Malnutrition in Head and Neck Cancer Patients as Assessed by Anthropometric and Body Composition Parameters
Received: 24-Jan-2023 / Manuscript No. snt-23-87844 / Editor assigned: 30-Jan-2023 / PreQC No. snt-23-87844(PQ) / Reviewed: 23-Feb-2023 / QC No. snt-23-87844 / Revised: 15-Mar-2023 / Manuscript No. snt-23-87844(R) / Published Date: 20-Mar-2023
Abstract
Background: Like other cancer patients, the patients having head and neck cancers also present with loss of weight. This may be because of cancer associated cachexia or more so because of difficulty in taking food. Hence determining the changes in anthropometric and body composition parameters assume significance not only for prognostication but also to look after the nutritional needs in these patients.
Methods: A total of seventeen cases were enrolled. Anthropometric along with body composition measurements were obtained at baseline, in the post-radiotherapy phase at 6 weeks and at 6 months of follow up period. Four patients were followed up for a period of one year.
Results: The study found that problem of malnutrition is common among head and neck cancer patients. Early in the course of disease, the fat stores are used up first and eventually there is breakdown of proteins leading to a decrease in both Fat Mass Index (FMI) and Fat Free Mass Index (FFMI).
Conclusions: The study findings emphasize that in head and neck cancer patients not only the cancer but also the adverse effects associated with its therapy may significantly increase the dynamics of malnutrition development in short periods of time and require adequate management.
Keywords
Head and neck cancer; Malnutrition; Body composition
Introduction
The study design was prospective observational, conducted in the departments of Physiology and Radiotherapy, in patients having histopatholgically proven head and neck cancers who reported to the Tertiary Cancer Centre, Indira Gandhi Medical College, Shimla.
Head and neck cancer is a group of cancers that starts in the mouth, nose, throat, larynx, sinuses, or salivary glands [1]. Symptoms for head and neck cancer may include weight loss, a lump or sore that does not heal, trouble swallowing, or a change in the voice [2]. There may also be unusual bleeding, facial swelling, or trouble breathing.
Head and Neck Cancer – Disease burden:
Head and Neck Squamous Cell Carcinoma (HNSCC) is the sixth most common cancer worldwide, with an annual incidence of approximately 900,000 cases and over 400,000 deaths annually [3]. In the United States, head and neck cancers accounted for 3 percent of malignancies with approximately 66,000 patients developing head and neck cancer annually and 14,600 dying from the disease [4]. In Europe there were approximately 250,000 cases (an estimated 4 percent of cancer incidence) and 63,500 deaths in 2012 [5]. The cancers were the ninth most-frequent cause of death from cancer [6]. In the United States, about 1% of people are affected at some point in their life, and males are affected twice as often as females [7]. The usual age at diagnosis is between 55 and 65 years. The average 5-year survival following diagnosis in the developed world is 42-64% [8-10].
In India according to National Cancer Registry Program of the Indian Council of Medical Research, squamous cell carcinomas of head and neck region account for 29.6% of all cancers in males (range 24.3% - 34.3%) and 11.84% of all cancers in females (range 10.5% - 15.5%) in different hospital registries [11]. Hospital Based Cancer Registries (HBCRs) provide important information related to magnitude of the cancer problem and efficacy of hospital practices in the management of cancer patients. The report highlighted the complex issues involved in cancer patient care in the Indian context. The HBCRs helped the institutes to know the magnitude of the cancer problem and exact status with reference to patient characteristics and management in the respective institutes. A high percentage of patients (67%) had clinically advanced disease at the time of presentation leading to poor survival. This emphasised the need for early detection and also for providing adequate palliative care. The report also highlighted the need for systemic recording of clinical information and underscored the difficulties in obtaining follow up details on a regular and sustained basis for evaluation of outcome of treatment. The report served as a guide to the treating oncologists, researchers and health administrators to look afresh into various aspects of cancer patient management.
In Tertiary Cancer Centre, Indira Gandhi Medical College, Shimla, the patients of head and neck cancers were 16% of all cancer patients [12].
Head and Neck Cancer – Sites and subtypes:
The sites affected by head and neck cancer are:
• Nose and Nasopharynx
• Mouth and Oropharynx
• Hypopharynx
• Larynx
Throat cancers are classified according to their histology or cell structure, and are commonly referred to by their location in the oral cavity and neck. This is because the prognosis is affected depending as to where the cancer appears (some head and neck cancers are more aggressive than others depending upon their location). The stage at which the cancer is first diagnosed also becomes a critical factor in the prognosis of throat cancer.
