Research Article Open Access
Major Depressive Disorder and Heroin-dependent Patients Share Decreased Frontal Gray Matter Volumes: A Voxel-Based Morphometry Study
Tumbwene Elieza Mwansisya1,2, Huiran Zhang1, Zhang Wang1, Guowei Wu1, Aimin Hu1, Peng Wang1, Chang Liu1, Haojuan Tao1 and Zhening Liu1*
1Mental Health Institute of the Second Xiangya Hospital, Key Laboratory of Psychiatry and Mental Health of Hunan Province, Central South University, Changsha, Hunan 410011, China
2College of Health Sciences, University of Dodoma, P.o Box 395 Dodoma, Tanzania
- *Corresponding Author:
- Zhening Liu
Mental Health Institute of the Second Xiangya Hospital
Key Laboratory of Psychiatry and Mental Health of Hunan Province
Central South University
Changsha, Hunan 410011, China
E-mail: zningl@163.com
Visit for more related articles at International Journal of Emergency Mental Health and Human Resilience
Abstract
Background: Heroin addiction has been occurring in comorbidity with depression. These conditions are thought to result from common neurobiological basis. However, to date, little is known on the common volumetric changes in the multiple gray matter regions in these two patients groups.
Methods: The study comprised samples of 15 depressed patients, 15 heroindependent subjects and 15, age- and sex-matched healthy controls. The gray matter volumes (GMV) abnormalities of these samples were identified and compared among them by using voxel-based morphometry method. The two resulted images were interpolated to locate the common areas of GMV alterations in the two subjects groups.
Results: Common GMV abnormalities were found in superior frontal gyrus (SFG), right Middle prefrontal cortex (MPFC) and Middle frontal gyrus (MFG) in both heroin-dependent subjects and patients with depression. Moreover, we found decreased GMV in MPFC to positively correlate with HAMD scores. The decreased GMV in SFG was found to be positively correlated with daily doses of heroin in patients with heroin addiction.
Conclusions: The Common disruptions of GMV in frontal lobe might be the neuroanatomical substrate for impairments of motivational drive, decision-making and behavioral control that characterizes individuals with heroin dependence and depression.
Keywords
Major depression, Heroin addiction, Voxel-based morphometry, Gray matter volumes.
Introduction
Studies on substance abuse, in particular, on heroin addiction have reported depression to be a highly prevalent psychiatric disorder (Brady & Sinha, 2005a,b). For instance, epidemiological data indicate that the twelve-month prevalence of major depression among heroin dependents to range from 23 % to 46% (Miller, Klamen, Hoffmann & Flaherty, 1996; Qiu et al., 2013; Rajkowska et al., 1999; Teesson et al., 2005). This data suggests the prevalence of major depression to range from twice to five times higher among heroin addicted individuals than in the general population (Brady & Sinha, 2005a).
Aside from epidemiological data, clinically, both of the two conditions have several commonalities in symptoms and impairments in brain regions. Acute or withdrawal of HD and MDD are characterized by irritability, sleep difficulties, anxiety, trouble with attention or concentration, motivational deficits, disordered affective processing, and impairments in decision-making (Brady & Sinha, 2005b; Maremmani et al., 2007). The commonalities of these symptoms can be related to that the pleasant effects of heroin use and depressive symptoms are exerted by stimulating or desensitization of the brain reward system, respectively, through receptors that are normally activated by endogenous opioids in response to reinforcers and stress (Naravane et al., 1997). Depression may result from repeated desensitization of the brain reward system as a response to intermittent withdrawal from dependence of heroin (a shortacting drug) or to stressful situations of being addict. Furthermore, withdrawal from opioids results in activation of locus coeruleus (a principal site for brain synthesis of brain noradrenaline) leading into enhanced noradrenergic postsynaptic responsiveness, which in turn leads into pathophysiology of clinical depression, anxiety and panic disorder (Berridge & Waterhouse, 2003; Sara, 2009). Thus, as in selfmedication hypothesis, depression or other painful stresses may lead into heroin dependence and heroin dependence may cause depression.
