Case Report Open Access
Malignant Glioma with Primitive Neuroectodermal Components: Clinical and Pathologic Features with Treatment Modalities of Five Cases
Tara Kimbason, Scott G Turner*, Syed Aj Kazmi, Edward Fourgas, Thomas Gergel, Lynn Belles, Angela Whitmire, Michel Lacroix and Steven A TomsDepartment of Neurology, Department of Neurosurgery, Department of Pathology, Department of Radiology, Geisinger Medical Center, Danville, PA, USA
- *Corresponding Author:
- Scott G Turner
Department of Neurosurgery
Geisinger Medical Center, Danville, PA, USA
Tel: 5702716590
Fax: 5702716663
E-mail: sgturner@geisinger.edu
Received Date: October 09, 2015 Accepted Date: October 19, 2015 Published Date: October 21, 2015
Citation: Kimbason T, Turner SG, Aj Kazmi S, Fourgas E, Gergel T, et al. (2015) Malignant Glioma with Primitive Neuroectodermal Components: Clinical and Pathologic Features with Treatment Modalities of Five Cases. J Clin Exp Pathol 5:255. doi: 10.4172/2161-0681.1000255
Copyright: © 2015, Kimbason T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
The classification of primary brain tumors poses many challenges. Glioblastoma multiforme (GBM) is the most common primary adult primary brain tumor, representing more than 50% of all cases. They are composed of cells of astrocytic origin and are characterized by infiltration into the brain parenchyma, making surgical cure impossible. Although local radiation and chemotherapy are routinely employed to treat these aggressive tumors, they invariably progress with survival on the order of two years or less. In contrast, primitive neuroectodermal tumors (PNET) typically occur in children and are composed of poorly differentiated neuroepithelial cells histologically similar to medulloblastoma which may disseminate through the cerebral spinal fluid (CSF). The response of PNETs to chemotherapy is variable but tends to be better than for GBM but usually poorer than for medulloblastoma.
Rarely, tumors with features of both malignant glioma and PNET occur, possibly arising from expansion of stem cell populations located within GBM. These mixed tumors pose not only a diagnostic challenge, but also a therapeutic challenge: while GBM is typically treated with alkylating agents, such as temozolomide or nitrosoureas, PNETs typically respond to platinum-based chemotherapy.
We report a series of five patients with this rare mixed tumor. All patients underwent resection followed by radiation and chemotherapy. Their clinical courses and treatments varied and one of the patients was treated with Optune Tumor Treating Fields (TTF). Their specific histologic features, radiographic presentation, and response to chemotherapy and TTF are discussed. We believe early, aggressive therapy with a combination of treatment modalities, including platinum-based chemotherapy may be beneficial for these rare, mixed tumors.
Keywords
GBM; PNET; TTF; MG-PNET; TMZ; FISH; Treatment
Abbreviations
ADC: Apparent Diffusion Coefficient; DWI: Diffusion-Weighted Imaging; FLAIR: Fluid-Attenuated Inversion Recovery; GBM: Glioblastoma Multiforme; GFAP: Glial Fibrillary Acidic Protein; H and E: Hematoxylin and Eosin; LMD: Leptomeningeal Disease; NSE: Neuron-Specific Enolase; sPNET: Supratentorial Primitive Neuroectodermal Tumor; mTMZ: Metronomic Temozolomide; TTF: Tumor-Treating Fields; XRT: Radiation Therapy; CSF: Cerebral Spinal Fluid; WHO: World Health Organization; MGMT: Methylquanine- DNA Methyltransferase
Introduction
The classification of primary brain tumors poses many challenges. Glioblastoma (GBM), the most common primary adult primary brain tumor, is composed of cells of astrocytic origin and is the most aggressive type of primary brain tumor. It occurs mostly in adults and is characterized by diffuse infiltration of the brain parenchyma, making recurrence common and surgical cure impossible.
The majority of glioblastomas are primary, developing very rapidly de novo, while secondary glioblastomas arise from the transformation of low-grade gliomas. The histopathological features of GBM are marked nuclear atypia, high mitotic index, and prominent microvascular proliferation with necrosis. Molecular markers such as epidermal growth factor receptor (EGFR), isocitrate dehydrogenase (IDH) 1 and 2 mutations, and methylguanine- DNA methyltransferase (MGMT) methylation status have played an important role in developing treatment plans and estimating survival and response to therapy.