Squamous-cell carcinoma:
Squamous-cell carcinoma is a cancer of the squamous cell – a kind of epithelial cell found in both the skin and mucous membranes. It accounts for over 90% of all head and neck cancers, including more than 90% of throat cancer [13]. Squamous cell carcinoma is most likely to appear in males over 40 years of age with a history of smoking coupled with heavy alcohol use.
Adenocarcinoma:
Adenocarcinoma is a cancer of epithelial tissue that has glandular characteristics. Several head and neck cancers are adenocarcinomas (either of intestinal or non-intestinal cell-type) [14].
Methodology
A total of seventeen cases were enrolled in the study. Anthropometric along with body composition measurements were obtained at baseline, in the post-radiotherapy phase at 6 weeks and at 6 months of follow up period. Four patients were followed up for a period of one year.
Anthropometrical and body composition measurements
Anthropometric measurements
(i) Height was measured without shoes to the nearest 0.1 cm by using a portable stadiometer with the participant standing upright with the back against the stadiometer and head in Frankfurt plane with heels together.
(ii) Weight was measured in light clothing and bare foot to the nearest 0.5 kg using digital weighing scale calibrated against a set of standard weights.
Body Mass Index (BMI was) calculated by Quetelet’s Index as weight in kilograms divided by height in meters square [15].
Waist circumference was taken as circumference of abdomen midway between lowermost rib and highest point of iliac crest at the end of gentle expiration and was measured in cms.
Hip circumference was taken as circumference of hips at the level of greater trochanters and measured in cm.
(vi). Heart rate and SpO2 was measured by using the calibrated Ssmed pulse oxymeter. The finger probe was applied to the left index finger to record the readings.
Body composition and Basal Metabolic Rate (BMR) measurements
Body composition and BMR was assessed by multi frequency bioelectrical impedance analysis using BODY STAT, Quad Scan 4000TM according to the recommendation in the National Institute of Health (NIH) technology assessment statement [16]. The basic principle of the method is that lean tissue, which consists essentially of electrolyte containing water, conducts the electrical current, whereas the fat acts as an insulator. The conductance of the body is therefore determined largely by the low-impedance lean tissues. Regression equations are then derived which relate impedance to Lean weight. Fat free mass and Total body water are measured by independent techniques. The technique is a non-invasive method which can be reliably used to determine changes in various body composition parameters at baseline and follow up.
Statistical analysis
It was a pilot study of cohort of seventeen patients of Head and Neck cancer undergoing Radiotherapy. Baseline investigations were done in all the patients. The anthropometric and body composition changes were analyzed at the end of radiotherapy which was at 6 weeks, then at 6 months and in a few patients one year follow up was done.
Changes in anthropometric and body composition parameters were analysed using Student t test which included paired single tail and paired two tailed t tests (done for continuous variables with normal distribution). For the t tests, the two tailed p value of <0.05 was taken as statistically significant. For single tailed tests the statistical significance of p value was also taken as <0.05, however more weight age was assigned to the two tailed test. Data was analyzed using Epiinfo version 7.2.5.0 statistical software.
The data was recorded and tabulated in a systemic manner and was subjected to statistical analysis. Following observations were made in the study:
Results
Patient characteristics
More males than females were affected, in a ratio of 14:3. 14 patients were smokers (82%) and 9 patients were both smokers and alcoholics (53%). So a high percentage of patients were smokers and alcoholics in this study.
Two patients (12%) had HTN and one patient (6%) had Type2 Diabetes Mellitus of 10 years duration. Two patients (12%) were found to have raised fasting blood sugar levels and were categorised as recently diagnosed Type2 DM. One patient had H/O ATT intake. There was no patient with H/O COPD or any other co-morbid conditions.
Median follow up of these patients was 6 months and ranged from 6 weeks to 1 year. Two patients were recruited late and hence only completed 6 weeks of follow up.
Oropharynx and oral cavity was the most common tumor site (10 patients, 58%), followed by hypopharynx and larynx (4 and 2 at 24% and 12% respectively). There were no case of nasopharyngeal carcinoma and one patient had Hodgkin’s Lymphoma with involvement of the cervical lymph nodes.
Patients were staged according to the American Joint Committee system on cancer staging. Majority of these patients (82%) were having advanced disease at presentation i.e. stage III and IV. Only 18% of patients had stage I & II disease.
Majority of the patients received higher radiation dose of 66Gy in 30 fractions plus boost dose. One patient of Hodgkin’s Lymphoma received lower dose of 20 Gy given in 30 fractions. The highest dose given was 70 Gy in 30 fractions plus boost in one patient.
