Case Report Open Access
Maltitol and Xylitol Sweetened Chewing-Gums Could Modulate Salivary Parameters Involved in Dental Caries Prevention
Thabuis Clémentine1*, Cheng Chuo Yue2, Wang Xiaoling2, Pochat Marine1, Han Alex3, Miller Larry4, Wils Daniel1 and Guerin-Deremaux Laetitia1
1Department of Biology and Nutrition, Roquette America Lestrem, France
2Department of Preventive Dentistry, Peking University School and Hospital of Stomatology, China
3Roquette China, Shanghai, China
4Sprim USA, San Francisco, USA
- Corresponding Author:
- Clémentine Thabuis
Ph.D, Roquette America Lestrem, France
E-mail: clementine.thabuis@roquette.com
Received Date: March 01, 2016; Accepted Date: March 25, 2016; Published Date: April 02, 2016
Citation: Clémentine T, Yue CC, Xiaoling W, Marine P, Alex H, et al., (2016) Maltitol and Xylitol Sweetened Chewing-Gums Could Modulate Salivary Parameters Involved in Dental Caries Prevention. J Interdiscipl Med Dent Sci 4:191. doi:10.4172/2376-032X.1000191
Copyright: © 2016 Clémentine T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at JBR Journal of Interdisciplinary Medicine and Dental Science
Abstract
Objectives
The effects of sugar-free chewing-gums (CG) sweetened with either 100% maltitol or 100% xylitol on salivary parameters were assessed in order to better understand the role polyols can play in dental caries prevention.
Methods
A double-blind, parallel, randomized, controlled study was conducted in China. Subjects (N = 258, age = 13 to 15 years old) were divided into 4 groups: 2 receiving polyols CG, containing respectively SweetPearl®-maltitol or XYLISORB®-xylitol, one placebo group receiving gum base and a negative control group not receiving any gum. CG were chewed for 30 days. Salivary parameters (flow rate, pH, glucan sucrase activity and free sialic acid concentration) were measured throughout the experimental period.
Results
All studied parameters were significantly modified with gum base compared with no-gum: salivary flow and pH increased. Glucan sucrase activity and saliva concentrations in free sialic acids decreased. Maltitol and xylitol CG showed even stronger effects on these parameters than gum base and led to a better resistance of the oral ecosystem to a sucrose challenge. This was tested in standardized in-vitro conditions.
Conclusion
Sugar-free CG sweetened with either maltitol or xylitol can similarly improve several parameters involved in global oral health and more specifically in dental caries prevention compared to gum base. The use of a gum base placebo allowed us to isolate the effects due to maltitol and xylitol themselves from those due to mastication, and to show that the effects were similar for both polyols.
Keywords
Dental caries; Maltitol; Polyol; Saliva
Introduction
According to a 2009 ILSI (International Life Science Institute) report [1], the main prerequisites involved in tooth decay development are tooth, dental plaque and substrate. Dental plaque results from the colonization of the teeth surface by oral bacteria from saliva. These bacteria are then able to form a biofilm on the tooth that is reinforced by bacterial product, salivary proteins and food substances from the diet. Some bacterial species can become predominant in the tooth colonization and among them, some produce acids inducing the dissolution of tooth mineral. Saliva could be considered as a primary defence mechanism against bacterial infection in the oral cavity [2]. It clears bacteria from the mouth by aggregation and flushing and by buffering and counteracting bacterial acid production in the oral ecosystem.
Saliva is produced in the salivary glands and composed of water, electrolytes, mucus, antibacterial compounds and various enzymes that play an important role in oral health management through saliva neutralization with carbonates and tooth remineralization [3]. More recently, studies showed that saliva could be considered as a diagnostic tool for different oral diseases through the identification and the quantification of newly discovered biomarkers [4,5]. The relationship between mastication and salivary flow rates has been widely studied [6], in particular in response to chewing gum [7-9]. The increase in saliva flow rates when chewing allowed a better clearance of bacteria and food debris from the oral cavity than ordinary salivary flow rate. Consequently, salivary flow rate is an useful parameter in assessing the beneficial impact of saliva stimulants on global oral health. Nevertheless, this parameter cannot be considered by itself, it has to be linked to the study of salivary pH. Indeed, a sucrose sweetened CG could induce an equivalent or even a higher salivary flow rate under stimulation than a sugar-free CG, but this phenomenon would be accompanied by a salivary pH decrease after 5 minutes of chewing (unpublished data). The simultaneous measurement of these two parameters is necessary to compare two CG efficiently.
Some other biomarkers were also isolated from the various salivary components, such as free sialic acids that are produced by oral bacteria and are important mediators for bacteria adhesion to the teeth, and consequently for dental plaque formation [1,10]. Similarly, glucan sucrase activity can be easily tested on saliva samples, and this parameter allows a direct quantification of acid producing bacteria, which are recognized as a major factor for dental caries development [11-13].
