA
Radiofrequency ablation (RFA) generates heat by application of high-energy frequency current through an electrode placed in the treatment site under CT-guidance. Frictional processes at the tip of the probe create heat (Joule effect), determining the destruction of adjacent tumour tissue. Bone provides a natural barrier for thermal energy, thus RFA is ideal for the treatment of lesions surrounded by bone. Depending on tumour size and location, one or more RF electrodes can be used. An additional probe performs continuous monitoring of the tissue temperature.
There are only a few studies assessing the curative aim of radiofrequency ablation in oncologic patients with bone metastases. For example, in the work of Deschamps et al. [13] 122 bone lesions from several primary cancers were treated: 74 metastases with RFA and 48 metastases with cryoablation. The aim was to reach a result as complete as possible in oligometastatic patients (group 1) and to prevent skeletal related events (SREs), including pain, fractures and nerve or spinal cord compression, in patients with long-life expectancy (group 2). The oneyear control rate was 67% and only 7 patients experienced SREs during the follow-up period. Also, an oligometastatic condition associated with metachronous and small-sized metastases, correlated with a reduction in the risk of treatment failure.
Radiofrequency ablation is often associated with stabilization by cementoplasty, especially for those bones - such as the vertebrae - which are easily subjected to compression stresses [12]. In the previously mentioned study, 38 lesions required additional cementoplasty.
More recently Wallace et al retrospectively reviewed their clinical experience aimed to evaluate the local control rate of bone metastases in patients treated with a combination of RFA and cementoplasty [14]. Patients who underwent radiotherapy were excluded, as well as those with entirely osteoblastic metastases. All the reviewed exams were performed during the 1-year follow-up. Criteria assessing local tumour control failure included: increased osteolysis or paravertebral tumour extension on CT, new or persistent enhancement of soft tissue extending into the epidural space, neural foramina or paravertebral space on MRI, and persistent FDG-uptake on PET/CT. Fifty-five tumours were treated, with estimated local control rates of 89% (41/46) at 3 months, 74% (26/35) at 6 months and 70% (21/30) at 1 year. In terms of systemic disease progression, the rates were 86% (32/37) at 3 months, 71% (22/31) at 6 months and 67% (18/27) at 1 year. Neither acute nor delayed post-procedural complications were documented during the median clinical follow-up of 34 weeks (according to the Society of Interventional Radiology classification). In 8 of 9 cases who did not achieve local tumour control after RFA and vertebral augmentation, the residual tissue was present in the posterior vertebral body and/or epidural space; this occurred due to the difficulty to treat these areas in a protective attempt to avoid damage to the adjacent nerve roots. In the ninth case a lesion of the anterolateral vertebral body was treated through a unipedicular approach and tumour recurrence occurred in the contralateral hemivertebral body.
CA
Cryoablation (CA) works through percutaneous probes that have two chambers, filled with compressed argon and helium. According to the Joule-Thomson effect, when the gases expand in the space surrounding the probe tip, a change in temperature occurs depending on the inversion temperature of the gases; the aim is to cool and thaw tissues, resulting in cell death. The volume of frozen tissue can be directly seen as a low density ice ball on CT; temperature on the surface of the ice ball is not lethal (0°C), thus the boundary should extend at least 5 mm beyond the target lesion in order to guarantee a complete ablation of the lesion itself. Furthermore, CA has an intrinsic analgesic effect on tissues, so it is well tolerated.
As far as indications and procedural technical recommendations are concerned, for curative cryoablation they are similar to those for radiofrequency ablation. Additional consolidation with cementoplasty is often necessary too [12].
In a single-institution retrospective study, McMenomy et al. [15] assessed the curative aim of cryoablation in 40 patients with 1 to 5 musculoskeletal metastases from different primary cancers, for a total of 52 metastasis. Nineteen of these were bone metastasis and 13/19 (68%) showed local control after treatment. Over the whole population overall survival (OS) rates were 91% at 1-year and 84% at 2-year followup, with a median OS of 47 months. One- and 2-year disease-free survival (DFS) rates were 22% and 7% respectively (median DFS = 7 months). Only two major complications were reported during the 40 procedures (5%).
As a part of their study, Littrup et al. [16] prospectively collected data on cryoablation procedures of 34 bone metastases (mean tumour size 4.6 cm) in 21 patients with oligometastatic disease (1-6 lesions). A further 217 soft tissue lesions were treated, classified according to the body region in retroperitoneal, superficial, intraperitoneal, head and neck. Any increase in ablation size or distinct development of asymmetric and/or nodular enhancement was considered as local treatment failure, and then distinguished between local tumour progression and recurrence, in order to differentiate incomplete tumour ablation from adjacent disease recurrence. At mean 11-month follow-up, total recurrence rate was 10% (26/251). Three recurrences (1.2%) occurred within the ablation zone, and therefore considered as local progression. Average time to recurrence was 4.9 months. In terms of bone metastases, the average follow-up period was 14.5 months, with a recurrence rate of 3% (1/34); this recurrence was marked as satellite and did not result from an incomplete treatment. A total of 5 major complications (2.3%) were referable to the procedure, two of which occurred during bone lesion treatment. These include pericardial effusion during chest wall ablation and prolonged peripheral nerve palsy following ablation of a sacral lesion.
