Management of Benign Prostatic Hyperplasia with Metformin, Letrozole and Scheduled Growth Hormone Injections: A Pathophysiology Oriented
Received: 08-Jun-2017 / Accepted Date: 26-Jun-2017 / Published Date: 30-Jun-2017
Abstract
Benign prostatic hyperplasia (BPH) is a common and bothersome condition characterized by prostate overgrowth causing slowly progressive lower urinary tract symptoms. It is highly prevalent in old age, and somewhat inescapable stigma of senility. Due to its major urologic manifestations, BPH has been conventionally assigned as a urologic disorder. Hence, it has remained an orphan medical entity in terms of pathogenesis and management. In fact, BPH, as a true metabolo-proteomic disorder, demands some relevant management strategies. Despite the known impacts of age, inheritance, senile changes in androgen to estrogen ratio, obesity, and metabolic disorders on development of BPH, the core pathogenic mechanisms that logically link and reasonably bring all those scattered findings together has not yet been meaningfully addressed. We believe that almost all informative pieces of BPH pathogenesis have been identified already. Thus, to revolutionize our understanding and to pave a definitely novel path towards medical therapy of BPH with a protocol consisting of metformin, letrozole and scheduled growth hormone injections, we only need to sort the available data out into the jigsaw of BPH pathogenesis picture.
Keywords: Benign Prostatic Hyperplasia; Metabolic Syndrome; Metformin; Letrozole; Growth hormone
165648Novel Perspectives with regard to BPH Pathogenesis
The maintenance of anthropometric measures of human body in general and specific internal organs in particular, depends on an extraordinarily system that governs the balance between cell proliferation and programmed cell death, that is, apoptosis. Even subtle disorder in this complex equation leads to either hypoplasia or hyperplasia. A wide variety of systemic growth hormones and diverse tissue specific growth factors mediate, moderate and modulate the cellgrowth cycle, proliferation, discrimination and ongoing cell refreshment. On the other hand, an extremely delicate physiologic process controls the rate of programmed cell death or apoptosis; a neat and non-inflammatory cell autolysis. Superior to these two processes stands a hitherto unknown supervising system that constantly counts the number of proliferated cells, momently measures the anthropometric organ dimensions, meticulously processes the captured data and finally makes sound and sensible commandments to maintain the physiologic equilibrium between cell proliferation and apoptosis. Even a trivial aberration in this ongoing process leads to long-term ultra-structural changes in cell morphology and eventually gross dysmorphism [1,2]. Among the long list of growth factors, insulin and insulin-like growth factor 1 (IGF-1) are the most abundant and the most potent signals inducing protein synthesis and mitogenesis. It is actually very important to know, that the IGF-1 also serves as the major cell-apoptosis modulating factor. IGF-1 prevents premature apoptosis or pathologic cell death in damaged cells through a sophisticated cell-refreshing process. The dual function of IGF-1 in cell proliferation and programmed cell death has made it the sine qua none of cellular life cycle scheduling system. Being so crucial in such a vital process, the tissue availability of active form of IGF-1 (the free IGF-1) is kept delicately regulated. Extremely short half-life of free IGF-1 (six minutes) makes it a vanishing tissue signal with rapid clearance. The unique solution to this problem is the synthesis of enough amounts of IGF-binding proteins. Therefore, the IGF-1 bioavailability is basically regulated by a few specific binding proteins, six or more of which are known to date. Among these binding proteins, IGFBP-3 has the highest affinity for IGF-1. There are various factors that markedly affect and sharply define the serum concentrations of IGF-binding proteins of which pituitary growth hormone (GH) is the most critical parameter driving the synthesis of IGF binding protein-3 by the liver. IGFBP-2 is the next binding protein in terms of binding affinity towards IGF-1. The serum level of IGFBP-2 is defined by hepatic cellular sensitivity to insulin.