Changes in the anthropometric & bodycomposition parameters:
The changes in the anthropometric & body composition parameters are depicted in tables 1 to 5.
| Parameter | Baseline (BL) (n=17) | Comparison of baseline with 6 weeks FU (n=17) |
Comparison of baseline with 6 months FU (n=15) |
Comparison of baseline with 12 months FU (n=4) |
|||
|---|---|---|---|---|---|---|---|
| BL | 6 wks | BL | 6 mths | BL | 12 mths | ||
| Mean Wt (kg) | 50 | 50 | 48 | 50 | 48 | 49.5 | 47 |
| Mean WC (cm) | 75 | 74 | 70.5 | 74 | 71.5 | 76.5 | 68.5 |
| Mean HC (cm) | 83 | 83 | 79 | 84 | 81 | 84 | 79 |
| Mean BMI | 19 | 19 | 18 | 19 | 19 | 20 | 18 |
| Pairs | Parameters | Sig. (p value) Single tail | Sig. (p value) Two tail |
|---|---|---|---|
| Pair 1 | BMI1 & BMI2 | .000 | .036 |
| Pair 2 | BMI1 & BMI3 | .023 | .610 |
| Pair 3 | BMI1 & BMI4 | .000 | .500 |
| Pair | Parameters | Sig. (p value) Single tail | Sig. (p value) Two tail |
|---|---|---|---|
| Pair 1 | Wt1 & Wt2 | .000 | .003 |
| Pair 2 | Wt1 & Wt3 | .003 | .508 |
| Pair 3 | Wt1 & Wt4 | .000 | .677 |
| Pair 4 | WC1 & WC2 | .001 | .012 |
| Pair 5 | WC1 & WC3 | .036 | .192 |
| Pair 6 | WC1 & WC4 | .000 | .410 |
| Pair 7 | HC1 & HC2 | .838 | .366 |
| Pair 8 | HC1 & HC3 | .838 | .366 |
| Pair 9 | HC1 & HC4 | .116 | .656 |
| Pairs | Parameters (Lean wt%) | Sig. (p value) Single tail | Sig. (p value) Two tail |
|---|---|---|---|
| Pair 1 | Lean wt1 & Lean wt2 | .000 | .395 |
| Pair 2 | Lean wt1 & Lean wt 3 | .196 | .104 |
| Pair 3 | Lean wt1 & Lean wt 4 | .000 | .205 |
| Pairs | Parameters | Sig. (p value) Single tail | Sig. (p value) Two tail |
|---|---|---|---|
| Pair 1 | Fat wt 1 & Fat wt2 | .000 | .425 |
| Pair 2 | Fat wt 1 & Fat wt3 | .000 | .031 |
| Pair 3 | Fat wt 1 & Fat wt4 | .000 | .265 |
| Pair 4 | BFMI1 & BFMI2 | .002 | .339 |
| Pair 5 | BFMI1 & BFMI3 | .195 | .047 |
| Pair 6 | FFMI1 & FFMI2 | .000 | .121 |
| Pair 7 | FFMI1 & FFMI3 | .008 | .349 |
| Pair 8 | TBW1 & TBW2 | .008 | .003 |
| Pair 9 | TBW1 & TBW3 | .098 | .428 |
| Pair 10 | ECW1 & ECW2 | .000 | .950 |
| Pair 11 | ECW1 & ECW3 | .098 | .005 |
| Pair 12 | ECW1 & ECW4 | .656 | .169 |
| Pair 13 | ICW1 & ICW2 | .056 | .799 |
| Pair 14 | ICW1 & ICW3 | .106 | .550 |
| Pair 15 | ICW1 & ICW4 | .000 | .881 |
| Pair16 | BMR/Wt1 & BMR/Wt 2 | .000 | .447 |
| Pair 17 | BMR/Wt1 & BMR/Wt3 | .002 | .024 |
| Pair 18 | BMR/Wt1 & BMR/Wt4 | .000 | .070 |
Discussion
Cancer is one of the leading most cause of morbidity and mortality worldwide. Hardly there is any family which has not borne its brunt, and when cancer hits it can hit hard. The burden on society caused by cancer is immense not only in terms of suffering of patients and to their caregivers, but also in the economic terms. As the modalities available to treat various cancers have increased so is the associated side effects and co-morbidities which deserve equal attention and care, if not more, as quality of life is a major issue in vast majority of these patients.