The main components of sugar-free CG are hydrogenated carbohydrates - also called polyols- used as sugar substitutes. Unlike sugars, they are not fermented by the microorganisms in the oral cavity and the dental plaque: they have all been tested and classified as nonacidogenic [13]. Three of the polyols most frequently used in sugar-free products are xylitol, maltitol and sorbitol. Even though xylitol has been the most extensively studied regarding dental properties, it seems that chewing sugar-free CG three or more times a day for prolonged periods of time may reduce caries incidence [14].
In the present study, the use of a gum base placebo group aimed at evaluating the role played by specific polyols (such as maltitol or xylitol) in the beneficial effects of sugar-free CG on salivary parameters involved in dental caries development. The placebo group allowed the normalization of the results on the mechanical effect of mastication.
Materials and Methods
All research procedures in this trial were performed in strict accordance with a predefined protocol that was approved by all researchers and a local ethic committee (named IRB food health committee from Shanghai Food Nutrition Society) including a signed informed consent before participation.
Subjects
Subjects were recruited from XuShe Middle School in YiXing, in the JiangSu Province of the People’s Republic of China. The school sent parents an information letter describing the study and asked for their child’s participation before the first visit.
The recruitment of subjects occurred one month before the beginning of the study and took 2 weeks, with an accrual rate of about 144 subjects a week. All volunteers completed the individual enrollment form. Enrollment was based on a two-step design. First, individuals were subjected to inclusion criteria; then, those who passed the inclusion step were checked for exclusion criteria.
In order to be included in the study, children had to fit the following criteria: be between 13 and 15 years-old, healthy and non-smokers; have their permanent dentition with the maximal number of teeth; neither eat CG or sugar-free candies on a regular basis; neither have active cavities, nor periodontal diseases, nor gingivitis, show a salivary Streptococcus mutans count inferior to 105 CFU/ml and a Decayed, Missing and Filled Teeth, DMFT, index of 1.2). The participants also had to have a normal salivary flow (>0.25 ml/min) and agree to use the provided toothpaste and toothbrush.
On the other hand, children were excluded from the study on the inclusion examination if they were currently participating in another trial, or had participated in a trial two months prior to this trial; had taken antibiotics during the preceding 4 weeks or anticipated consumption of the same; wore an orthodontic appliance, took medication/food that may affect salivary flow ( such as 75% sodium fluoride paste, 8% glycerol Stannous fluoride solution, Acidic phosphate fluoride solution (APF), fluorine-containing gelatine or Green tea). They should not have high blood pressure, a disease of the hematopoietic system, diabetes, hyperthyrea, kidney disease, hepatitis, xerostomia, Sjogren Syndrome.
Finally 288 parental consenting male and female children aged 13- 15 years old, with normal salivary was included for this study. Eligible subjects were then randomized and started on the allocated study group as soon as they were enrolled. They were randomly allocated to four groups (N = 72 per group) according to a gender and baseline plaque index stratification after the 2 weeks of recruitment and 16 days prior to the beginning of the study. Before the beginning of the CG supplementation, there was a 2-week period with no consumption of sugar-free or sugar based gum/candy, followed by a 2-day washout period during which volunteers in the CG groups (maltitol, xylitol and gum-base groups) chewed gum-base CG identically to what will be performed during the study. The no gum group did not have wash out period because it did not consume any CG.
Study design
This double-blind, randomized, placebo-controlled, parallel group, single-centred study utilized two treatment groups, one placebo group and one control group. The group receiving maltitol CG chewed 2 pellets 5 times a day for 10 minutes. Pellets were chewed after each meal (breakfast, lunch, snack and dinner) and before going to bed in lieu of brushing. The same supplementation schedule was also followed by the xylitol group. For technical reasons, the gum base pellets of the gum base control group were produced to obtain the same quantity of gum base to chew in one control pellet as in two maltitol or xylitol pellets. Consequently, the gum base group chewed one pellet on the same schedule as the two other groups. Finally, the no-gum control group received no CG. Every subject consumed gum base CG during the 2 days preceding Randomization. In order to control CG intake and chewing duration, 3 daily intakes out of 5 took place during class hours. During the study, the children were asked not to consume sugar-free products; to refrain from using mouth wash or dental floss throughout the study and to respect one tooth brushing per day (in the morning) consistent with the Chinese norm [15] using herbal tooth paste and tooth brushes that were given to all of them. The treatment period lasted 30 days.
Product recipe
Out of 4 experimental groups, 3 were supplemented with CG, two of which were polyol CG. A xylitol CG was made up of 0.35g gum base (Cafosa S.A., Barcelona, Spain), 0.5g xylitol (XYLISORB®, Roquette Frères, Lestrem, France), 0.01g maltitol syrup (LYCASIN HBC 80/55, Roquette Frères, Lestrem, France), 0.004g glycerine (The Dow Chemicals Company, Midland, U.S.A.) and 0.009g spearmint flavour (Mane S.A., Le Bar-sur-Loup, France). A maltitol CG was made up of 0.35g gum base (Cafosa S.A., Barcelona, Spain), 0.49g maltitol (SweetPearl®, Roquette Frères, Lestrem, France), 0.02g maltitol syrup (LYCASIN HBC 80/55, Roquette Frères, Lestrem, France), 0.004g glycerine (The Dow Chemicals Company, Midland, U.S.A.) and 0.009g spearmint flavour (Mane S.A., Le Bar-sur-Loup, France). A gum base control CG was made up of 0.7g gum base (Cafosa S.A., Barcelona, Spain), 0.24g talc ( Talc de Luzenac, Rio Tinto Minerals-Luzenac Operations, Luzenac, France) and 0.03 g spearmint flavour (Mane S.A., Le Bar-sur-Loup, France).