Furthermore, the volumetric changes in circumscribed brain areas have been associated with MDD (Ballmaier et al., 2004; Rounsaville, Dolinsky, Babor & Meyer, 1987) and HD (Goldstein & Volkow, 2011; Liu, Matochik, Cadet & London, 1998; Wang et al., 2012).
Despites these studies suggesting co-morbidity as well as similarities in clinical symptoms to result from alteration in brain structure, yet, very few studies, if any, have evaluated the common volumetric changes in multiple gray matter regions in these two patients groups. Interestingly, Magnetic Resonance Imaging (MRI) has suggested to be able to provided discrimination between gray matter, white matter and cerebral spinal fluid, and a precise quantification of structural changes (Tanabe et al., 2009).
Heroin dependence and major depressive disorder are of particular concern because may result in a vicious cycle and worsen the treatment outcomes of each other. Heroin dependence can cause depressive symptoms (Lin et al., 2012). On the other hand, depressive symptoms in individuals with heroin dependence may lead to higher relapse rates and suicidal tendencies (Darke & Ross, 2002; Trivedi, Hollander, Nutt & Blier, 2008), poor physical health (Teesson et al., 2005), impaired psychosocial functioning, and diminished quality of life (Kennedy & Paykel, 2004).
Furthermore, heroin dependence and major depressive disorder has reported to cause cognitive disruptions (Garavan & Hester, 2007), which are associated with gray matter volume (GMV) disruptions (Zhang et al., 2012). Consequently, cognitive disruptions may result in impairments in decision-making and behavior inhibition that are potential to accelerate these conditions to poor prognosis, complicated abstinence or increased attrition from treatment (Garavan & Hester, 2007). Certainly, depressive symptoms and heroin dependence without effective preventive and treatment measures, its combined effects may increase related risk to adverse life events, morbidity and mortality. Thus, a study which investigates the shared alterations in GMV in these patients groups could be helpful in finding a biomarker which eventually may lead to development of better preventive, diagnostic, and therapeutic strategies.
Major depressive disorders and heroin dependence symptomatology are conceptualized to reflect alterations in brain structures (Markou, Kosten & Koob, 1998). Previous neuroendocrine and neuroimaging studies reported dysregulastion of frontal-limbic system in major depression disorders and substance use disorders. For instance, Goldstein et al. (2004) reported mesostriatal-mesocorticol dopamine system, a crucial pathway for processing stress and reward to be a common neurobiological process that mediate the link between depression and addictive behaviors (Teesson et al., 2005; Trivedi, Hollander, Nutt & Blier, 2008). Furthermore, MRI studies have associated heroin dependence with diminished GMV in certain brain regions such as prefrontal cortex, temporal lobe, thalamus, insular gyrus, cingulate cortices, and left supplementary motor cortex (Liu et al., 2009; Reid et al., 2008; Yuan et al., 2009). Similarly, in depression, studies reported substantial reduction of GMV in certain specific brain regions such as left hippocampal gyrus, the cingulate gyrus, and thalamus, dorsolateral prefrontal cortex, Superior and inferior temporal gyrus, amygdala, and pre- and post-central gyrus (Drevets, Price & Furey, 2008; Li et al., 2010; MacQueen et al., 2003; Vasic, Lawrence & Clark, 2008). These evidences suggest overlapping of numerous neurobiological abnormalities in individuals with major depression disorders and heroin dependence.
Indeed, previous neuroimaging studies on MDD and HD have been inconsistent. The heterogeneity of these results might be related to differences in clinical characteristics of the sample such as illness duration, severity, comorbidity and medication (Bora, Fornito, Pantelis & Yucel, 2012). For instance, a recent MRI study using Voxel-based morphometry (VBM) analysis, characterized structural deficits in heroin users on methadone treatment with co-morbid depression (Lin et al., 2012). In comparison to healthy controls, this study found diminished GMV in medial frontal gyrus, post-central gyrus, inferior frontal gyrus, sub-colossal cingulate gyrus in the left hemisphere, and right cerebellar declive. However, major depression Bora, Fornito, Pantelis & Yucel, 2012; Du et al., 2012; Koolschijn et al., 2009; Vasic, Walter, Hose & Wolf, 2008) and heroin dependence (Liu et al., 2009; Qiu et al., 2013; Yuan et al., 2010) has been independently associated with diminished GMV. Therefore, interpretations of the results from these study samples pose a question to whether or not are related to the effect of depression or heroin dependence, independently. Thus, a study with independent samples of subjects with heroin-dependence, major depressive disorder and healthy controls might be helpful to mitigate this effect by identifying and comparing the common subtle changes of GMV from these samples.