In this study more males than females were affected, in a ratio of 14:3.This could be related to increased abuse of tobacco and alcohol in the male population of the state as compared to females. Also a high percentage of patients in the study were smokers and alcoholics, though no sex and age matched controls were available for direct comparison. Most of the patients completed 6 months of follow up (15 out of 17). Oropharynx and oral cavity was the most common tumor site (10 patients, 59%), followed by hypopharynx and larynx at (24% and 11% respectively). There were no case of nasopharyngeal carcinoma and one patient had Hodgkin’s Lymphoma with involvement of cervical lymph nodes. Most of these patients (82%) were having advanced disease at presentation i.e stage III and IV. Majority of the patients received a higher RT dose above 66 Gy as they were having advanced stage III & IV disease. This is in consonance with the current RT protocols which advocate hitting once and hitting hard, with the aim to limit recurrences in these patients.
Malnutrition and changes in the anthropometric & body composition parameters in the course of disease
Significant differences were found during the follow up periods in anthropometric parameters. Total weight, waist circumference, hip circumference and BMI parameters changes are depicted in Table 1. BMI of most of these patients was borderline low (less than twenty) at baseline and subsequent follow ups indicating a malnourished state. The reason of this could be because of the fact that most of the patients (82%) had advanced disease at presentation (Stage III & IV) and hence showed various degrees of malnourishment which progressed subsequently at each follow up.
Statistically significant values were found in the weight (p values of .000, .003 and .000) and waist circumference (p values of .001, .036 and .000) in all the follow ups in single tailed test. However hip circumference only showed significant difference in the third follow up (p value of .000). Statistically significant differences were found in weight (p value of .003) and waist circumference (p value of .012) in the first follow up among these patients when two tailed test was used.
Having a lesser BMI made this group of patients vulnerable to subsequent malnutrition. Statistically significant differences were found in BMI in all the follow ups among these patients (p values of .000, .023 and .000) when single tailed test was used. In the two tailed test statistically significant correlation was found in BMI in the first follow up only among these patients (p value of .036).
The body mass composition parameters were also analysed using paired analysis with single tailed and two tailed student t tests (Tables 2 to 5). The parameters showed significant statistical differences in the lean body mass in the 6 weeks follow up and one year follow up but not in the 6 months follow up when single tailed test was used. However when two tailed test was used there were no significant difference in the follow up period. The lean mass is composed of body mass minus the fat mass. It includes muscle mass as well as mass of bones and bodily fluids.
The fat weight in percent also showed a significant difference. There was a progressive and consistent increase in body fat percentage at the expanse of lean mass (bearing reciprocal relationship) and the differences were statistically significant in single tailed test in all the follow up periods. In the two tailed test significant difference was seen only in the second follow up (p value of 0.031).
The other body composition parameters which showed significant changes were BFMI, TBW, ECW, ICW, BMR/Body Wt, as depicted in Table 5. BFMI is calculated by the formula: Body Fat/Ht2 in metres, FFMI by : Lean wt./Ht2 in metres. The sum of BFMI and FFMI gives BMI. Though the FFMI did not show any significant change but it was less than the desirable levels as depicted in the table. An FFMI higher than 17 (for women) and 20 (for men) is considered desirable. However the mean values were way less in our patients in all the follow ups indicating that these patients were having increased protein catabolism.
In two tailed test significant differences were seen in BFMI in the second follow up, TBW in the first follow up, ECW in the first follow up, BMR/Wt. in the second follow up. The BFMI showed decrease as fat stores are depleted before the muscle proteins are metabolized for the energy needs. The TBW and ECW showed a decline which may be because of dehydration associated with vomiting in this phase due to the administration of concurrent chemotherapy regime.
Malnutrition developing in the course of malignant disease can be defined as a clinical condition that results from systemic inflammation caused by the underlying disease (cancer) and characterized by an imbalance of energy, protein, and other nutrients that results in measurable changes in the body composition, weight loss, and deterioration of the body’s physical activity [17-19]. Cachexia (wasting syndrome) is a multifactorial syndrome characterized by unintentional weight loss with a progressive loss of muscle mass with or without adipose tissue loss that cannot be completely reversed by conventional nutritional therapy. In addition, cachexia is characterized by a negative protein and energy balance caused by a disturbed food intake and abnormal metabolism featuring increased energy expenditure, insulin resistance, lipolysis, and proteolysis, which intensify weight loss and are induced by systemic inflammatory factors. Untreated malnutrition may progress to cachexia, and the first symptom of this is often the loss of muscle mass. It is known however that not all patients suffering from malnutrition develop cachexia, while all patients with cachexia develop malnutrition to varying degrees [20-22]. On the other hand sarcopenia is characterized by the loss of muscle mass and muscle strength and it is is primarily a disease of the elderly. The development of sarcopenia may also be associated with conditions that are not exclusively seen in older persons, such as malnutrition and cachexia. The loss of muscle mass is typical of cachexia, but most sarcopenic individuals are not cachectic. Patients having no weight loss, no complaints of anorexia and without any systemic inflammatory response may well be sarcopenic [23, 24].