Since polyol CG contained more ingredients in weight than gum base CG, the recipes were adjusted so that the amount of gum base per intake was the same for all products. One gum base pellet contained 0.7 g of gum base whereas one pellet of polyol CG contained 0.35g of gum base. Consequently, children in the maltitol and xylitol groups chewed 2 pellets per intake whereas those in the gum base control group chewed one pellet per intake.
Methods
Measurements were performed at baseline on 2 days before the first day of supplementation (day 0), and during the last 2 days of supplementation. Several days of clinical measurements were necessary to allow the various measurements and sample collections (Figure 1). During the two last and two first clinical days, the children were asked to refrain entirely from brushing their teeth in order to observe the effects of CG on dental plaque formation (data under reviewing process).
Non-stimulated saliva collection: Saliva was collected during 10 minutes at baseline and after 14 and 28 days of CG supplementation to perform glucan sucrase activity tests, free sialic acid quantifications and assess the in-vitro acidification ability of saliva (sucrose challenge). Unstimulated saliva was collected by placing a plastic pipette in the buccal area and applying gentle suction. Samples were collected mid-morning, at least 1 hour after breakfast, and transported to the laboratory in a refrigerated container within 4 hours.
Glucan sucrase activity testing: This test aimed at measuring sucrose cleavage by saliva over 30 minutes of incubation using a spectrophotometer (Wuxi Qian-Rong High-Speed Analyzer Co., Ltd). Results were expressed in unit of glucose per minute.
Free sialic acid testing: The saliva samples were heated at 100ºC in a boiling water bath for 4 min to inactivate bacterial neuraminidases, then centrifuged at 10,000×g for 5 min and dialyzed against distilled water overnight at 5ºC. The thiobarbituric acid method was used to analyze free sialic acids as previously described [10].
Measurement of in-vitro acidification ability of saliva (sucrose challenge): 0.2 ml of non-stimulated saliva (collected at baseline and after 30 days of CG supplementation) was mixed with 10 ml of culture broth (casein peptone) containing sucrose and incubated at 30ºC during 21 hours. At different time points (9, 15, 18 and 21 hours), pH was measured to assess the acidification ability of the saliva collected in each study group.
Stimulated saliva collection: Saliva was collected during 10 minutes at baseline and after 27 days of CG supplementation to perform salivary flow rate measurements and salivary pH measurements. The participants were asked to chew the CG corresponding to their allocation group. To evaluate salivary flow rate, subjects were asked to sit passively and expectorate into pre-weighed plastic containers on each time periods (0-1 min, 1-2 min, 2-3 min, 3-4 min, 4-5 min, 5-6 min, 6-7 min, 7-8 min, 8-9 min, 9-10 min) as the saliva accumulated on the floor of the mouth. Salivary volume was determined gravimetrically, and salivary flow rates were expressed in g per minute. pH values were evaluated directly with a digital pH meter (671P, Janco, USA). This pH meter was able to determine the pH value of saliva by using a combined calomel electrode for recording and known buffers (pH 4, 7, 9) for calibration. Areas under the curve (AUC) were calculated for saliva pH and salivary flow rate kinetics at baseline and at day 28 using the trapezoidal rule.
Statistical analysis
The primary outcome of this study was plaque index measurement according to the Index method of Quigley and Hein (data under reviewing process). The secondary outcomes were salivary pH and salivary flow measurements, the quantification of free sialic acid and of sucrase activity.
Simulation-based methods which postulate various scenarios for effect size - based on dichotomizing the Quigley and Hein score and using odds ratios – indicated a benefit for the treated groups compared with the no-gum control group and gum base placebo group. These simulation methods showed that it was necessary to enrol 288 participants to allow dichotomizing the Quigley and Hein score and obtain a 17% attrition rate with a statistical power of at least 90%.
Means and standard deviations were used to describe continuous outcomes. Comparisons between the four groups were conducted with univariate analyses such as ANOVA or Kruskal Wallis as appropriate. To measure continuous outcome, constancy of the error variance structure was tested, and a least-squares-based modelling approach was employed to correctly identify the potential effects in both treated groups against the control group and the placebo group. Multiple comparisons for plaque pH kinetics, longitudinal comparison and area under the curve were controlled using Duncan’s and Dunnette's approaches. Overall significance level for the statistical tests of differences among the groups was set to 0.05.