The present study, aimed to characterize the volumetric changes of multiple gray matter regions in groups of subjects with major depressive disorder and heroin dependence as compared to healthy controls. We analyzed the whole-brain GMV by using an unbiased VBM technique. Based on the previous research findings, we hypothesized that the GMV in the frontal and temporal regions of depression and heroin-dependent individuals would be inversely related to that of healthy controls. We also, hypothesized that heroin-dependent and major depression subjects would have shared abnormalities of GMV as compared to healthy controls.
Materials and Methods
Participants
Using the Structured Clinical Interview patient version for DSM-IV (SCID-P), 15 treatment naïve patients with major depression were recruited from in-patient and out-patient units of the Department of Psychiatry, the Second Xiangya Hospital of Central South University and were well- matched in terms of age and gender with 15 heroin-dependent patients who were recruited from the Addiction Recovery Center-Changsha Mental Health Hospital of Changsha City, Hunan province, PR China.
The common inclusion criteria for the two patients groups were: a) age between 18 and 45; b) Han Chinese ethnicity; c) nine years of education or above; d) right-handed by a determination of hand preference. The special inclusion criteria for a group with major depressive disorder were: a) patients met DSM-IV criteria for major depressive disorder; b) the total scores of Hamilton Depression Rating Scale-17 (HAMD-17)>17. The heroin-dependent group met the following special inclusion criteria: a) met DSM-IV criteria for heroin dependence; b) self-reported active heroin use for at least 6 months; c) not engaged in any drug treatment therapy ; d) positive urine test for heroin on admission; e) willingness to begin methadone maintenance treatment (MMT) after participation in the study. The exclusion criteria were: a) any contraindications to MRI scanning; b) alcohol or substance abuse history for depression group and poly substance abuse (other than nicotine use) for heroin dependence; c) disorder of consciousness more than five minutes; d) chronic neurological disorders or severe physical diseases; e) psychiatric disorders in the first-degree relatives; f) psychiatric disorders other than depression in patients with depression or heroin dependence in heroin-dependent subjects g) comorbidity of major depression and heroin dependence as ascertained by DSM-IV criteria and HAMD-17 scores ≥ 14 (Stein et al., 2004).
Fifteen healthy controls (HC) were recruited from a community sample. The inclusion and exclusion criteria were the same as those of the two patients groups except that the healthy controls did not meet the DSM-IV diagnostic criteria of any psychiatric disorders by SCID non-patient version.
All subjects gave informed consent for participation in the study after the risks and benefits of their participation were explained in detail. The study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University.
Image Acquisition
Scans were performed on a 1.5-Tesla GE Signa Twinspeed MR scanner (General Electric Medical System, Milwaukee, Wisconsin, USA) equipped with high-speed gradients. A birdcage head coil was used for radio frequency transmission and reception of magnetic resonance signal. Subjects lay supine in the scanner, and their head was snugly fixed with foam pads provided by the manufacturer to minimize head motion. Besides, their bilateral auditory meatuses were also plugged with ear plugs to minimize the influences of noise. High-resolution whole brain volume T1-weighted images were acquired sagittally with a three-dimensional spoiled gradient echo (SPGR) pulse sequence: repetition time (TR) = 12.1ms, echo time (TE) = 4.2ms, flip angle = 15°, field of view = 240×240 mm2, acquisition matrix = 256×256, thickness = 1.8 mm, gap=0, number of excitations = 172 slices
Image Analysis
Voxel-based morphometry pre-processing and statistical analysis
All structural images were processed by voxel-based morphometry (VBM5) toolbox (http://dbm.neuro.uni-jena.de/vbm) on Statistical Parametric Mapping 5 (SPM5; Welcome Department of Cognitive Neurology, London, UK) running on MATLAB7.2. The segmentation, normalization and modulation were completed in one step, resulting in modulated gray matter (GM). In the normalization, all images were spatially normalized to the Montreal Neurological Institute (MNI) T1-weighted template. All the gray matter images were re-sampled with a final voxel size of 1×1 ×1mm3 and then smoothed with an 8-mm Gaussian kernel.