Following the initiation of the cancerous processes and its further progression, the key function of the body, which is nutrition, becomes impaired. In the physiological state, nutrition is precisely controlled by mechanisms regulating hunger, satiety, and molecular and biochemical pathways responsible for the supply of substances that provide energy to tissues and then their use for the needs of intracellular metabolism. The proper functioning of the above mechanisms allows the body to maintain a dynamic balance between the processes of energy-providing substance consumption and their use for cellular metabolism, as well as the storage of their surplus (catabolism-anabolism balance) [25, 26]. The disturbance of said balance by changing cellular metabolism in favor of catabolic processes leads to a gradual disruption of the quantitative and qualitative composition of energy-providing substances in the body, which leads to an impaired function of cells, tissues, and organs, and, consequently, the whole body, with effects of varying intensity occurring in cancer patients [27]. In light of recent studies, disorders of the catabolic-anabolic balance in the course of neoplastic disease are attributed to both the developing cancer and the body of the patient trying to defend itself against the “intruder”. The developing tumor initially mainly consumes the energy substrates circulating in the host’s blood—carbohydrates, fats, and proteins—to satisfy hyper anabolic processes that are consequent to rapid and uncontrolled cell proliferation. Along with the disease progression in the patient’s body, the cancer’s metabolic needs also increase, which requires the supply of increasing amounts of energy and building materials [28]. At this stage, the energy needs of the tumor can only be meet by the release of fats and proteins stored in the patient’s adipose and muscle tissues under the influence of lipolytic and proteolytic factors secreted by cancer cells [29]. On the other hand, the body, which is being starved due to gradual diminution of energy-providing substances, is forced to cover its own energy needs at the expense of a further loss of adipose and muscle tissues. In addition, in response to the developing pathology, the patient’s body produces a number of pro-inflammatory cytokines (IL-1, IL-6, and TNF-α) [30]. Although they act as alarm and defence mechanisms of the body, their long-term release and persistently high level in the body lead to a negative effect on adipose and muscle tissue. Currently, many researchers postulate the development of generalized inflammation as one of the key mechanisms leading to the development of malnutrition and cachexia [31, 32]. The current definition of nutritional disabilities also includes the unfavourable effects of the therapy (surgery, chemotherapy, RT, and CRT) on the nutritional status of these cancer patients.
The problem specific to malnutrition in head and neck cancer patients
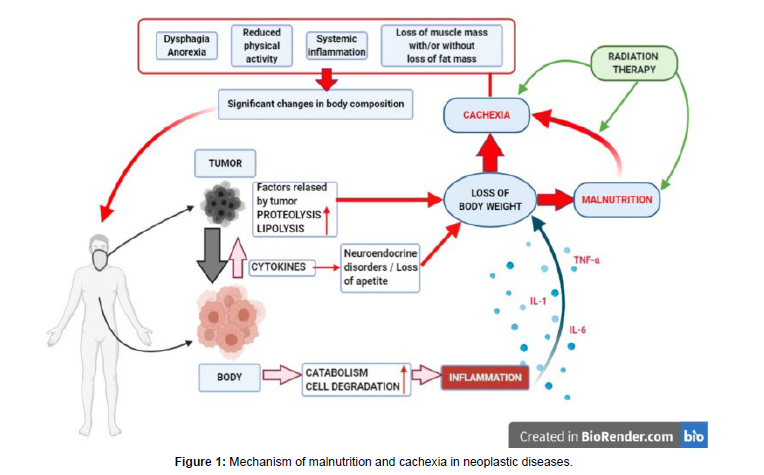
The mechanism of malnutrition and cachexia in neoplastic diseases postulated by most researchers and definition complemented by the impact of RT on the nutritional status of the HNC patients are presented in the Figure 1. The figure outlines the mechanism of malnutrition and cachexia in neoplastic diseases as postulated by most researchers. The classic definition has been complemented by the unfavorable impact of RT on the nutritional status of the HNC patients [33].
The result of the above-described metabolic disorders developing under the influence of the ongoing cancer process is disruption of the body’s caloric balance, the development of inflammation, a loss of cell mass, and a change in the body composition that can lead to the development of malnutrition or wasting of the body [34, 35].
It is now known that malnutrition, sarcopenia, and cachexia in cancer patients constitute an unfavorable clinical spectrum associated with deterioration in the quality of life and a worse response to the applied therapy, as well as a shorter survival time [36]. Malnutrition of various degrees is reported in 50–80% of cancer patients, and about 20–30% of patients in the terminal stage of the disease do not die of cancer, but due to long-term wasting of the body, which is no longer able to support the functions of vital organs due to the depletion of energy substrates [37, 38].