Results
Analyses were performed at baseline and after 4 weeks of CG supplementation in order to allow for group comparisons, longitudinal comparisons and for the visualisation of both immediate and long term effects in the same study. As mentioned previously (data under reviewing process), our study design allowed a 17% attrition rate. We observed that 11% of the eligible subjects were dropped out from the study, but our statistical prevision was validated (Nfinal = 258). The main reason for drop out was compliance in both control and placebo groups. As gum base was not tasty, the gum base group showed 15 drop out subjects. In the control group, as they did not receive any treatment, the children could forget about the study, the no-gum group showed 9 drop out subjects. Clinical examination indicated lack of presence of any significant differences among the groups with respect to these characteristics and very healthy indicators among these children. Randomization was performed to obtain 4 groups of 72 children containing 36 males and 36 females with a statistically similar plaque index score within the groups (from 2.24 ± 1.26 to 2.41 ± 1.29). At the end of the study, the placebo group contained 57 children (28 males and 29 females) and exhibited an average plaque index score of 2.26 ± 1.43. The control group contained 63 children (32 males and 31 females) and exhibited an average plaque index score of 2.44 ± 1.23. The xylitol group contained 66 children (32 males and 34 females) and exhibited an average plaque index score of 2.41 ± 1.32. The maltitol group contained 68 children (33 males and 35 females) and exhibited an average plaque index score of 2.32 ± 1.25. This shows that the drop outs did not affect the group homogeneity.
Salivary flow and pH at baseline and after 4 weeks of CG supplementation
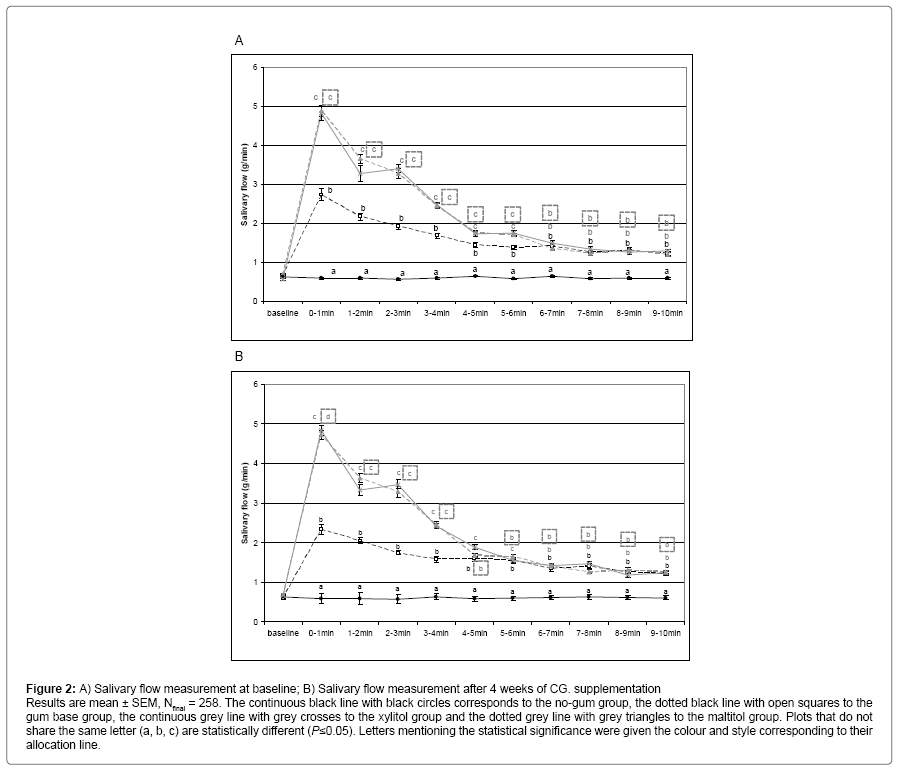
During the wash out period, the eligible children were tested for their saliva flow rate under gum base stimulation, the four groups exhibited similar stimulated flow rate (data not shown). At baseline, the no-gum group exhibited a constant salivary flow whereas in the gum base chewing group, salivary flow increased significantly throughout the 10 minute long saliva collection, clearly showing the effect of mastication on saliva production. Both polyol CG induced higher salivation than in both gum base and no-gum groups (Figure 2A). After 4 weeks of CG supplementation, the salivary flow evolution of the different groups under stimulation was strictly similar to what was observed at baseline (Figure 2B).
Figure 2: A) Salivary flow measurement at baseline; B) Salivary flow measurement after 4 weeks of CG. supplementation Results are mean ± SEM, Nfinal = 258. The continuous black line with black circles corresponds to the no-gum group, the dotted black line with open squares to the gum base group, the continuous grey line with grey crosses to the xylitol group and the dotted grey line with grey triangles to the maltitol group. Plots that do not share the same letter (a, b, c) are statistically different (P≤0.05). Letters mentioning the statistical significance were given the colour and style corresponding to their allocation line.