MWA
During microwave ablation (MWA), a high-frequency electromagnetic field is produced at the tip of the antenna inserted into the tumour. The water dipoles in the adjacent tissue continuously realign with the oscillating microwave field, and their consequent rotation generates frictional heat. MWA is independent of changes in tissue impedence and the negative cooling effect of blood flow in the near vessels (heat sink effect) is little. Consequently, MWA has emerged as an alternative to RFA, because of its ability to achieve faster results with larger ablation zones. However, MWA is a newer technique and it is characterized by a higher learning curve due to the heterogeneity of clinical systems (antenna design, wavelengths, and power output of generators). Thus clinical data and experience are minimal.
Literature data on the curative potential of microwave ablation in patients with bone metastasis are still limited, but encouraging. Aubry et al. [17] found that CT-guided MWA in patients with oligometastatic bone disease had good short-term anti-cancer effects. In their study a total amount of 16 lesions were treated: 6 osteolytic metastases, 5 osteoblastic metastases and 5 soft tissue sarcomas. The results were assessed through contrast-enhanced MRI at 1, 3, 6 and 12 months after the procedure, and treatment success was defined as ≥ 80% necrosis. At 1- and 3-month follow-up the success rate was about 80%; at 6 and 12 months following MWA it was 76.9% and 63.6% respectively. No major complications were described during the procedures.
HIFU
Magnetic resonance-guided high intensity focused ultrasound (MR-HIFU) is a recently-emerged technique that focuses acoustic energy on a small target volume under MR guidance, in order to perform thermal ablation. Real-time monitoring of the temperature increase in the adjacent soft tissue, and of the corresponding thermal damage, allows immediate optimization of treatment delivery. In addition to temperature increase in the target volume, the introduced ultrasonic pulse (sonication) causes mechanical effects, namely gas formation (cavitation effect). Consequently, brief pauses between individual sonications are necessary to avoid uncontrolled reflections and deformation of the ultrasound beam.
Much experience with MR-HIFU has been gained with the treatment of some soft tissue tumours, such as symptomatic uterine fibroids [18]. Cortical bone is characterized by a high absorption of ultrasound and low thermal conductivity, thus the maximum energy deposition occurs in the region of periosteal localized pain fibers. This is a very important advantage in the treatment of painful bone lesions. However, little information is available on HIFU as a curative option for bone metastases. In a recent prospective study, Napoli et al. assessed palliation of painful bone metastases achieved with MR-HIFU, while also confirming its potential for local tumour control [19]. Targeted lesions were located in the ilium (10/18; 55.6%), the scapula (3/18; 16.7%), the transverse process of the T7 vertebra (1/18; 5.6%) and the extremities (4/18; 22.2%). An increase of bone density with restoration of cortical borders was observed in 5 of the 18 treated patients. In terms of local control, complete and partial responses (according to MD Anderson criteria) were obtained in 2 and 4 patients respectively, during a 3-month follow-up. Contrast-enhanced images were used to quantify the non-perfused volume (NPV), as defined by the percentage of cancer tissue volume enhancing before treatment and not enhancing after treatment: NPV remained substantially stable during the followup, and no significant difference was found between responders and non-responders.
Palliative Treatment
The main goal of palliative treatment is not the ablation of the tumour, but rather its aim should be to obtain pain relief and improvement in patients’ quality of life. However, despite the availability of several traditional approaches, not all patients benefit equally from the same treatment option.
The effect of ablation to achieve immediate pain reduction is based on thermal destruction of pain receptors, debulking of bone lesions and reduction of tumour-related pain mediators such as TNF. In this regard, pain palliation is achieved through ablation of the boundary surface between normal bone and neoplastic tissue, which is the most aggressive part of the tumour [6].
Patient selection for successful loco-regional treatment of bone metastases is very important. There are no well-defined criteria to submit patients to palliative ablation, yet there is a recommendation for patients with pain, evaluated as greater than 4 points in a 10-point visible analogue scale (VAS), with one or two predominant pain locations. Less eligible candidates are oncologic patients with disseminated bone disease, as well as those with osteoblastic metastases [20].
Another important aim of palliative treatment in bone metastatic patients is the prevention of skeletal related events (SREs), first of which are pathologic fractures. Osteolytic metastases located in weightbearing regions, such as vertebral body, acetabulum or condyles, can be treated with a combination of local ablation and consolidative treatment like cementoplasty [21].
RFA
Over the last two decades, at least 25 studies involving more than 400 patients have appeared in the literature regarding the role of radiofrequency ablation in pain palliation [22]. Among these, two multicentre trials, assessing the efficacy of RFA as a palliative approach in patients with painful bone metastases, have produced significant results.