HNC patients are at a high risk of developing malnutrition and the high rate of malnutrition in these patients is governed by the following factors: anatomical location of the tumor, the degree of its infiltration affecting mastication and digestion processes and the toxicity of the various therapies [37, 39]. Approximately 50–70% of HNC patients have malnutrition of varying degrees, and progressive weight loss is often one of the commonest symptoms of cancer [40]. Although there are diagnostic tools based on clinical scales (Subjective Global Assessment and Malnutrition Universal Screening Tool), anthropometric measurements , electrical bioimpedance , or dual energy X-ray absorptiometry, which are able to detect malnutrition, sarcopenia, or cachexia with various degrees of sensitivity and specificity before or after treatment, there are still no objective predictive markers that would allow patients at the highest risk of developing malnutrition or cachexia during therapy to be initially selected [41-43].
Malnutrition, cachexia, and sarcopenia developing as a side effect of various therapies employed for cancer treatment is currently a significant but still inaccurately studied clinical problem. RT or CRT, which are given to limit or destroy the tumor tissue, unfortunately also damage healthy tissues, resulting in either the development of malnutrition or aggravation of the already existing malnutrition, ultimately leading to cachexia [44, 45]. The negative effect of therapy on the nutritional status of HNC patients is confirmed by the high percentage of malnutrition (44–88%) found after the completion of therapy. Toxicity caused by RT leads to gastrointestinal disorders, such as dry mouth, stomatitis, pain, vomiting, diarrhoea, and taste disorders, all of these leading to poor food intake. In addition, there is also a negative impact on the patient’s psyche. Patients may have anxiety with unwillingness to eat as eating is associated with physical pain that accompanies the process of mastication and swallowing [46]. These side effects of RT lead to a nutritional and energy deficit. This promotes a state of anabolic/catabolic mismatch in the body resulting in a gradual loss of body mass. There is remodelling (changes in the body composition) associated with progressive proteolysis and/or lipolysis of muscle and/or fat tissue [47, 48]. The most recent metaanalysis conducted demonstrated an unfavourable impact of RT-based therapy on sarcopenia incidence; its prevalence ranged from 6.6 to 64.6% pre-treatment and 12.4 to 65.8% post-treatment [49]. However, the incidence of malnutrition observed in HNC patients in oncological departments can reach upto 80% [50, 51]. The presence of malnutrition, cachexia, and sarcopenia in these patients herald an unfavourable prognosis leading to higher morbidity and mortality and affecting the quality of life. Therefore, it is necessary to identify patients with a high risk of these syndromes [52]. The problem of malnutrition developing as a result of therapies is so important that the classical definition of malnutrition and cachexia has been expanded to also include the applied therapy beyond the factors related to the presence of a tumor and metabolic disorders in the body. It is believed that any involuntary weight loss ≥ 5% within 1 month is a reliable indicator of malnutrition associated with hospitalization and the applied treatment [53]. If the loss of weight and decrease in BMI is taken as criteria of malnutrition, a significant proportion of our patients fall into this category.
In this study we found that problem of malnutrition is common among head and neck cancer patients. Early in the course of disease, the fat stores are used up first and eventually there is breakdown of proteins leading to a decrease in both FMI and FFMI. The findings emphasize that not only the cancer but also the adverse effects associated with its therapy may significantly increase the risk of malnutrition development, within a short span of time, such as during the duration of radical RT and require adequate management.
Conclusions
There were changes found in the anthropometric and body composition parameters on follow up. Many patients were malnourished having BMI at borderline levels and in particular showed decrease in BFMI, FFMI and Lean mass% consequent to increased fat and proteins break down. Therefore it is recommended that problem of malnutrition developing as a result of the disease and its treatment, and its impact on the patients’ life, be evaluated critically and managed effectively.
The limitations of this study were small sample size and a curtailed follow up period. Nonetheless the findings of this study were unequivocal in demonstrating the problem of malnutrition in HNC patients which should be identified early and managed properly to improve upon overall morbidity and mortality.
Ethics approval and consent to participate
Ethics approval of research protocol: The research protocol and patient information document was submitted to ethics committee of Indira Gandhi Medical College, Shimla, Himachal Pradesh, India. The same was reviewed and approved by the institutional ethics committee vide letter no. HFW (MC-II) B (12) ETHICS/2020/-13902 dated 31-08- 2020. A written signed consent was obtained from all the participants. Study was performed in accordance with the declaration of Helsinki.