Areas under the curve (AUC) were also calculated to compare the different groups during saliva stimulation (Table 1). At baseline, saliva production over 10 minutes was significantly increased by gum base compared to no-gum (P<0.05), and this increase was significantly higher for both maltitol and xylitol groups. Results were similar at the end of supplementation.
| Group comparison | At baseline (D1) | After 27 days of supplementation (D27) | ||
|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | |
| No-gum | -0.56a | 4.04 | -0.49a | 3.72 |
| Gum base CG | 19.69b | 5.31 | 19.57b | 4.74 |
| Maltitol CG | 31.36c | 4.74 | 31.11c | 5.78 |
| Xylitol CG | 33.22d | 7.42 | 31.66c | 5.61 |
Table 1: Areas under the curve (AUC) for salivary flow measurements at baseline (D1) and after 4 weeks of supplementation (D27).
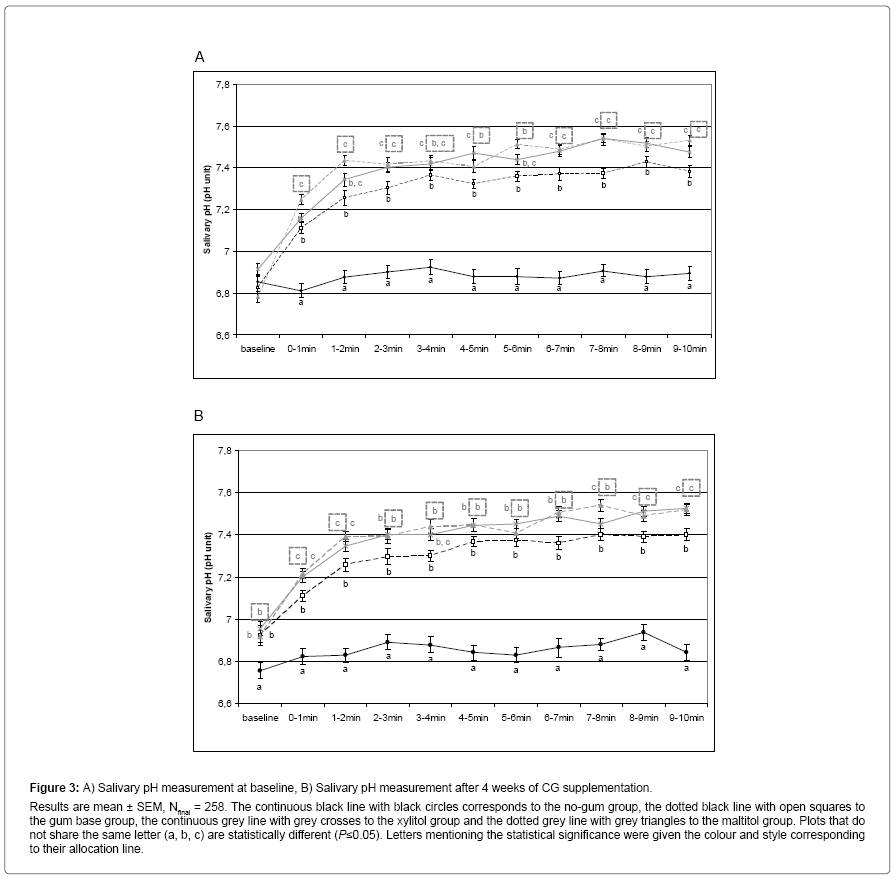
During saliva stimulation, pH was also measured every minute. At baseline, the different groups exhibited the same pH at the beginning of saliva stimulation. The pH remained constant throughout the stimulation for the no-gum group whereas gum base CG induced an increase in pH during the first four minutes of stimulation. This increase was even higher for both maltitol and xylitol CG than for gum base CG (Figure 3A). After the first four minutes and throughout the rest of the stimulation, saliva pH remained higher for the gum base group than for the no-gum group and even higher for both maltitol and xylitol groups. At the end of the supplementation, pH evolution during saliva stimulation was similar to what was obtained at baseline, except for the fact that salivary pH was significantly higher at the beginning of stimulation (Figure 3B).
Figure 3: A) Salivary pH measurement at baseline, B) Salivary pH measurement after 4 weeks of CG supplementation. Results are mean ± SEM, Nfinal = 258. The continuous black line with black circles corresponds to the no-gum group, the dotted black line with open squares to the gum base group, the continuous grey line with grey crosses to the xylitol group and the dotted grey line with grey triangles to the maltitol group. Plots that do not share the same letter (a, b, c) are statistically different (P≤0.05). Letters mentioning the statistical significance were given the colour and style corresponding to their allocation line.
As for salivary flow, areas under the curve (AUC) for salivary pH were calculated to compare the different groups during saliva stimulation (Table 2). At baseline, saliva pH was significantly higher in the gum base group than in the no-gum group (P<0.05) and it was even significantly higher for the maltitol group (not for xylitol). At the end of the supplementation, results were similar to baseline except for the fact that both polyol groups were significantly different from gum base (but not from each other).
| Group comparison | At baseline (D0) | After 30 days of supplementation (D30) | ||
|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | |
| No-gum | 0.17a | 5.58 | 2.04a | 6.12 |
| Gum base CG | 9.26b | 6.22 | 7.54b | 6.96 |
| Maltitol CG | 12.52c | 5.21 | 9.75c | 4.3 |
| Xylitol CG | 9.81b | 5.72 | 8.727b | 5.43 |
Table 2: Areas under the curve (AUC) for salivary pH measurements at baseline (D1) and after 4 weeks of supplementation (D27).