The first study [23] by Goetz et al considered 43 patients with painful refractory bone metastases between 1 and 18 cm in size, who either failed or were poor candidates for standard treatments. The patients were included if they had one or two painful sites, and a score of at least 4 on the VAS over a 24-hour period. An improvement following treatment of at least two points from the worst pain was considered successful. During the follow-up period at 4, 12 and 24 weeks, 41 subjects (95%) experienced pain relief, as well as a reduction in the consumption of analgesics at 8 and 12 weeks. Adverse events were seen in 3 patients, which include cutaneous burn, transient bowel and bladder incontinence after the treatment of a sacral lesion, and acetabulum fracture.
The second study involved 6 centers belonging to the American College of Radiology Imaging Network (ACRIN) [24]. Fifty-five patients with a single painful (score >50 on a 1–100-point scale) bone metastasis underwent radiofrequency ablation. These lesions measured between 2 and 8 cm in size (average 5.2 cm), and were located in the pelvis, chest wall, spine and extremities. Patients with haematological diseases, lesions of 9 cm or greater, impending pathological fractures or with spinal canal infiltration were excluded. The subjects who previously underwent chemotherapy or external beam radiation were admitted if the therapy was suspended at least 2 weeks or 1 month respectively, before ablation. Mean pain score before ablation was 54 (range 51-91) out of 100. Pain relief was achieved in all cases during the follow-up period, with an average reduction in pain intensity of 26.9 points at 1 month and 14.2 points at 3 months. Moreover, there was an improvement in patients’ clinical status, with an increase of 19.9 and 14.9 points from pre-RFA score at 1- and 3-month followup respectively. Major complications were reported in 3 of 55 (5.4%) patients, which consisted of refractory pain and neural damage.
These two multicenter clinical trials have demonstrated the efficacy and safety of RFA for the palliation of painful bone metastases, though differences in pain relief results have also emerged, which may be due to several factors, for example patient selection criteria or technical aspects linked to the procedures (i.e. different types of RF electrodes used).
In fact the variability of patient eligibility criteria and the procedural differences between these two studies may explain the relatively decreased pain relief achieved in the ACRIN study. For example, 74% of patients in the Goetz et al trial underwent previous radiation therapy to the tumour site, compared to the 24% of population in the ACRIN study. On the other hand, no significant difference in pain relief was found between patients who previously received external beam radiation therapy and those who underwent ablation alone, although a combination of the two techniques showed a higher efficacy in the palliation of chest wall masses. Moreover, procedural pain management and control in the Goetz et al trial was accomplished with either general anaesthesia or conscious sedation, while in the ACRIN trial conscious sedation was used for the majority of cases. It is possible that this difference could have influenced the volume of tissue destruction, due to the painfulness limiting the procedure. Finally, differences in the types of treated tumours could account for the differential results of the two studies; although the most common primary tumours were the same in both studies (lung, colon and renal carcinomas), in the Goetz et al trial there was a higher proportion of other tumours, some of which with more favourable biology (desmoid, paraganglioma, meningioma, thyroid, prostate, breast).
More recently Wallace et al. [25] retrospectively reviewed their experience with RFA applied to achieve pain relief in 72 patients suffering spinal bone metastases from several primary cancers, with a total amount of 110 skeletal lesions. Cement was also instilled into the vertebral body for structural support in 105 cases (95%). Patients with osteoblastic metastases, as well as those with lesions causing spinal instability or spinal cord compression, were excluded. For each patient, pre-procedural worst pain score was assessed on the day of ablation, and then 1 and 4 weeks after treatment, using a 10-point scale (Numeric Rating Scale). A reduction of 2 or more points was considered as partial relief, while complete relief was defined when a post-procedural score ≤ 1.89% of the treated patients (64/72) had at least moderate pain (≥ 4 out of 10) prior to the procedure, with a mean score of 8.0. All patients achieved pain palliation after the procedure, including those who did not receive previous or concurrent external beam radiation therapy or corticosteroids injections. Mean pain scores were 3.9 after 1 week and 2.9 after 4 weeks, which translated into complete relief rates of 23% and 45% respectively. The 4-week follow-up pain score corresponded to a decrease in the use of pain medication in 31% of patients, as well as an increase in activity in 50% of patients. According to the Society of Interventional Radiology guidelines, no major complications related to RFA were reported. Three of the 5 lesions that were ablated - but not augmented - fractured within 12 months.
CA
Cryoablation of musculoskeletal metastases has produced similar rates of pain palliation compared with RFA [26]; yet it presents several advantages over RFA, including intrinsic direct analgesic properties, preservation of tissue collagenous architecture and potential treatment of larger tumours due to the excellent imaging-guided monitoring [27].