In the presence of insulin resistance and the related hyperinsulinemia, hepatic synthesis of IGFBP-2 is critically curtailed. In idiopathic growth hormone deficiency, serum IGFBP-3 markedly decreases, whereas in acromegaly (high GH state) IGFBP-3 concentrations surge dramatically. Thus, it is of paramount importance to bear in mind the crucial role of serum GH on serum IGFBP-3 secretion [3-8]. It is also necessary to know that, pituitary GH secretion declines significantly after fourth decade of life and this constant decrease in serum GH, parallels the aging and senility. Abdominal obesity and the related insulin resistance are the other wellknown effectors regarding blunted pituitary GH production and decreased IGFBPs. The serum and tissue IGFBP concentrations are also modulated by a series of serum and tissue proteases called IGFBPs’ proteases. Among various sub-types of IGFBP proteases known so far, prostate specific antigen (PSA) is the most potent IGFBP protease expressed only in prostate tissue. It is also quite useful to note, that PSA gene transcription is basically regulated by prostate tissue free androgen concentrations. PSA is an IGFBP3 protease first found in seminal plasma and known to be synthesized by prostatic epithelial cells. PSA gene is positively regulated by the androgen receptor stimulation and has been studied as a model for androgen-regulated genes. Thus, the synthesis of PSA in prostate and its unique regulatory role as an IGFBP protease is currently a well- established issue [9,10], and so is the IGF-1/IGF-1 receptor/IGFBPs/IGFBP protease system as a regulatory mechanism in cell growth and programmed cell death in the prostate gland.
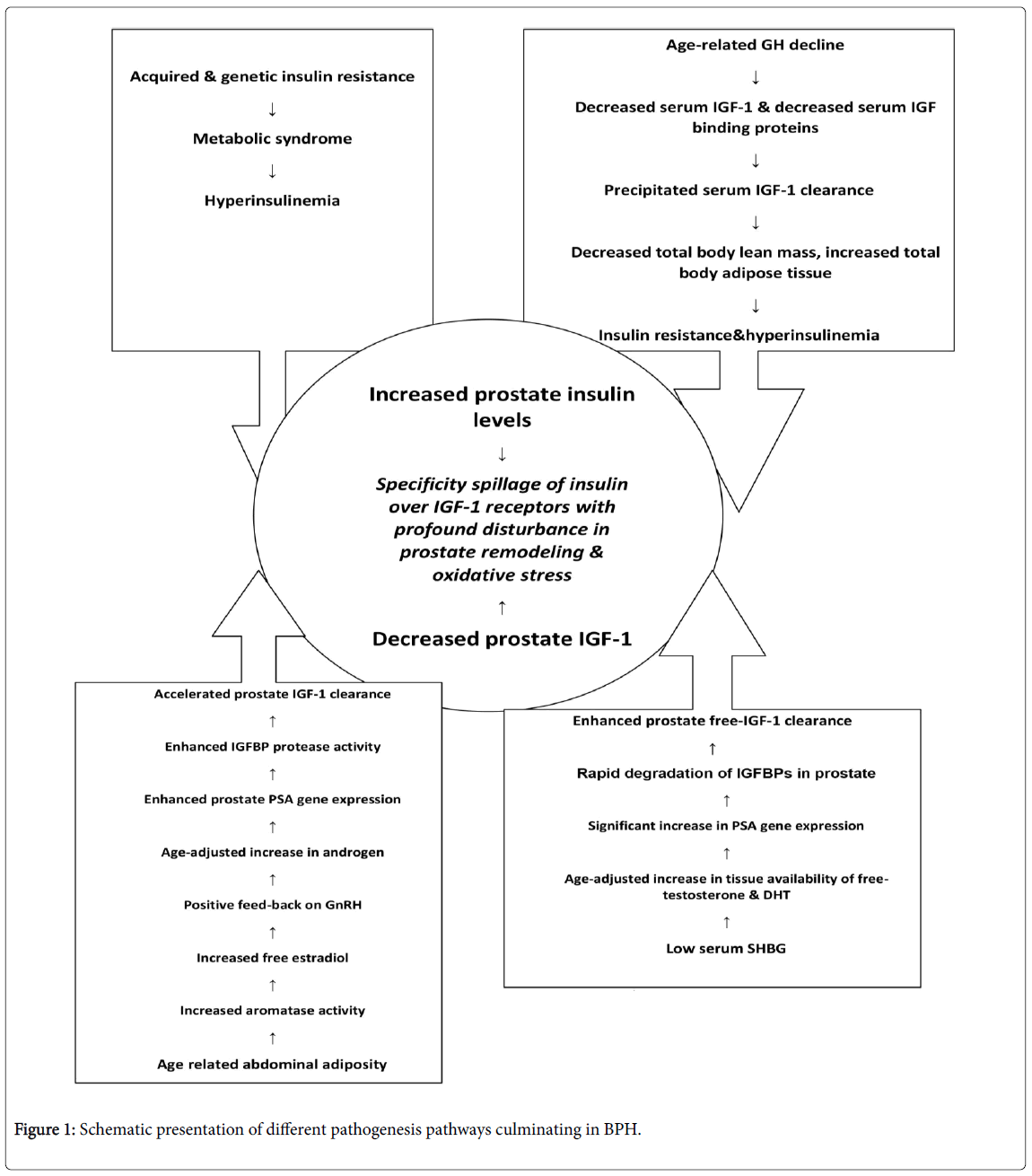
Being provided with these scientific facts, one would be able to put them together and puzzle out the exact location of each piece in the Jigsaw of BPH pathophysiology. The critical anatomic location of hyperplastic prostate, engulfing the proximal urethra, has made it a clinically important urologic disorder. Hence, in terms of pathogenesis and logical management strategies, BPH has remained somewhat an "orphan" disease in urology domain. What really happens to the body during senescence on the whole, and to the prostate in particular, that derails the normal prostate remodeling? This is the most critical question to be answered at the molecular biology level. The very first consequence of aging is the gradual but steady decline in 24-hour pituitary growth hormone secretion rate. The personal genetic make - up and, of course, the eating habits and life style will define the severity of the age-related GH secretion slope. The predictable outcome of the age-related GH shortage is the concordant loss of lean body mass, and precipitation of adipose tissue, particularly centripetal in distribution; a well-known physiologic process commonly pointed to as middle-agespread. The net effect of this middle-age change in body compartments (coincident with BPH kick-off) is the emergence of a mild physiologic insulin resistance and reciprocal compensatory hyperinsulinemia [11-15]. It has been, also, documented that obesity and insulin resistance markedly blunt both physiologic and provoked pituitary GH secretion. Thus, the whole body is pulled into a progressive vicious cycle of decreased GH secretion, proneness to adiposity, aggravation of insulin resistance, and parallel decline in serum IGF-1 and IGFBP-3. Numerous studies have demonstrated that IGFBP-3 synthesis is directly driven by