Biomarkers showing plaque bacteria adhesion: glucan sucrase activity and free sialic acid qua ntifications in saliva
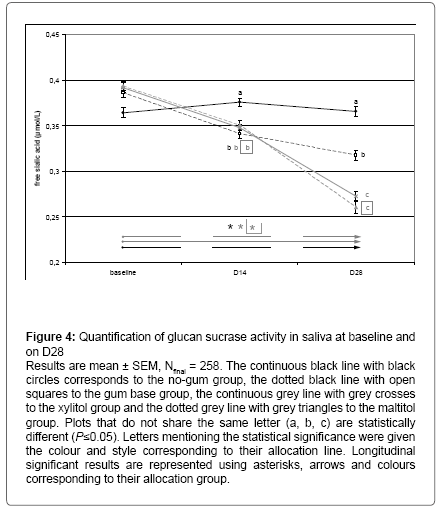
After 2 weeks of CG supplementation, a significant decrease in the activity of the glucans sucrase enzyme was observed in the maltitol (-19% , P < 0.05 compared to gum base and -37%, P < 0.05 compared to no-gum) and xylitol (-28% , P < 0.05 compared to gum base and -43%, P < 0.05 compared to no-gum) groups compared to both control and placebo groups. This effect was significantly stronger in the xylitol group than in the maltitol group (-10%, P < 0.05). Moreover, the gum base group showed a significantly lower sucrase activity than the no-gum group (Figure 4, -22%, P < 0.05). After 4 weeks of CG supplementation, results were similar to D14 except for the fact maltitol and xylitol groups were not significantly different from each other , while still being significantly different from gum base group (-25%, P < 0.05). Gum base chewing induced a decrease in sucrase activity compared to the no-gum group (-40%, P < 0.05, Figure 4).
Figure 4: Quantification of glucan sucrase activity in saliva at baseline and on D28
Results are mean ± SEM, Nfinal = 258. The continuous black line with black circles corresponds to the no-gum group, the dotted black line with open squares to the gum base group, the continuous grey line with grey crosses to the xylitol group and the dotted grey line with grey triangles to the maltitol group. Plots that do not share the same letter (a, b, c) are statistically different (P≤0.05). Letters mentioning the statistical significance were given the colour and style corresponding to their allocation line. Longitudinal significant results are represented using asterisks, arrows and colours corresponding to their allocation group.
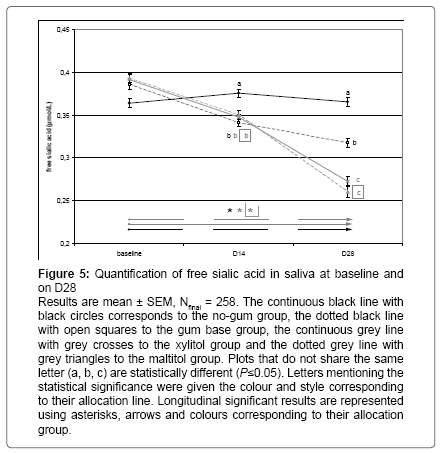
After 2 weeks of CG supplementation, a significant decrease was observed in the 3 supplemented group (-8%, P < 0.05 compared to nogum, see Figure 5). After 4 weeks of CG supplementation, a significant decrease in free sialic acid was observed in both polyol groups (-17%, P < 0.05 compared to gum base and -28%, P < 0.05 compared to no-gum). Gum base chewing induced a decrease in free sialic acid compared to the no-gum group (-15%, P < 0.05, Figure 5).
Figure 5: Quantification of free sialic acid in saliva at baseline and on D28
Results are mean ± SEM, Nfinal = 258. The continuous black line with black circles corresponds to the no-gum group, the dotted black line with open squares to the gum base group, the continuous grey line with grey crosses to the xylitol group and the dotted grey line with grey triangles to the maltitol group. Plots that do not share the same letter (a, b, c) are statistically different (P≤0.05). Letters mentioning the statistical significance were given the colour and style corresponding to their allocation line. Longitudinal significant results are represented using asterisks, arrows and colours corresponding to their allocation group.
In-vitro acidification ability of saliva (sucrose challenge)
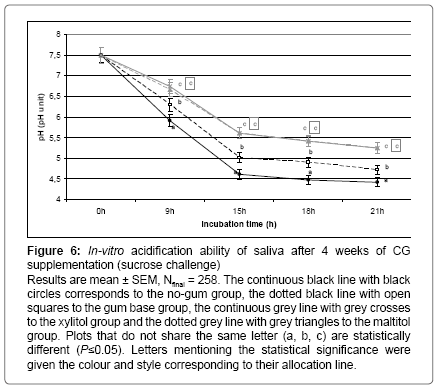
This in-vitro test was performed at baseline and at the end of the CG supplementation in order to visualize the basal acidification ability of saliva in standardized conditions. At baseline, similar acidification kinetics were observed in every group over the 21 hours of incubation with sucrose. The pH value decreased from 7.5 to 4.5 (data not shown). At the end of the supplementation, saliva exhibited various acidification abilities depending on the supplementation group. After 9, 15, 18 or 21 hours of incubation, the decrease in pH was significantly lower in the gum base group than in the no-gum group. This decrease was even lower in both polyol groups than in the gum base group (P < 0.05, see Figure 6).