In the largest available study supporting the therapeutic aim of CA, Callstrom et al. [28] treated 61 patients with 1 or 2 painful bone metastases and a pre-treatment pain score of 4 or higher on a 10-point scale. Lesions in close proximity to neural structures or those causing impending fractures were excluded. A total of 69 tumours ranging from 1 to 11 cm (average 4.8 cm) in size were cryoablated. Response was assessed through the Brief Pain Inventory-short form; prior to cryoablation the mean score for worst pain in a 24-hour period was 7.1/10, which then decreased to 5.1/10 after 1 week, 4/10 after 4 weeks, and 1.4/10 after 24 weeks. A reduction in the use of analgesics was obtained in 83% of patients. Only one patient experienced a major complication (osteomyelitis at the site of ablation), thus confirming the safety of CA.
A recent case series including 14 oncologic patients with a total number of 31 vertebral metastases from different tumours, and a significant pain score (≥ 4 out of 10), showed statistically significant improvement in pain and reduction of analgesic usage at 1-week, 1-month and 3-month follow-up [29]. Local tumour control was also achieved in 30/31 ablations (96.7%) during a median follow-up of 10 months (range 1-24 months). Moreover, this was the first study assessing the efficacy of cryoablation in the palliation and local control of osteoblastic painful metastases; and only one single case report was excluded [30]. No major complications were reported, while 2 patients had post-procedural radicular pain that required corticosteroid injections.
In a retrospective single-center review by McArthur et al. [31], 16 patients with a single bone metastasis from different tumours underwent cryoablation. All reported improvement in pain within 1 week following the procedure and at 3-month follow-up. In 2 cases there was a near-to-complete pain relief, with a 10/10 pre-procedural pain score dropping to 1-2/10 at 3 months, while one patient reported only mild pain improvement at 3 months, with a 2-point reduction in pain levels. Over the total of patients, 93.8% had tumour arrest or shrinkage on follow-up CT. Post-treatment CT examinations demonstrated marginal enhancement at the ablation site in 62.5% of cases, although only one patient had interval growth. A single case of neuroapraxia was reported, which resolved within 48 hours.
MWA
Microwave ablation of bone lesions has emerged as an alternative to RFA in some cases: since MWA does not rely on the flow of current but rather on the induction of an electromagnetic field, it is independent of changes in tissue electrical impedance, thus allowing achieving faster results with larger ablation zones [20]. However it is a rather new technology, so there are fewer studies compared to other techniques, and therefore clinical data is still limited.
In a retrospective study, Kastler et al. [32] suggested that MWA may be advantageous for palliation of sclerotic bone metastases. MWA was performed on 17 patients with 20 painful spinal metastatic tumours from different primary cancers, measuring from 12 to 70 mm in size. Concurrent cementoplasty was performed in 9 cases. A significant reduction in pain was achieved, shown by a pre-procedural VAS score of 7.4/10 that dropped to post-procedural scores of 1.9, 2.2 and 2.3 at 1-month, 3-month and 6-month time points respectively. Eleven patients suspended pain medications, while the remaining 6 replaced opioid agents with non-steroidal anti-inflammatory drugs. No major complications were reported.
In a further study 35 patients were treated with MWA plus cementoplasty, achieving a mean reduction in VAS scores of 90% at 1- and 6-month follow-up (0.7 and 0.6 respectively, vs. 6.8 at baseline) [33]. All patients were discharged in stable conditions and without complications 24 hours after the procedure.
More recently Wei et al. [34] confirmed that MWA in combination with osteoplasty could represent an efficacious and safe treatment for extra-spinal skeletal metastases. In their study researchers treated 26 lung-cancer metastatic patients with refractory bone pain localized to one or two regions, life expectancy ≥3 months, and a tumour size no larger than 6 cm. A total of 33 lesions localized in the iliums (11), acetabulums (9), ischiums (8), femurs (2), clavicle (1), sacrum (1) and tibia (1) were ablated and treated with cementoplasty. The effectiveness was evaluated by a 10-point VAS: the mean pre-procedural score was 7.4, which then decreased to 1.5 after 1 month, 0.9 after 3 months and 1.2 after 6 months. A significant reduction in the average use of mean opioids was also shown in the results. Due to the occurrence of additional bone metastases, two patients experienced pain increase after 5 months; MWA was performed again, in combination with osteoplasty, which then resulted in complete pain relief. According to the International Working Group on Imaging-Guided Tumour Ablation, 2 major complications (7.7%) were reported (a local infection and a pathologic fracture), while the minor complication rate was 23.1% (6/26).
HIFU
Preliminary clinical experience with MRI-guided high intensity focal ultrasound for palliative treatment of pain was described in 11 patients with extra-spinal bone metastases (osteolytic, osteoblastic and mixed) by Gianfelice et al. [35]. The mean pre-procedural VAS score was 6.0, and all patients reported progressive improvement of pain at 1- and 3-month follow-up, with VAS scores dropping to 1.3 and 0.5 respectively. Seven patients no longer needed treatment for pain, and the remaining 4 patients reduced their intake of analgesics by at least 50%. In 5 cases there was an increase of bone density at the site of treated osteolytic metastases, suggesting a potential consolidative role of HIFU. No adverse events were reported.