The GMV differences were tested with analysis of covariance (ANCOVA) model co-varying for total intracranial volume. Images were masked with an absolute threshold 0.1. Combined height thresholds at p<0.001 and extent threshold at k>100 voxels were set to identify the significant clusters among the three groups. The GMV volume value for each cluster showing significant differences in the ANOVA analysis was obtained for each participant. Post hoc analysis was performed by pair-wise comparison. We report all significant main effects (p<0.001) in the results section.
Statistical analysis for clinical features
We performed one-way ANOVA to compare age and years of education, and χ2 test to compare gender distribution among the three groups. Two-sample t tests were used to compare the duration of illness, HAMD scores between the two patients groups. We performed a linear correlation analysis to detect associations between the values of GMV in the two patients groups that we obtained from the significant clusters in VBM analysis and the HAMD scores, daily dosage of heroin and methadone.
Results
Description of demographic and clinical characteristics
At the time of scanning, the depression patients were treatment naïve for medication and the heroin-dependent subjects were also not engaged in any drug treatment. The demographic and clinical characteristics for groups with major depression, heroin-dependence and healthy controls is summarized in Table 1, There was no statistical significant difference among the three groups regarding to age, years of education and gender composition ( all p>0.05). There was statistical significant difference between major depression and heroin dependent in the total scores of HAMD-17 (T = 9.513, p<0.001) and course of disease (T = -2.554, p = 0.019).
| Characteristic | Group (mean ± SD) * | ||
|---|---|---|---|
| depression | heroin dependence | healthy controls | |
| Age (years) | 27.53 ± 7.43 | 30.47 ± 6.17 | 30.60 ± 6.77 |
| Education (years) | 12.07 ± 1.79 | 10.40 ± 1.55 | 11.87 ± 2.50 |
| Gender (male/female) | 10/5 | 10/5 | 10/5 |
| Duration of illness (months) † | 34.33 ± 25.13 | 74.53 ± 55.54 | - |
| HAMD-17 scores † | 27.07 ± 6.25 | 8.87 ± 3.98 | - |
| Dose of heroin (g/d) | - | 1.26 ± 1.09 | - |
| HAMD-17 = Hamilton Depression Rating Scale-17; SD = standard deviation. * Unless otherwise indicated. † p<0.05 |
|||
Table 1: Demographic and clinical characteristics for patients with major depression, heroin dependence and healthy controls
Morphometry analysis
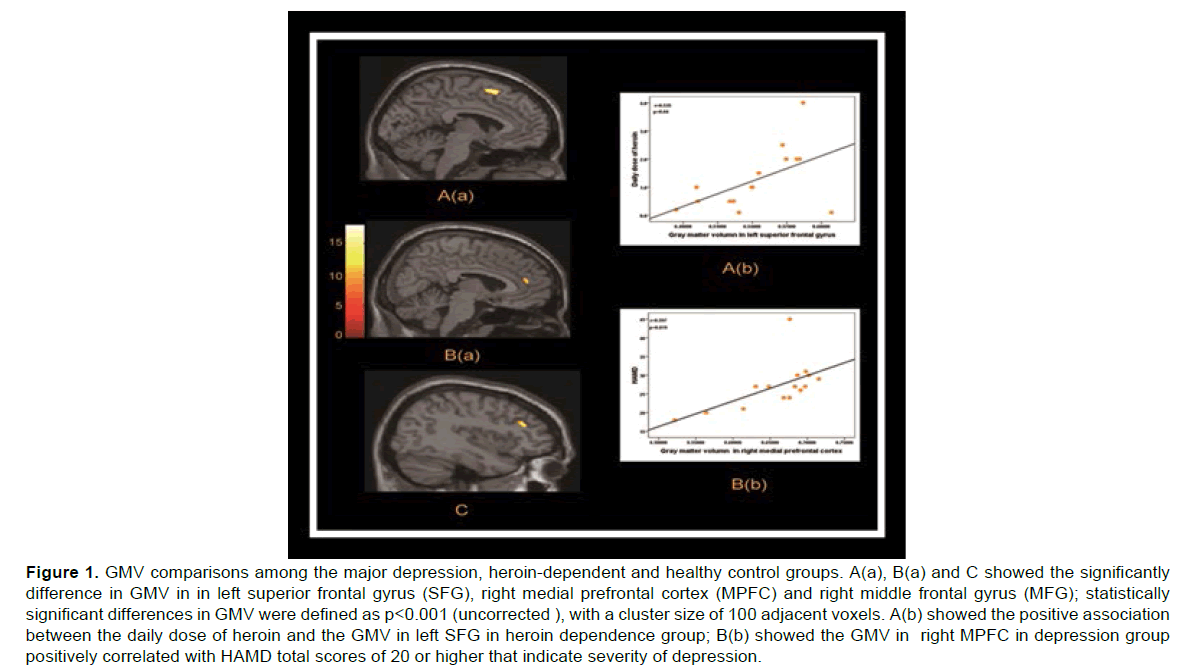
Table 2 shows the Brain regions with common GMV changes between major depressive disorder and heroin-dependent subjects as compared to healthy control group. When the results from VBM analysis were compared between the three groups: major depression, heroin-dependent and healthy control group, the significant differences in GMV were found Brain regions with common GMV changes between major depressive disorder and heroin-dependent subjects as compared to healthy