Prognosis depends upon the response to a limited number of available treatments. For example, other WHO grade IV tumors such as germinomas and medulloblastomas are fatal if untreated but with appropriate radiation and chemotherapy their 5-year survival rates exceed 60% in germinomas and 80% in medulloblastomas [1]. A majority of patients with GBM, especially the elderly, do not survive longer than a year from time of diagnosis [1]. Though local radiation and chemotherapy such as temozolomide (TMZ) are routinely employed to treat these aggressive tumors, they become resistant to the therapy and invariably progress with overall survival on the order of two years or less.
In contrast to GBM, primitive neuroectodermal tumors (PNET) are typically found supratentorially and are more common in children. They are histologically similar to medulloblastomas and are composed of poorly differentiated neuroepithelial cells [1]. It is not uncommon for them to spread through the cerebral spinal fluid (CSF), requiring craniospinal radiation in addition to platinum-based chemotherapy [2].
Case Reports
No history of prior low-grade glioma (secondary GBM) was noted and all patients had a Karnofsky performance status of 90-100% at baseline and 80% or greater at progression. Their clinical courses varied and CSF dissemination was seen in two patients; one underwent re-resection.
The clinical characteristics and neuroimaging and immunohistochemical findings of the five patients are summarized in Table 1.
Case 1:
A 72 year-old man presented with forgetfulness and ataxia. Magnetic resonance imaging (MRI) showed a large, enhancing, multilobulated predominantly cystic mass in the right temporal lobe. Following resection and radiation therapy (RT), he received 4 cycles of metronomic TMZ before developing multi-focal recurrence of his tumor with apparent leptomeningeal spread on imaging (CSF was negative for malignant cells). Due to the multifocality of his recurrence, re-resection was not recommended and he switched to bevacizumab. He passed away several months later, one year after his initial diagnosis.
Case 2:
A 48 year-old man presented with a new onset seizure. MRI showed a large peripherally enhancing left frontal lobe mass with multiple areas of suspected petechial hemorrhage. He had a subtotal resection followed by RT and TMZ and 4 cycles of metronomic TMZ before his tumor recurred. He then switched from TMZ to bevacizumab and completed 5 cycles with a good radiographic response, but acutely declined clinically and chose to pursue hospice care.
Case 3:
A 71 year-old man presented with a month of intermittent dizziness and MRI revealed a large multi-loculated necrotic mass in the left cerebellar hemisphere. He underwent resection followed by RT with TMZ and 11 cycles of metronomic TMZ before radiographic recurrence was noted 15 months from diagnosis. One month later, he passed away while in hospice.
Case 4:
A 42 year-old man presented with headache and his MRI showed an enhancing mass in the left frontal lobe. He underwent a gross total resection followed by RT and TMZ. Thereafter, he enrolled in the Novocure-tumor treating fields (TTF) trial and received metronomic TMZ along with TTF. At 11 months from initial diagnosis, his tumor recurred and he underwent a second resection followed by bevacizumab with ifosfamide, carboplatin, and etoposide (ICE) in addition to TTF. He later developed leptomeningeal disease for which he was treated with one cycle of intrathecal etoposide and cytarabine. Two months later, he passed away, 24 months from initial diagnosis.
Case 5:
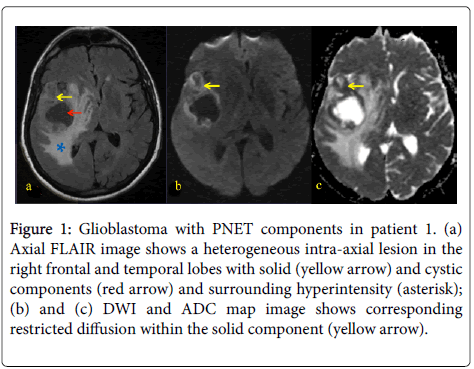
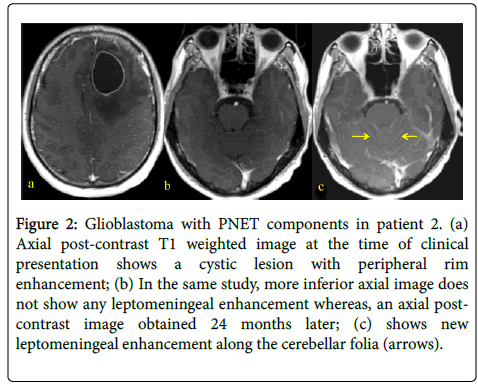
A 31 year-old woman presented with left-sided weakness. Her MRI showed a large cystic lesion with an enhancing mural nodule in the right frontal lobe (Figure 1). She underwent resection followed by RT and TMZ followed by 12 months of metronomic TMZ. She subsequently developed radiographic recurrence and started TTF, though it was discontinued after 2 months due to patient discomfort (Figure 2). She therefore started bevacizumab and has recently started carboplatin for after evidence of further recurrence.