Competing Interests
The authors declare that they do not have any financial or nonfinancial competing interests.
References
- Curado MP, Hashibe M (2009) Recent changes in the epidemiology of head and neck cancer. Current opinion in oncology 21:194-200.
- Zackrisson B, Mercke C, Strander H, Wennerberg J, Cavallin-Ståhl E (2003) A systematic overview of radiation therapy effects in head and neck cancer. Acta Oncologica 42:443-461.
- Global Cancer Observatory (2021) International agency for Research on Cancer. World Health Organization.
- Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics. CA: Cancer J Clin 71:7-33.
- Gatta G, Botta L, Sánchez MJ, Anderson LA, Pierannunzio D, Licitra L, et al. (2015) Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur J Cancer 51: 2130-2143.
- Gupta B, Johnson NW, Kumar N (2016) Global epidemiology of head and neck cancers: a continuing challenge. Oncology 91:13-23.
- Ridge JA (2015) Head and Neck Cancer, An Issue of Surgical Oncology Clinics of North America. E-Book. Elsevier Health Sciences 24: 15-16.
- Davies L, Welch HG (2006) Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg 135:451-457.
- Marur S, Forastiere AA (2008) Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc 83: 489-501.
- Johnson NW, Amarasinghe HK (2016) Epidemiology and aetiology of head and neck cancers. In Head and neck cancer 1-57.
- Mathur P, Sathishkumar K, Chaturvedi M, Das P, Sudarshan KL, et al.(2020) ICMR-NCDIR-NCRP Investigator Group. Cancer statistics, 2020: report from national cancer registry programme, India. JCO Global Oncology 6: 1063-1075.
- Gupta M, Vats S, Bhattacharyya T, Seem RK, Gupta M, et al. (2015) Prospective randomized trial to compare the outcome and tolerability of delivering the same total dose of radiation in 61/2 weeks versus 51/2 weeks’ time in head and neck cancers. South Asian J Cancer 4:118-122.
- Johnson DE, Burtness B, Leemans CR, Lui VW, Bauman JE, et al. (2020) Head and neck squamous cell carcinoma. Nat. Rev Dis Primers 6:1-22.
- Adams GL, Duvall AJ (1971) Adenocarcinoma of the head and neck. Arch Otolaryngol 93: 261-270.
- Status WP (1995) The use and interpretation of anthropometry. WHO technical report series 854: 1-452.
- NIH technology assessment statement (1994) Bioelectric impedance analysis in body composition measurement. U.S.D.H.H.S: Bethesda, USA:1-35
- Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KC (2018) Cancer-associated cachexia. Nature reviews Disease primers 4: 1-8.
- Fox KM, Brooks JM, Gandra SR, Markus R, Chiou CF (2009) Estimation of cachexia among cancer patients based on four definitions. Journal of oncology.
- Hersberger L, Bargetzi L, Bargetzi A, Tribolet P, Fehr R, et al. (2020) Nutritional risk screening (NRS 2002) is a strong and modifiable predictor risk score for short-term and long-term clinical outcomes: secondary analysis of a prospective randomised trial. Clin Nutr 39: 2720-2729.
- Morley J, Argilés J, Bales C, Baracos V, Guttridge D (2008) Cachexia: a new definition. Clin Nutr 27: 793-799.
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, et al. (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12: 489-495.
- Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, et al. (2017) ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr 36:1187-1196.
- Steinbeck L, Ebner N, Valentova M, Sandek A, Bekfani T, et al. (2013) C-terminal agrin-fragment as a novel diagnostic marker for muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure. Eur Heart J 17: 1283-1293.
- Aapro M, Arends J, Bozzetti F, Fearon K, Grunberg SM, et al. (2014) Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Ann Oncol 25: 1492-1499.
- Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, et al. (2010) Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG)“cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 29: 154-159.
- Yoshida T, Delafontaine P (2015) Mechanisms of cachexia in chronic disease states. Am J Med Sci 350: 250-256.
- Donohoe CL, Ryan AM, Reynolds JV (2011) Cancer cachexia: mechanisms and clinical implications. Gastroenterology research and practice.
- Zhou J, Zhao LJ, Watson P, Zhang Q, Lappe JM (2010) The effect of calcium and vitamin D supplementation on obesity in postmenopausal women: secondary analysis for a large-scale, placebo controlled, double-blind, 4-year longitudinal clinical trial. J Nutr Metab 7: 1-9.
- Tisdale MJ (2009) Mechanisms of cancer cachexia. Physiological reviews 89: 381-410.
- Petruzzelli M, Wagner EF (2016) Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev 30: 489-501.