control groups in left superior frontal gyrus (SFG), right medial prefrontal cortex (MPFC) and right middle frontal gyrus (MFG) (p<0.001). Compared to healthy control group, post-hoc analyses showed significantly lower GMV in the two patients groups (healthy controls vs. Depression, in SFG, p<0.001; in MPFC, p<0.001, in MFG, p<0.001; Healthy controls vs. Heroin-dependent, in SFG, p<0.001; in MPFC, p = 0.001; in MFG, p = 0.001). However, there was no significant difference between the depression group and heroin dependence group (Depression vs. Heroin-dependent, in SFG, p = 0.0281; in MPFC, p = 0.508; in MFG, p = 0.235). Linear correlation analyses (Figure 1) showed that the GMV in MPFC in depression group was positively correlated with HAMD score (r = 0.597, p = 0.019) and the GM volume in SFG in heroin dependence group was positively correlated with daily dose of heroin (r = 0.535, p = 0.04).
| Brain area | Cluster | Z value | P value | MNI coordinate | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Left superior frontal gyrus | 319 | 4.40 | <0.001 | -4 | 15 | 57 |
| Right medial prefrontal cortex | 262 | 4.05 | <0.001 | 41 | 33 | 23 |
| Right middle frontal gyrus | 174 | 3.50 | <0.001 | 6 | 45 | 14 |
Table 2: Brain regions with common GMV changes between major depressive disorder and heroin-dependent subjects as compared to healthy control groups.
Figure 1: GMV comparisons among the major depression, heroin-dependent and healthy control groups. A(a), B(a) and C showed the significantly difference in GMV in in left superior frontal gyrus (SFG), right medial prefrontal cortex (MPFC) and right middle frontal gyrus (MFG); statistically significant differences in GMV were defined as p<0.001 (uncorrected ), with a cluster size of 100 adjacent voxels. A(b) showed the positive association between the daily dose of heroin and the GMV in left SFG in heroin dependence group; B(b) showed the GMV in right MPFC in depression group positively correlated with HAMD total scores of 20 or higher that indicate severity of depression.
Discussion
In this study, compared to healthy controls, we found the common brain regions with decreased GMV in superior frontal gyrus (SFG), right Middle prefrontal cortex (MPFC) and Middle frontal gyrus (MFG) in both heroin-dependent individuals and patients with depression. Moreover, we found decreased GMV in right MPFC to positively correlate with HAMD scores. Whereas, the decreased GMV in left SFG was found to correlated positively with daily doses of heroin in heroin-dependent subjects. These support our hypothesis that heroin-dependent and major depression subjects have shared frontal GMV alterations compared to health controls. The present findings also are consistent with other structural and functional neuroimaging studies which found disruptions of the frontal cortex in individuals with repeated drug abuse including heroin-dependence (Upadhyay et al., 2010; Vasic, Walter, Hose & Wolf, 2008; Verdejo-Garcia, Rivas-Perez, Lopez-Torrecillas & Perez-Garcia, 2006).