Figure 1: Glioblastoma with PNET components in patient 1. (a) Axial FLAIR image shows a heterogeneous intra-axial lesion in the right frontal and temporal lobes with solid (yellow arrow) and cystic components (red arrow) and surrounding hyperintensity (asterisk); (b) and (c) DWI and ADC map image shows corresponding restricted diffusion within the solid component (yellow arrow).
Figure 2: Glioblastoma with PNET components in patient 2. (a) Axial post-contrast T1 weighted image at the time of clinical presentation shows a cystic lesion with peripheral rim enhancement; (b) In the same study, more inferior axial image does not show any leptomeningeal enhancement whereas, an axial postcontrast image obtained 24 months later; (c) shows new leptomeningeal enhancement along the cerebellar folia (arrows).
Pathology
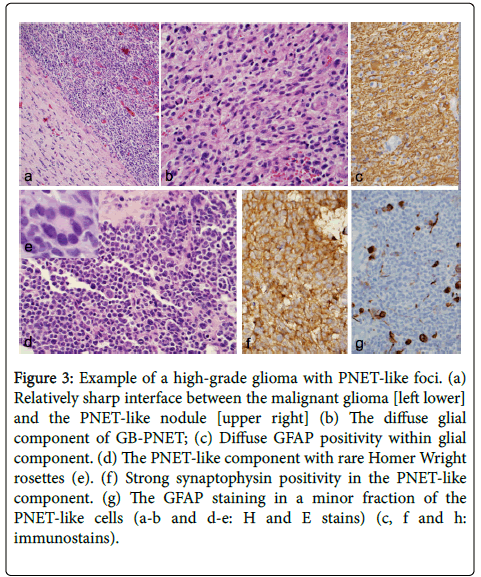
No history of prior low-grade glioma (i.e., secondary GBM) was reported in our patients. The infiltrative glioma component was consistent with GBM in four cases and gliosarcoma in patient 3. In one case the astrocytic cells are gemistocytic type with abundant cytoplasm and eccentric nuclei. In cases where tumor infiltrated the cortex, secondary structures of Scherer were seen. The PNET-like component typically appeared as demarcated, markedly hypercellular nodules (Figure 3) and was composed of cells with scant cytoplasm with hyperchromatic round to oval to slightly irregular nuclei. The tumor in these areas showed brisk mitoses and apoptotic bodies. Homer-Wright rosettes were seen in patient 3 and epithelioid differentiation was seen in patient 4.
Figure 3: Example of a high-grade glioma with PNET-like foci. (a) Relatively sharp interface between the malignant glioma [left lower] and the PNET-like nodule [upper right] (b) The diffuse glial component of GB-PNET; (c) Diffuse GFAP positivity within glial component. (d) The PNET-like component with rare Homer Wright rosettes (e). (f) Strong synaptophysin positivity in the PNET-like component. (g) The GFAP staining in a minor fraction of the PNET-like cells (a-b and d-e: H and E stains) (c, f and h: immunostains).
Immunostaining demonstrated two glioma components with diffusely positive GFAP and Olig2 (not performed in 2 cases) while mostly negative in the primitive component. The PNET-like component in all cases displayed immunoreactivity to one or more neuronal markers (Figure 3), including synaptophysin and neuronspecifsic enolase (NSE) and were negative for glial markers. The extent of positivity for neuronal markers was always greater in the PNET-like foci compared with the adjacent diffuse glioma, whereas the expression of glial markers was always greater in the latter element (Figure 3). These PNET-like foci also demonstrated a very high MIB-1 labeling index.
Immunostaining for synaptophysin was strong in three cases, focal in patient 3, and negative in patient 4; however this case was positive for CD56. Nonetheless, the finding of strong and diffuse positivity within primitive nodules was supportive of the diagnosis. Nuclear p53 expression was seen in patients 1 and 4 and was limited to PNET areas. Immunostaining for mIDH1 (R132H) was performed in patient 3 and was negative.
FISH studies showed all cases were negative for EGFR gene amplification. In patients 2 and 3, FISH analysis was also performed for n-myc status and was negative for amplification.
Two patients expressed MGMT gene promoter methylation, an important predictive factor for benefit of TMZ in GBM.