Figure 6: In-vitro acidification ability of saliva after 4 weeks of CG supplementation (sucrose challenge)
Results are mean ± SEM, Nfinal = 258. The continuous black line with black circles corresponds to the no-gum group, the dotted black line with open squares to the gum base group, the continuous grey line with grey crosses to the xylitol group and the dotted grey line with grey triangles to the maltitol group. Plots that do not share the same letter (a, b, c) are statistically different (P≤0.05). Letters mentioning the statistical significance were given the colour and style corresponding to their allocation line.
Discussion
As previously published concerning plaque parameters (data under reviewing process), the present study included a placebo group in order to observe the influence of mastication on saliva parameters. The nogum group reflected no treatment at all, whereas the gum base group allowed the isolation of the effects of polyols and its comparison to that of chewing alone.
Salivary flow was immediately modified by sugar-free CG upon first intake (Figure 2). Mastication induced an increase in salivary flow observable thanks to the gum base group. Both maltitol and xylitol sweetened CG induced a larger increase in salivary flow, most likely because the taste of polyol can stimulate salivation. Salivary flow was measured by weighting expectorated saliva every minute. Part of the increase in weight of saliva has to be attributed to the dissolution of both polyols in the saliva. Nevertheless, polyol weight would not be sufficient to explain the significant difference in salivation between both maltitol and xylitol groups and the gum base group during the first seven minutes of chewing. This exact same phenomenon was repeated after 4 weeks of a daily CG consumption showing no adaptation of the children’s mouth to sugar-free CG. The increase in salivary flow would have been similar with a sucrose sweetened CG [16] but it would have induced acidification due to sucrose fermentation [17]. The increase in salivary flow allowed a higher mouth clearance and a higher remineralization under neutral pH conditions [1].
The biochemical parameters assessed were the main biomarkers of the occurrence and production of oral pathogenic bacteria such as Streptococcus mutans [10,11,18]. Consequently, measuring free sialic acid concentration and glucan sucrase activity at baseline and after 2 or 4 weeks of CG supplementation could reflect the progressive decrease in oral pathogenic bacteria in dental plaque due to the decrease in adhesion factors as previously published (data under reviewing process). Throughout the 4 weeks of supplementation, the oral ecosystem has changed on a chemical (salivary pH) and on a biological (bacteria occurrence) level. Saliva is one of the components regulating this ecosystem through its mechanical clearing of the mouth and its buffering power. It has been observed that 4 weeks of CG supplementation were able to increase this last ability in saliva. Indeed, at the end of the experimental period, when saliva was collected and incubated in-vitro with sucrose during 21 hours, the saliva of children supplemented with both maltitol or xylitol CG demonstrated a significantly lower decrease in pH ( Figure 6). This effect of chewing sugar-free CG on a daily basis was also observable through the higher salivary pH even before starting saliva stimulation (Figure 3B). These last results underline the fact that the frequent use of polyol sweetened CG could induce changes in saliva and dental plaque triggering a better resistance of the oral ecosystem against acidification during a sucrose challenge.
Physiological effects of sugar-free CG have already been demonstrated through the study of the impact of CG containing xylitol, sorbitol, erythritol and maltitol on dental caries prevalence [19-23]. The anticariogenic and cariostatic properties of polyols were also demonstrated in in-vitro studies [24-27].
In conclusion, compared to both placebo and control groups, maltitol and xylitol CG induced a higher salivation rate accompanied by a higher salivary pH after the first intake. The four-week supplementation with both polyol CG led to a significant decrease in the activity of glucan sucrase and in the concentration of free sialic acid in saliva revealing the lower occurrence of oral pathogenic bacteria. In addition, it induced a better resistance of the oral ecosystem to sucrose challenge. All together, these results demonstrate that both maltitol and xylitol CG can improve mouth clearance and salivary parameters involved in dental caries development.
Acknowledgement
This study was fully sponsored by Roquette (Lestrem, France). Paper was written by CT. The protocol and analyzes were performed by CYC and XW. The protocol was established by MP, AH, DW and LGD. LM performed statistical analyses. Results were interpreted by CT and LGD.
References
- Van loveren C (2009) Oral and dental health: prevention of dental caries, erosion, gingivitis and periodontitis. ILSI Europe Concise Monograph series.
- Mandel ID (1989) Impact of saliva on dental caries. Compend Suppl 13: S476-S481.
- Edgar WM (1990) Saliva and dental health. Clinical implications of saliva: report of a consensus meeting. Br Dent J 169: 96-98.