serum growth hormone levels. The concomitant decrease in IGF-1 and its major binding protein (IGFBP-3) result in an accelerated tissue IGF-1 clearance. An age-related phenomenon that deprives the cells from a vitally important cell-protecting, cellrefreshing and cell-proliferating regulatory factor and, also, the unique cell-surface signal in charge of programmed cell death or apoptosis. Now, let us scuba-dive further deep and take a closer look into the core concept of BPH pathophysiology. It is now crystal clear that the two major growth factors of the body, IGF-1 and insulin, originally stem from a common ancestral gene. Proinsulin and IGF-1 have very many amino acid sequences in common along their peptide chains. There is also, a striking resemblance between insulin receptor and IGF-1 receptor, so that the majority of their amino acid sequences are identical, especially on their biologically vital domains. Apart from those similarities, there is a hybrid IGF-1/insulin receptor variant in which one alpha-beta chain of insulin receptor is hinged to one alphabeta chain of IGF-1 receptor. Once two hormones own similar structures and bind nearly identical receptors, they could biologically cross-talk in particular circumstances.
This well-established and fully-described phenomenon is called specificity spill-over. For example, growth hormone excess in acromegaly could easily interact with prolactin receptors and causes euprolactinemic galactorrhea. Exactly the same phenomenon happens to insulin and IGF-1. In a setting of insulin resistance, the compensatory insulin surplus, tsunamically spills over the IGF-1 receptors all over the body including the prostate gland. In this context, insulin does not physiologically interact but mingles with IGF-1 receptor. Therefore, it results in an aberrant signal transduction and a hodgepodge cross-talk, leading to a disorganized signaling through which some of insulin biologic functions and some of IGF-1 biologic activities are expressed. Another must-to-known issue is the age-related physiologic process of relative but steady decline in serum androgens in general and for testosterone in particular. It has been shown that with progressive age-related insulin resistance and precipitous hyperinsulinemia, sex-hormone binding globulin (SHBG) synthesis by the liver is decreased. Therefore, despite the relative decline in serum total testosterone, the tissue free-testosterone availability is relatively sustained. Central obesity of aging gives rise to enhanced aromatase activity, thereby, more and more androgens are converted to estrone and estradiol; an elderly phenomenon that results in increased estrogen to androgen ratio, and a well-known risk for BPH development [16]. It has, also, been shown that in a setting of relative increase in tissue availability of free testosterone, and the permissive effect of ample prostate estrogen concentrations, PSA gene expression is markedly enhanced. This might be considered as the unique explanation for gradual increase in serum PSA levels with aging and the philosophy behind the Massachusetts male aging study in which the increased risk of BPH was significantly associated with higher serum free PSA levels [17]. As it was previously mentioned, PSA with its basic role as an IGFBP protease, shortens the prostate tissue IGF-1 half- life substantially. The collision of insulin resistance syndrome (metabolic syndrome) with pervasive age-related insulin resistance causes a marked augmentation of hyperinsulinemia.