Results
Their response to the standard care of high grade glioma was assessed by using the Macdonald criteria [5,6]. Clinical success was defined as increased time to progression-free period and overall mean survival time. Clinical characteristics, neuroimaging, and immunohistochemical findings of the five patients are summarized in Table 1.
| Case | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age (yrs)/Sex | 71/M | 49/M | ?70/M | 42/M | 31/F |
| Initial presenting symptoms | Forgetfulness and ataxia | New onset of seizures | Intermittent dizziness | Headaches | Left-sided weakness |
| Karnofsky score at baseline/recurrence | 90/80 | 100/100 | 100/?80 | 100/100 | 100/100 |
| MRI findings: Location, size and characteristics | Right temporal lobe multilobulated, cystic, necrotic 4.1 x 5.6 x 4.6 cm |
Left frontal lobe 5.5 cm mass w/ necrosis, hemorrhage |
Left cerebellum Multiloculated, Cystic &hemorrhagic necrotic 5.5x3.5x1 cm Irregular rim enhancement |
L frontal lobe 4x4.7x3.6 cm cystic & necrotic smooth rim enhancement |
R fontal lobe Cystic (50x4.1x3.3 cm) w/ irregular rim enhancement Mural component (2.5x2.1x1.5 cm) |
| Glial markers: GFAP Olig-2 (is proneural? Or proglial?) CD56 | +GFAP +CD56 (N-CAM) |
++GFAP +CD56 (N-CAM) |
+GFAP +Olig-2 |
rare GFAP Variable CD56 (Dr. K’s interpretation) |
++CD56 +GFAP |
| Neuronal markers: Synaptophysin Neuron-specific enolase (NSE) P53 ?CD56 ?Olig-2 | ++Synap +P53 +CD56 high MIB-1/Ki-67 labeling index |
+Synap +CD56 |
+Synap +NSE |
Rare Synap (Dr. K’s interpretation) +P53 |
++CD56 +Synap |
| MGMT immunostain Score | Positive 12.06 |
Negative | Negative | Negative | Positive 58.94 |
| Other immunostain | Neg EGFR ampl | Gain of MYC (chromo 8) Neg MYC-IGH fusion Neg EGFR ampl |
MYC-N (10%) Neg EGFR ampl |
High polysomy Neg EGFR ampl |
Neg EGFR ampl |
| Number of cycle of mTMZreceived after XRT/TMZ | 5 | 40 | 11 | ?0 | 12 |
| TTF + mTMZ | No | No | No | Yes | No |
| TTF | No | No | No | Yes | Briefly, 1 mth |
| Platinum-based therapy | No | No | No | Yes | Yes |
| Bevacizumab initiated after progression | No | Yes | No | Yes | Yes |
| Other chemotherapy initiated after progression | None, quick deterioration placed on hospice care | None | None, quick deterioration and hospice care | ifosfamide, carboplatin, etoposide | None |
| Intrathecal infusion | No | No | No | Yes, 1 cycle of etoposide and cytarabine | No |
| Non-specific enhancement along surgical resection | Yes | Yes | Yes | No | Yes |
| Leptomeningeal enhancement | Yes | No | No | No | No |
| CSFanalysis for spread | Negative | N/A | N/A | positive | N/A |
| Karnofsky score at baseline/recurrence | 90/80 | 100/100 | 100/?80 | 100/100 | 100/100 |
| Time to radiographic progression (mth) | 9.5 | 10 | 15 | 11 | 18 |
| Overall survival from recurrence (mth) | 4 | 9.5 | 1 | 14 | Alive at 2 months |
| Overall mean survival since diagnosis (mth) | 12 | 12.5 | 16 | 24 | Alive at 18 months |
Table 1: The clinical characteristics and neuroimaging and immunohistochemical findings of the five patients.
Discussion
This is a retrospective observational study of five adult patients with histologically confirmed GBM with PNET components. These rare mixed tumors with features of both malignant glioma and PNET are thought to arise from expansion of stem cell populations located within the GBM or by neuroblastic or neuronal metaplasia [2]. Astrocytes can de-differentiate in vitro by overexpressing c-MYC [3,4,5] suggesting one possible mechanism by which this may occur. They pose a diagnostic challenge due to the variability of their clinical and radiographic presentations, as well as a therapeutic challenge since GBM is typically treated with alkylating agentssuch as temozolomide [4] while PNETs respond to platinum-based chemotherapy [6].
Perry et al. reported 53 patients with GBM/PNET with an overall mean survival similar to that for high-grade glioma (9.1 months) [3] Of our four deceased patients, the overall mean survival ranged from 12 to 24 months from initial diagnosis and the overall survival from initial recurrence ranged from 1 month to 14 months. There was longer survival with more aggressive treatment (the addition of carboplatin as well as one cycle of intrathecal chemotherapy). Perry et al. also reported metastases in 40% of cases [2], which is the same as in our series (one confirmed with CSF cytology one leptomeingeal enhancement on imaging).