The first phase III trial studying HIFU on patients with painful bone metastases was presented by Hurwitz et al. [36]. Targeted tumours were device-accessible, located in ribs, extremities (excluding joints), pelvis, shoulders, or posterior aspects of spinal vertebra below L2. A group of 147 oncologic patients were randomly assigned, in a 3:1 ratio, to MR-HIFU sonication treatment or placebo: at 3-month follow-up a response rate of 64.3% was found in the HIFU arm, compared with 20.0% in the placebo-treated group. Fast pain response was achieved through HIFU, since pain relief was observed within 3 days from the procedure in about two thirds of responders. The most common minor complication was pain during treatment (32%), while major adverse events were observed in 3% of treated patients, which included pathological fractures, neuropathy and skin burn.
In their prospective study, Napoli et al. [19] treated 18 consecutive patients with MR-HIFU, showing a higher response rate 3 months following the procedure: the overall pain response rate was 89%, with complete pain relief in 72% of patients.
Recently, through an international consensus statement, a number of recognised experts in focused ultrasound reviewed all the available data in literature, confirming the effectiveness and safeness of HIFU in the treatment of painful extra-spinal bone metastases, especially for those lesions that were refractory to conventional therapeutic modalities (for example external beam radiation treatment) [37]. To date, treatment of vertebral metastases with HIFU is not performed, due to the proximity to spinal cord and its potential thermal damage.
CP
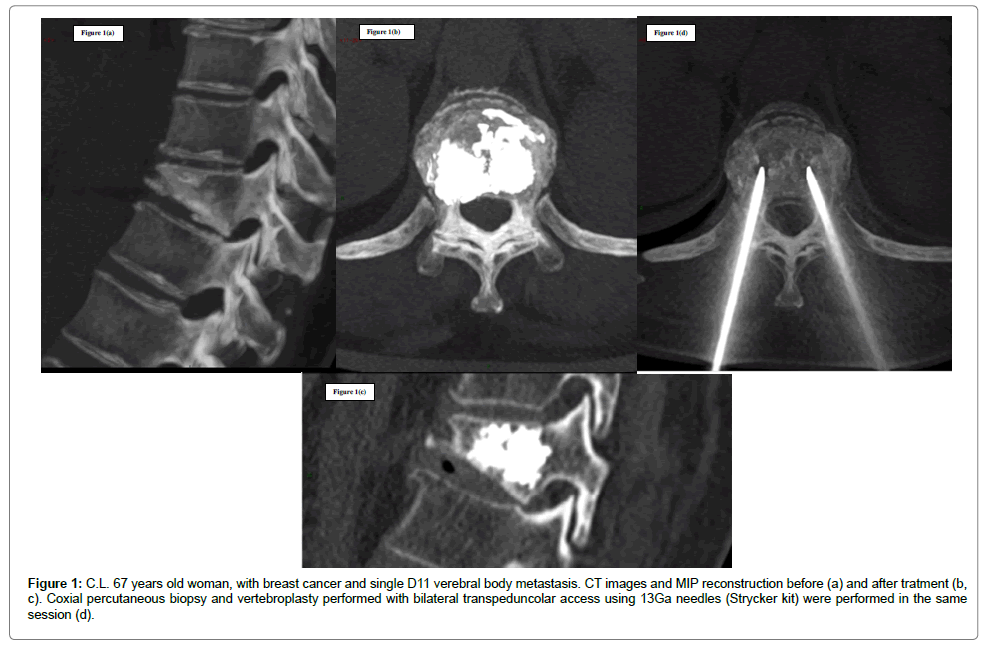
Cementoplasty, or vertebroplasty when it involves the spine, consists of percutaneous injection of polymethyl-methacrylate (PMMA) cement into the bone, most commonly used for benign vertebral body compression fractures [21]. This treatment is increasingly performed to improve the structural integrity of lytic lesions in weight-bearing bones, both in the axial and appendicular skeleton, in order to stabilize them and minimize the risk of pathologic fractures. Once injected, PMMA polymerizes through an exothermic reaction, causing local temperature to rise. As a result, cementoplasty determines not only micro- and macro-fractures consolidation, but also pain relief through the destruction of nociception terminals (Figure 1). However, the cytotoxic effects of PMMA polymerization are limited and for such reason, if procedures are performed with a curative purpose, cementoplasty should always be preceded by an ablative treatment [6].
Figure 1: C.L. 67 years old woman, with breast cancer and single D11 verebral body metastasis. CT images and MIP reconstruction before (a) and after tratment (b, c). Coxial percutaneous biopsy and vertebroplasty performed with bilateral transpeduncolar access using 13Ga needles (Strycker kit) were performed in the same session (d).
As far as timing is concerned, cementoplasty can be performed immediately after RFA or MWA, whereas for cryoablation the operator must wait for the ice ball to melt before proceeding, so that polymerization of the PMMA is not affected [38].