The gradual increase in concentrations of a powerful tissue specific protease (PSA) results in marked increase in tissue IGF-1 clearance rate. Increased serum insulin on a background of IGF-1 shortage, provides the ideal situation for insulin to freely spill over the IGF-1 receptors; a full-scale turmoil at the level of operating tissue growth factors which results in a true metabolo-proteomic disorder of prostate gland. In such a biologic chaos, insulin imposes its powerful mitogenic effects and growth factor properties on prostate tissue, but when it comes to extremely sophisticated process of cell apoptosis, insulin appears to be rather naive compared to the native apoptosis signal, which is de novo tissue IGF-1. The net result of the new biologic treaty is a kind of cell proliferation to cell apoptosis mismatch. A state of enhanced cell replication with poorly programmed cell death, a pathophysiologic process that we have, technically called it dysapoptosis. This pathologic process brings about a cell-senescence status with surreptitious but ongoing cellular oxidative stress, a doomed deviation of cell death from a neat, non-inflammatory one towards the gloomy, ruinous and pro-inflammatory cell destruction. This is the reasonable explanation for all those papers addressing the presence of marked inflammation in prostate gland in BPH [18-28]. With escalating pro-inflammatory condition and smoldering hypoxia and oxidative stress, the hypoxia-inducible factor-1 gene is also expressed which in due course results in tissue expression of a host of other abnormal local growth factors. A fully-featured scenario of derailed prostate remodeling process and overt inflammation that push the poor elderly man into the whirling and pulling vicious cycle of symptomatic prostate hyperplasia (Figure 1). One might argue that, whilst majority of already discussed pathogenic processes are systemic in nature, why prostate gland is so deeply affected though? The answer is utterly straightforward because prostate is the exclusive place where PSA gene is expressed; a powerful protease that destroys the IGFbinding proteins to the point of rapid elimination and marked curtailment of tissue IGF-1 bioavailability, leaving insulin as the sole growth factor in charge of prostate remodeling.
Hints towards the Novel Management Strategies for BPH
Based on the orderly reviewed pathogenic mechanisms, one might be able to suggest an absolutely novel path towards the radical treatment of BPH. Considering the major pathophysiologic processes related to unleashed prostate hyperplasia, that is, GH-IGF1shortage, insulin resistance syndrome with related hyperinsulinemia, and abnormal estrogen to androgen ratio, the logical perspectives towards BPH management sounds fairly straightforward.
Tackling the above mentioned pathophysiologic processes with an insulin sensitizer (Metformin) to loosen up the insulin resistance and the related hyperinsulinemia [29], an aromatase inhibitor (Letrozole) to balance the estrogen/androgen levels, and supervised, wellscheduled shots of somatotropine (Human Growth Hormone) to boost the serum and tissue IGF-1 concentrations might predictably break the described vicious cycle of BPH development. We hope this opinion could draw the attention of some eminent researchers in wellequipped endocrine, metabolic and urology departments, and to be considered as a platform for future clinical trials in this field.
Conflict of Interest
None
Acknowledgements
None
References
- Thompson TC, Yang G (2000) Regulation of apoptosis in prostate disease. Prostate Suppl 9: 25-28.
- Kyprianou N, Tu H, Jacobs SC (1996) Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum Pathol 27: 668-675.
- Neuhouser ML, Schenk J, Song YJ, Tangen CM, Goodman PJ, et al. (2008) Insulin-like growth factor-I, insulin-like growth factor binding protein-3 and risk of benign prostate hyperplasia in the prostate cancer prevention trial. Prostate 68: 1477-1486.
- Monti SL, Di Silverio F, Lanzara S, Varasano P, Martini C, et al. (1998) Insulin-like growth factor-I and -II in human benign prostatic hyperplasia: relationship with binding proteins 2 and 3 and androgens. Steroids 63: 362-366.
- LeRoith D, Werner H, Beitner-Johnson D, Roberts CT Jr. (1995) Molecular and cellular aspects of theinsulin-like growth factor I receptor. Endocr Rev 16:143-163.
- Grimberg A, Cohen P (2000) Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis.J Cell Physiol 183: 1-9.
- Fiorelli G, De Bellis A, Longo A, Giannini S, Natali A, et al. (1991) Insulin-like growth factor-I receptors in human hyperplastic prostate tissue: characterization, tissue localization, and their modulation by chronic treatment with a gonadotropin-releasing hormone analog. J Clin Endocrinol Metab 72:740-746.