PNETs have a high risk of metastases through CSF dissemination and treatment typically includes craniospinal radiation as well as platinum-based chemotherapy [7,8,9] if patients do not respond with temozolomide [2]. Thus, an approach combining alkylating agents and platinum-based therapy may be needed to adequately treat these rare tumors with consideration of craniospinal radiation or intrathecal chemotherapy to address CNS dissemination.
Optune (formerly called Novo-TTF) is a portable device in which an alternating electric field is generated throughout the brain by scalp electrodes and is believed to exert its effects by disrupting dividing tumor cells during mitosis. This treatment was found to be as effective as physician's choice chemotherapy for recurrent GBM [6,10] and was therefore approved by the Federal Drug Administration for use after GBM recurrence. An interim analysis [11,12,13] of the trial in which Patient 4 was enrolled reported the effects of TTF in newly diagnosed GBM and showed a dramatic improvement in progression-free and overall survival in patients treated with both chemotherapy and TTF. Patient 4 had a good response with combined multimodal therapy including TTF and had the longest mean survival, suggesting that TTF may also be effective against this tumor type.
In summary, our case series supports the view that GBM with PNET should be treated aggressively using a multimodal approach including maximal surgical resection, radiation therapy, and chemotherapy including temozolomide and bevacizumab which work well with malignant glioma and possibly platinum-based chemotherapy to better address the PNET component. Spinal imaging should be considered while maintaining vigilance for CSF spread. In addition, we present long-term follow-up data suggesting that TTF may be effective against this tumor type, as well. Further study with long-term followup is needed to optimize treatment and predict outcome for this rare tumor type.
References
- McLendon RE, Judkins AR, Eberhart CG, Fuller GN, Sarkar C, Ng HK (2007) Central nervous system primitive neuroectodermal tumours. In: WHO Classification of Tumours of the Central Nervous System. DN Louis, H Ohgaki, OD Wiestler, WK Cavenee (eds.), pp. 141-146.
- Reddy AT, Janss AJ, Phillips PC, Weiss HL, Packer RJ (2000) Outcome for children with supratentorial primitive neuroectodermaltumors treated with surgery, radiation, and chemotherapy. Cancer 88: 2189-2193.
- Perry A, Miller CR, Gujrati M, Scheithauer BW, Zambrano SC, et al. (2009) Malignant gliomas with primitive neuroectodermaltumor-like components: a clinicopathologic and genetic study of 53 cases. Brain Pathol 19:81-90
- Stupp R, Mason WP, van den Bent MJ, Weller MJ, Fisher B, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987-96
- Johnston DL, Keene DL, Lafay-Cousin L, Steinbok P, Sung L, et al. (2008) Supratentorial primitive neuroectodermaltumors: a Canadian pediatric brain tumor consortium report. J Neurooncol 86:101-108.
- Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, et al. (2012) NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. European Journal of Cancer 48:2192-2202.
- Lassman AB, Dai C, Fuller GN, Vickers AJ, Holland EC (2004) Overexpression of c-MYC promotes an undifferentiated phenotype in cultured astrocytes and allows elevated Ras and Aktsignaling to induce gliomas from GFAP-expressing cells in mice. Neuron Glia Biol 1:157-163.
- Federal Drug Administration NovoTTF-100A System - P100034.
- Louis D, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, et al. (2007) Review: The 2007 WHO Classification of Tumours of the Central Nervous System. ActaNeuropathol 114: 97-109
- Song X, Andrew Allen R, Terence Dunn S, Fung KM, Farmer P, et al. (2011)Gliblastoma with PNET-like components has a higher frequency of isocitrate dehydrogenase 1 (IDH1) mutation and likely a better prognosis than primary glioblastoma. Int J ClinExpPathol 4:651-660.
- Chinot OL, Macdonald DR, Abrey LE, Zahlmann G, Kerloëguen Y, et al. (2013) Response Assessment Criteria for Glioblastoma: Practical Adaptation and Implementation in Clinical Trials of Antiangiogenic Therapy. CurrNeurolNeurosci Rep 13:347.
- Stupp R, Wong E, Scott C, et al. (2014) Interim analysis of the EF-14 trial: a prospective, multicenter trial of NovoTTF-100A together with temozolomide compared to temozolomide alone in patients with newly diagnosed GBM. 19th Annual Meeting of the Society for Neuro-Oncology, Miami, FL.
- Kawasoe T, Takeshima H, Yamashita S, Mizuguchi S, Fukushima T, et al. (2015) Detection of p53 mutations in proliferating vascular cells in glioblastomamultiforme. J Neurosurg. 122:317-323
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 13044
- [From(publication date):
December-2015 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 12071
- PDF downloads : 973