Consolidative treatments are most often performed on axial loading locations, including spine and acetabular regions, which are the areas mainly subjected to compressive forces. In these cases cement injection could be sufficient to achieve bone consolidation.
Conversely, in the appendicular skeleton torsional forces are also involved, especially for long bone diaphysis, implying that further strategies of consolidation such as endo-medullary nailing or external fixation should be adopted along with cementoplasty. The risk of impending fractures for bone metastases of the limbs may be quantified through Mirels’ score [39]. Consolidative treatments should be offered to patients at high risk, with a score of 9 or higher.
In the current literature coming from several multicenter series and prospective randomized trials, there is strong evidence supporting safeness and effectiveness of vertebroplasty for palliation of painful spinal metastases.
In a large prospective multicenter trial by the European VErtebroplasty RESearch Team (E.VE.RES.T), 4547 patients with vertebral compressive fractures due to several pathologies (osteoporosis, trauma, metastases) underwent vertebroplasty [40]. Pain entity was evaluated through a 10-point VAS, at baseline, 48 hours and 12 months following the procedure, and a reduction of 2 or more points was considered successful. A significant pain relief was observed in 88% of patients within 48 hours from the procedure. In the bone metastases group, including 644 patients, the average pre-treatment VAS score of 8.3 dropped to 2.4 at 48-hour follow-up, while no significant change was noted at 12-month follow-up (mean VAS score 2.9). No major complications were reported, whereas minor complications were observed in 32.9% of cases, first of which was venous leakage (20.5% of the cases).
The Cancer Patient Fracture Evaluation study [41] prospectively enrolled 134 oncologic patients with 1 to 3 vertebral compressive fractures, moderate pain (≥ 4 out of 10) and Rolande Morris disability (RDQ) score >10. They were randomly assigned to kyphoplasty (n = 70) or non-surgical conservative management (n = 64). A significant RDQ improvement was obtained in the first group, while the improvement was minimal in the non-surgical arm, as demonstrated by an average decrease of 8.3 vs 0.1 points respectively. This result was impressive, to the point that strong ethical reasons allowed for crossover from the non-surgical group to the kyphoplasty one. However, although the greater beneficial effects of kyphoplasty over conservative treatment were evidently demonstrated, statistical significance tended to vanish with time, probably due to the relatively low number of patients remaining in the conservative treatment group. As far as adverse events are concerned, two subjects from the kyphoplasty group were involved: one patient had an intraoperative non-Q-wave myocardial infarction, and one had a new fracture in the adjacent level of treatment.
Several authors assessed the efficacy of balloon kyphoplasty (BKP) compared to simple vertebroplasty (SVP) for pain palliation of bone metastases. Recently Bae et al. [42] reviewed over 10 years of their experience in the stabilization of metastatic vertebral compression fractures. Three hundred forty-two patients who underwent SVP (238) or BKP (104) for painful metastatic compression fractures from solid cancer were included. For the BKP group, the mean preoperative VAS score was 5.8, which was then reduced on average by 2.7 points after the procedure. Effective improvement in VAS score (≥ 3 points) was achieved in 206 patients (60%). Although the pre-treatment degree of compression was significantly higher in the kyphoplasty arm (47%) compared to the simple vertebroplasty arm (30%), no differences were observed in terms of VAS score improvement between the two groups. In conclusion, kyphoplasty showed better results in terms of pain reduction.
In regards to cementoplasty for pain palliation of extra-spinal bone metastases, the evidence originating from prospective and retrospective case series is scientifically less strong.
A systematic review of the current evidence by Cazzato et al. [43] demonstrated the safeness and effectiveness of percutaneous long bone cementoplasty (PLBC) for palliation of malignant lesions. Overall, 13 papers were included, with a total of 382 patients treated. Among these, 196 patients with 223 metastatic lesions (average 45 mm in size) underwent PLBC. The work obtained from 10 of the 13 articles using the 10-point VAS to assess pain, provided data suitable for statistical analysis: in this subgroup, consisting of 115 patients, a pain control rate of 95.6% was achieved (68.2% high improvement and 27.4% mild improvement), showing an extremely significant correlation between PLBC and pain relief. In a further subgroup of patients considered from 7 papers, researchers also evaluated functional improvement, which was achieved in 77.9% of cases. PLBC showed a low rate of complications: 16 patients (8%) experienced a secondary fracture, whereas other complications (haematomas, infections, PMMA leakage) were reported in 5 cases (2%).
Moreover, PLBC can be combined with other interventional and non-interventional procedures. In his review, Cazzato reports that previous external beam radiation therapy was performed in 39% of cases, while percutaneous thermal ablation was combined with cementoplasty treatment in 6% of patients. Besides the Mirels’ score [39], another way to quantify the risk of impending femoral fractures was proposed by Deschamps et al. [44] patients with cortical involvement > 30 mm or a previous fracture of the lesser trochanter should receive some form of bone stabilization. Therefore, it was shown that in combination with PLBC, 32 of the 196 patients received minimally invasive endomedullary stabilization [43], which resulted more suitable than any surgical technique, given the generally poor prognosis reserved to these patients.