- Giannobile WV, Beikler T, Kinney JS, Ramseier CA, Morelli T, et al. (2009) Saliva as a diagnostic tool for periodontal disease: current state and future directions. Periodontol 2000 50: 52-64.
- Goncalves LD, Soares MR, Nogueira FC, Garcia C, Camisasca DR et al. (2010) Comparative proteomic analysis of whole saliva from chronic periodontitis patients. J Proteomics 73: 1334-41.
- Johnson DA, Sreebny LM (1973) Effect of increased mastication on the secretory process of the rat parotid gland. Arch Oral Biol 18: 1555-1557.
- Dawes C, Kubieniec K (2004) The effects of prolonged gum chewing on salivary flow rate and composition. Arch Oral Biol 49: 665-669.
- Jenkins GN, Edgar WM (1989) The effect of daily gum-chewing on salivary flow rates in man. J Dent Res 68: 786-790.
- Polland KE, Higgins F, Orchardson R (2003) Salivary flow rate and pH during prolonged gum chewing in humans. J Oral Rehabil 30: 861-865.
- Mishiro YI, Kirimura KL (1964) Accumulation of free sialic acid in the mixture of whole saliva and sugar. J Dent Res 43: 1258.
- Ruby JD, Goldner M, Hargreaves JA(1978) Streptococcus mutans, an assessment of its physiological potential in relation to dental caries. Rev Can Biol 37: 273-289.
- Ryan CS, Kleinberg I (1995) A comparative study of glucose and galactose uptake in pure cultures of human oral bacteria, salivary sediment and dental plaque. Arch Oral Biol 40: 743-752.
- Wursch P, Koellreutter B (1982) Maltitol and maltotriitol as inhibitors of acid production in human dental plaque. Caries Res 16: 90-95.
- Van loveren C (2004) Sugar alcohols: what is the evidence for caries-preventive and caries-therapeutic effects? Caries Res 38: 286-293.
- Zhu L, Petersen PE, Wang HY, Bian JY, Zhang BX (2003) Oral health knowledge, attitudes and behaviour of children and adolescents in China. Int Dent J 53: 289-298.
- Dawes C, Macpherson LM (1992) Effects of nine different chewing-gums and lozenges on salivary flow rate and pH. Caries Res 26: 176-182.
- Manning RH, Edgar WM (1993) pH changes in plaque after eating snacks and meals, and their modification by chewing sugared- or sugar-free gum. Br Dent J 174: 241-244.
- Edwardsson S (1970) The caries-inducing property of variants of Streptococcus mutans. Odontol Revy 21: 153-157.
- Holgerson PL, Sjostrom I, Stecksen-Blicks C, Twetman S (2007) Dental plaque formation and salivary mutans streptococci in schoolchildren after use of xylitol-containing chewing gum. Int J Paediatr Dent 17: 79-85.
- Lee EJ, Jin BH, Paik DI, Hwang IK (2009) Preventive effects of sugar-free chewing gum containing maltitol on dental caries in situ. Food Science and Biotechnology 18: 432-435.
- Makinen KK, Bennett CA, Hujoel PP, Isokangas PJ, Isotupa KP, et al. (1995) Xylitol chewing gums and caries rates: a 40-month cohort study. J Dent Res 74: 1904-1913.
- Manning RH, Edgar WM, Agalamanyi EA (1992) Effects of chewing gums sweetened with sorbitol or a sorbitol/xylitol mixture on the remineralisation of human enamel lesions in situ. Caries Res 26: 104-109.
- Twetman S (2009) Consistent evidence to support the use of xylitol- and sorbitol-containing chewing gum to prevent dental caries. Evid Based Dent 10: 10-11.
- Amaechi BT, Higham SM, Edgar WM (1999) Caries inhibiting and remineralizing effect of xylitol in vitro. J Oral Sci 41: 71-76.
- Bowen WH, Pearson SK (1992) The effects of sucralose, xylitol, and sorbitol on remineralization of caries lesions in rats. J Dent Res 71: 1166-1168.
- Kawanabe J, Hirasawa M, Takeuchi T, Oda T, Ikeda T (1992) Noncariogenicity of erythritol as a substrate. Caries Res 26: 358-362.
- Miake Y, Saeki Y, Takahashi M, Yanagisawa T (2003) Remineralization effects of xylitol on demineralized enamel. J Electron Microsc (Tokyo) 52: 471-476.
Relevant Topics
- Cementogenesis
- Coronal Fractures
- Dental Debonding
- Dental Fear
- Dental Implant
- Dental Malocclusion
- Dental Pulp Capping
- Dental Radiography
- Dental Science
- Dental Surgery
- Dental Trauma
- Dentistry
- Emergency Dental Care
- Forensic Dentistry
- Laser Dentistry
- Leukoplakia
- Occlusion
- Oral Cancer
- Oral Precancer
- Osseointegration
- Pulpotomy
- Tooth Replantation
Recommended Journals
Article Tools
Article Usage
- Total views: 11117
- [From(publication date):
April-2016 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10165
- PDF downloads : 952