- Cohen P, Peehl DM, Rosenfeld R (1998) Insulin-like growth factor 1 in relation to prostate cancer and benign prostatic hyperplasia.Br J Cancer 78: 554-546.
- Rajah R, Katz L, Nunn S, Solberg P, Beers T, et al. (1995) Insulin-like growth factor binding protein (IGFBP) proteases: functional regulators of cell growth. Prog Growth Factor Res 6: 273-284.
- Cohen P, Peehl DM, Graves HC, Rosenfeld RG (1994) Biological effects of prostate specific antigen as an insulin-like growth factor binding protein-3 protease. J Endocrinol 142: 407-415.
- Defronzo RA (1981) Glucose intolerance and aging. Diabetes Care 4: 493-501.
- Davidson MB (1979) The effect of aging on carbohydrate metabolism: a review of the English literature and a practical approach to the diagnosis of diabetes mellitus in the elderly. Metabolism 28: 688-705.
- Fink RI, Kolterman OG, Olefsky JM (1984) The physiological significance of the glucose intolerance of aging. J Gerontol 39:273-278.
- Loeb S, Kettermann A, Carter HB, Ferrucci L, Metter EJ, at al. (2009) Prostate volume changes over time: results from the Baltimore Longitudinal Study of Aging. J Urol 182: 1458-1462.
- Berry SJ, Coffey DS, Walsh PC, Ewing LL (1984) The development of human benign prostatic hyperplasia with age. J Urol 132: 474-479.
- Krieg M, Nass R, Tunn S (1993) Effect of aging on endogenous level of 5 alpha-dihydrotestosterone, testosterone, estradiol, and estrone in epithelium and stroma of normal and hyperplastic human prostate. J Clin Endocrinol Metab 77: 375-381.
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB (1994) Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 151: 54-61.
- Hammarsten J, Högstedt B (2001) Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur Urol 39: 151-158.
- Ciccone MM, Scicchtano P, Cameli M, Cecere A, Cortese F, et al. (2014) Endothelial function in pre-diabetes, diabetes and diabetic cardiomyopathy: A review. J. Diabetes Metab 5:364.
- Gorbachinsky I, Akpinar H, Assimos DG (2010) Metabolic syndrome and urologic diseases. Rev Urol 12: 157-180.
- Giovannucci E, Rimm EB, Chute CG, Kawachi I, Colditz GA, et al. (1994) Obesity and benign prostatic hyperplasia. Am J Epidemiol 140: 989-1002.
- Parsons JK, Carter HB, Partin AW, Windham BG, Metter EJ, et al. (2006) Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab 91: 2562-2568.
- Kramer G, Marberger M (2006) Could inflammation be a key component in the progression of benign prostatic hyperplasia?. Curr Opin Urol 16:25-29.
- Theyer G, Kramer G, Assmann I, Sherwood E, Preinfalk W, et al. (1992) Phenotypic characterization of infiltrating leukocytes in benign prostatic hyperplasia. Lab Invest 66: 96-107.
- Irani J, Levillain P, Goujon JM, Bon D, Doré B, Aubert J (1997) Inflammation in benign prostatic hyperplasia: correlation with prostate specific antigen value. J Urol 157: 1301-1303.
- Potts JM, Pasqualotto FF (2003) Seminal oxidative stress in patients with chronic prostatitis. Andrologia 35: 304-308.
- Grosman H, Fabre B, Lopez M, Scorticati C, Lopez SM, et al. (2015) Complex Relationship Between Sex Hormonnes, Insulin Resistance and Leptin in Men with and without Prostatic Disease. The Aging Male 19: 40-45.
- Dobrek L, Thor PJ (2015) Benign Prostatic Hyperplasia-progress in pathophysiology and management. Pol Merkur Lekarski 39: 263-270.
- Wang Z, Xiao X, Ge R, Li J, Johnson CW, et al. (2017) Metformin Inhibits the Proliferation of Benign Prostatic Epithelial Cells. PLOS one 12: e0173335.
Citation: Abbas Tavakolian Arjmand (2017) Management of Benign Prostatic Hyperplasia with Metformin, Letrozole and Scheduled Growth Hormone Injections: A Pathophysiology Oriented. Epidemiology (Sunnyvale) 7:313.
Copyright: © 2017 Arjmand AT. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 4614
- [From(publication date): 0-2017 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 3774
- PDF downloads: 840