Conclusion
With the increase of overall survival rate of oncologic patients and the improvement in sophistication and accuracy of imaging techniques, the number of patients detected with bone metastases is growing. In this scenario, minimally invasive image-guided procedures have become an important tool in the palliative and potentially curative management of these patients. The role of interventional radiology in the curative treatment of malignant bone lesions is limited to the few patients with oligometastatic bone disease and good life expectancy. These techniques are in fact more frequently performed with a palliative aim, in the attempt to achieve pain relief and prevent skeletal-related events. Aside from the objective advantage of being minimally invasive, interventional radiology strategies can also be safely combined with standard treatments, including chemotherapy and external beam radiation therapy.
In conclusion, to our knowledge, the data available in the literature on the safety and efficacy of ablation procedures mainly originates from retrospective case series. Thus there is great interest for prospective randomized clinical trials, in order to establish well-defined therapeutic plans and standardize the choice of treatment among the minimally invasive procedures available today, and performed by interventional radiologists on a regular basis.
References
- Tubiana-Hulin M (1991) Incidence, prevalence and distribution of bone metastases. Bone 12: S9-S10.
- Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12: 6243s-6249s.
- Saad F, Lipton A, Cook R, Chen YM, Smith M, et al. (2007) Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 110: 1860-1867.
- Lutz S, Berk L, Chang E, Chow E, Hahn C, et al. (2011) Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Rad Oncol Biol Phys 79: 965-976.
- Ahmed M, Brace CL, Lee FT, Jr, Goldberg SN (2011) Principles of and advances in percutaneous ablation. Radiol 258: 351-369.
- Cazzato RL, Buy X, Grasso RF, Luppi G, Faiella E, et al. (2015) Interventional Radiologist's perspective on the management of bone metastatic disease. Eur J Surg Oncol 41: 967-974.
- Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, et al. (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interven Rad 25: 1691-1705.
- Weichselbaum RR, Hellman S (2011) Oligometastases revisited. Nature Rev Clin Oncol 8: 378-382.
- Milano MT, Katz AW, Zhang H, Okunieff P (2012) Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Rad Oncol Biol Phys 83: 878-886.
- Kurup AN, Callstrom MR (2016) Increasing role of image-guided ablation in the treatment of musculoskeletal tumors. Cancer J 22: 401-410.
- Kurup AN, Morris JM, Callstrom MR (2017) Ablation of musculoskeletal metastases. Am J Roentgenol 209: 713-721.
- Gangi A, Tsoumakidou G, Buy X, Quoix E (2010) Quality improvement guidelines for bone tumour management. Cardiovasc Interven Rad 33: 706-713.
- Deschamps F, Farouil G, Ternes N, Gaudin A, Hakime A, et al. (2014) Thermal ablation techniques: a curative treatment of bone metastases in selected patients?. Eur Radiol 24: 1971-1980.
- Wallace AN, Tomasian A, Vaswani D, Vyhmeister R, Chang RO, Â et al. (2016) Radiographic local control of spinal metastases with percutaneous radiofrequency ablation and vertebral augmentation. Am J Neurorad 37: 759-765.
- McMenomy BP, Kurup AN, Johnson GB, Carter RE, McWilliams RR, et al. (2013) Percutaneous cryoablation of musculoskeletal oligometastatic disease for complete remission. J Vasc Interven Rad 24: 207-213.
- Littrup PJ, Bang HJ, Currier BP, Goodrich DJ, Aoun HD, et al. Â (2013) Soft-tissue cryoablation in diffuse locations: feasibility and intermediate term outcomes. J Vasc Interven Rad 24: 1817-1825.
- Aubry S, Dubut J, Nueffer JP, Chaigneau L, Vidal C, et al. Â (2016) Prospective 1-year follow-up pilot study of CT-guided microwave ablation in the treatment of bone and soft-tissue malignant tumours. Eur Radiol 27: Â 1477-1485.
- Trumm CG, Napoli A, Peller M, Clevert DA, Stahl R, et al. (2013) MR-guided focused ultrasound. Current and future applications. Der Radiologe 53: 200-208.
- Napoli A, Anzidei M, Marincola BC, Brachetti G, Ciolina F, et al. (2013) Primary pain palliation and local tumor control in bone metastases treated with magnetic resonance-guided focused ultrasound. Invest Radiol 48: 351-358.
- Ringe KI, Panzica M, von Falck C (2016) Thermoablation of bone tumors. In RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren 188: 539-550.
- Gangi A, Guth S, Imbert JP, Marin H, Dietemann JL (2003) Percutaneous vertebroplasty: indications, technique, and results. Radiographics 23: e10-e10.
- Rosenthal D, Callstrom MR (2012) Critical review and state of the art in interventional oncology: benign and metastatic disease involving bone. Radiol 262: 765-780.
- Goetz MP, Callstrom MR, Charboneau JW, Â Farrell MA, Maus TP, et al. (2004) Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol 22: 300-306.