- de Matos-Neto EM, Lima JD, de Pereira WO, Figuerêdo RG, Riccardi DM, et al. (2015) Systemic inflammation in cachexia–is tumor cytokine expression profile the culprit?. Front Immunol 6: 629.
- Porporato PE (2016) Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 5: 200.
- Seelaender M, Laviano A, Busquets S, Püschel GP, Margaria T, et al. (2021) Inflammation in cachexia.
- Naumann P, Eberlein J, Farnia B, Liermann J, Hackert T, et al. (2019) Cachectic body composition and inflammatory markers portend a poor prognosis in patients with locally advanced pancreatic cancer treated with chemoradiation. Cancers 11: 1655.
- Ni J, Zhang L (2020) Cancer cachexia: Definition, staging, and emerging treatments. Cancer Manag Res 12: 5597.
- Grundmann O, Yoon SL, Williams JJ. Malnutrition, Cachexia, and Quality of Life in Patients with Cancer. Handbook of Famine, Starvation, and Nutrient Deprivation 943-59.
- Von Haehling S, Morley JE, Anker SD (2010) An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle 1:129-33.
- Penet MF, Bhujwalla ZM (2015) Cancer cachexia, recent advances, and future directions. J. Cancer 21:117.
- von Haehling S, Anker SD (2014) Prevalence, incidence and clinical impact of cachexia: facts and numbers-update 261-263.
- von Meyenfeldt M (2005) Cancer-associated malnutrition: an introduction. Eur J Oncol Nurs 9:35-38.
- Andreoli A, De Lorenzo A, Cadeddu F, Iacopino L, Grande M (2011) New trends in nutritional status assessment of cancer patients. Eur Rev Med Pharmacol Sci 15: 469-480.
- Dev R (2018) Measuring cachexia-diagnostic criteria. Annals of palliative medicine 8: 24-32.
- Sadeghi M, Keshavarz-Fathi M, Baracos V, Arends J, Mahmoudi M, et al. (2018) Cancer cachexia: diagnosis, assessment, and treatment. Critical reviews in oncology/hematology 127:91-104.
- Langius JA, Doornaert P, Spreeuwenberg MD, Langendijk JA, Leemans CR, et al. (2010) Radiotherapy on the neck nodes predicts severe weight loss in patients with early stage laryngeal cancer. Radiother Oncol. 97: 80-85.
- Unsal D, Mentes B, Akmansu M, Uner A, Oguz M, et al. (2006) Evaluation of nutritional status in cancer patients receiving radiotherapy: a prospective study. Am J Clin Oncol 29: 183-188.
- Gorenc M, Kozjek NR, Strojan P (2015) Malnutrition and cachexia in patients with head and neck cancer treated with (chemo) radiotherapy. Reports of Practical Oncology and Radiotherapy 20: 249-258.
- Haghjoo S (2015) Malnutrition associated with head and neck cancers. Rev Clin Med145:76-79.
- Almada-Correia I, Neves PM, Mäkitie A, Ravasco P (2019) Body composition evaluation in head and neck cancer patients: a review. Front Oncol 9:1112.
- Findlay M, White K, Stapleton N, Bauer J (2021) Is sarcopenia a predictor of prognosis for patients undergoing radiotherapy for head and neck cancer? A meta-analysis. Clin Nutr 40: 1711-1718.
- Koom WS, Do Ahn S, Song SY, Lee CG, Moon SH, et al. (2012) Nutritional status of patients treated with radiotherapy as determined by subjective global assessment. Radiat Oncol J 30:132.
- Planas M, Álvarez-Hernández J, León-Sanz M, Celaya-Pérez S, Araujo K, et al. (2016) Prevalence of hospital malnutrition in cancer patients: a sub-analysis of the PREDyCES® study. Supportive Care in Cancer 24:429-435.
- Mowe M, Bosaeus I, Rasmussen HH, Kondrup J, Unosson M, et al. (2006) Nutritional routines and attitudes among doctors and nurses in Scandinavia: a questionnaire based survey. Clin Nutr 25: 524-32.
- Powrózek T, Dziwota J, Małecka-Massalska T (2010) Nutritional Deficiencies in Radiotherapy-Treated Head and Neck Cancer Patients. J Clin Med 10:574.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Sood V, Gharu Y, Sharma A, Vats S, Gupta M (2023) Malnutrition in Head and Neck Cancer Patients as Assessed by Anthropometric and Body Composition Parameters. J Nutr Sci Res 8: 190.
Copyright: © 2023 Sood V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 3176
- [From(publication date): 0-2023 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 2871
- PDF downloads: 305