- Dupuy DE, Liu D, Hartfeil D, Hanna L, Blume JD, et al. (2010) Percutaneous radiofrequency ablation of painful osseous metastases. Cancer 116: 989-997.
- Wallace AN, Greenwood TJ, Jennings JW (2015) Radiofrequency ablation and vertebral augmentation for palliation of painful spinal metastases. J Neuro-oncol 124: 111-118.
- Tsoumakidou G, Koch G, Caudrelier J, Garnon J, Cazzato RL, et al. (2016) Image-Guided spinal ablation: a review. Cardiovasc Interven Rad 39: 1229-1238.
- Cazzato RL, Garnon J, Ramamurthy N, Koch G, Tsoumakidou G, et al. (2016) Percutaneous image-guided cryoablation: current applications and results in the oncologic field. Med Oncol 33: 140.
- Callstrom MR, Dupuy DE, Solomon SB, Beres RA, Littrup PJ, et al. (2013) Percutaneous image-guided cryoablation of painful metastases involving bone: multicenter trial. Cancer 119: 1033-1041.
- Tomasian A, Wallace A, Northrup B, Hillen TJ, Jennings JW (2016) Spine cryoablation: pain palliation and local tumor control for vertebral metastases. Am J Neurorad 37: 189-195.
- de Freitas RM, de Menezes MR, Cerri GG, Gangi A (2011) Sclerotic vertebral metastases: pain palliation using percutaneous image-guided cryoablation. Cardiovasc Interven Rad 34: S294-299.
- McArthur TA, Narducci CA, Lander PH, Lopez-Ben R (2017) Percutane image-guided cryoablation of painful osseous metastases: a retrospective single-center review. Curr Prob Diag Rad 46: 282-287.
- Kastler A, Alnassan H, Aubry S, Kastler B (2014) Microwave thermal ablation of spinal metastatic bone tumors. J Vasc Interven Rad 25: 1470-1475.
- Pusceddu C, Sotgia B, Fele RM, Ballicu N, Melis L (2016) Combined microwave ablation and cementoplasty in patients with painful bone metastases at high risk of fracture. Cardiovasc Interven Rad 39: 74-80.
- Wei Z, Zhang K, Ye X, Yang X, Zheng A, et al. (2015) Computed tomography-guided percutaneous microwave ablation combined with osteoplasty for palliative treatment of painful extraspinal bone metastases from lung cancer. Skeletal Rad 44: 1485-1490.
- Gianfelice D, Gupta C, Kucharczyk W, Bret P, Havill D, et al. (2008) Palliative treatment of painful bone metastases with MR imaging-guided focused ultrasound. Radiol 249: 355-363.
- Hurwitz MD, Ghanouni P, Kanaev SV, Iozeffi D, Gianfelice D, et al. (2014) Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J National Cancer Inst 106(5).
- Huisman M, ter Haar G, Napoli A, Hananel A, Ghanouni P, et al. (2015) International consensus on use of focused ultrasound for painful bone metastases: Current status and future directions. Int J Hyperthermia 31: 251-259.
- Kurup AN, Morris JM, Schmit GD, Atwell TD, Schmitz JJ, et al. (2015) Balloon-assisted osteoplasty of periacetabular tumors following percutaneous cryoablation. J Vasc Interven Rad 26: 588-594.
- Mirels H (2003) Metastatic disease in long bones: A proposed scoring system for diagnosing impending pathologic fractures. Clin Ortho Rel Res 415: S4-S13.
- Anselmetti GC, Marcia S, Saba L, Muto M, Bonaldi G, et al. (2012) Percutaneous vertebroplasty: multi-centric results from EVEREST experience in large cohort of patients. Eur J Rad 81:4083-4086.
- Berenson J, Pflugmacher R, Jarzem P, Zonder J, Schechtman K, et al. (2011) Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol 12: 225-235.
- Bae JW, Gwak HS, Kim S, Joo J, Shin SH, et al. (2016) Percutaneous vertebroplasty for patients with metastatic compression fractures of the thoracolumbar spine: clinical and radiological factors affecting functional outcomes. Spine J 16: 355-364.
- Cazzato RL, Palussiere J, Buy X, Denaro V, Santini D, et al. (2015) Percutaneous long bone cementoplasty for palliation of malignant lesions of the limbs: a systematic review. Cardiovasc Interven Rad 38: 1563-1572.
- Deschamps F, Farouil G, Hakime A, Barah A, Guiu B, et al. (2012) Cementoplasty of metastases of the proximal femur: is it a safe palliative option? J Vasc Interven Rad 23: 1311-1316.
Citation: Faiella E, Santucci D, Vullo GL, Zobel BB, Grasso RF (2018) Management of metastatic bone disease: an update of the role of Interventional Radiology. OMICS J Radiol 7:299. DOI: 10.4172/2167-7964.1000299
Copyright: © 2018 Faiella E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4612
- [From(publication date): 0-2018 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 3710
- PDF downloads: 902