In the current study, we found common multiple decreased GMV in frontal cortex (left SFG, right MPFC and MFG) in both individuals with heroin-addiction and patients with depression as compared to healthy controls. These brain regions with significant differences are responsible for intermingling executive cognitive functions; such that MFG is responsible for high level executive functions including decisions, arousal and motivation (Camchong et al., 2011) and MPFC for emotional processing and motivation aspects of behavior (Drevets, Bogers & Raichle, 1997) and left SFG for working memory (du Boisgueheneuc et al., 2006). Certainly, from this evidence it is plausible that disruption of these brain regions might potentially related to manifestation of similar symptoms in both of the two patients’ groups; including symptoms like irritability, sleep difficulties, anxiety, trouble with attention or concentration, motivational deficits, disordered affective processing and impairments in decision-making (Brady & Sinha, 2005b; Maremmani et al., 2007). In addition, these symptoms have reported to occur in both; depression and heroin addiction patients groups.
In the current study, the daily dose of heroin in heroin-dependent group was found to be positively correlated with the GMV alterations in left SFG brain region. This means that the severity the use of heroin among heroin-dependent subjects, the greater the abnormal decrease in GMV of the SFG region. Subsequently, the left SFG has reported to be the key component of the neuronal network of working memory; and its disruption has been associated with impairments in working memory (du Boisgueheneuc et al., 2006). Moreover, this brain region has found to be associated with the mechanism of heroin craving under cue-exposure (Bonson et al., 2002; Li et al., 2012). Since, working memory is a system for temporarily storing and managing information that are required to carry out complex cognitive tasks such as learning, reasoning, and comprehension (du Boisgueheneuc et al., 2006); therefore, it is certainly conceivable that impairments of GMV in left SFG would play role in maintaining daily doses of from individuals with heroin addiction regardless of the consequences. The present study findings are in agreement with a recent longitudinal study which found decreased gray matter density in left SFG at baseline in individuals with heroin addiction; interestingly, this was the only region which reversed fairly to normal after one month of abstinence (Wang et al., 2012). Taken altogether, these results entail that future measures for heroin addiction prevention, abstinence and therapeutics that blocks the response of SFG to heroin-related cues would be presumably protective factor to sustained heroin abuse.
The HAMD scores were found to be positively correlated with GMV in right MPFC in the group with depression, but not in other parts of the frontal lobe. This means that the higher HAMD scores for depressive symptoms the greater the GMV abnormalities in the right MPFC brain region. This is steady with report of other studies (Bremner et al., 2002; Wagner et al., 2008). Structural and functional MRI studies implicated the role of MPFC to be emotional processing and motivation aspects of behavior (Drevets et al., 1997; Farb, Anderson, Bloch & Segal, 2011b; Wagner et al., 2008). Consequently, it is intuitively reasonable that excessive disruption of the right MPFC would be fundamentally involved in severity of depressive symptoms. Recently, a longitudinal functional MRI study reported MPFC to be a biomarker for risk of relapse in patients with depression, probably, it is related to over activation in response to rumination thoughts ( Farb, Anderson, Bloch & Segal, 2011a). Nevertheless, abnormalities in this region has found to be restored successful after antidepressants treatment (Nahas et al., 2010). Inconsistently, some studies reported increased amygdala metabolism to be associated with depression severity (Abercrombie et al., 1999; Drevets et al., 2002).
This study has several limitations; first, the sample was relatively small which may influence generalizability and power of the study. Also, this has limited further analysis in relation to subgroups. For instance, Hastings et al. (2004) reported influence of gender on GMV disruption in major depression; such that total amygdala GMV reduction occurred in females but not in males. This poses a question to our present findings to whether male and females would show differences. Because of the limited sample size we consider this as preliminary findings. Thus, future studies with large sample size are warranted. Second, since the study design is a case-control, the observed interaction between heroin-dependent and depression subjects could be due to other moderating factors such as shared genetics or environment vulnerability of both disorders. The longitudinal study with more stringent methods can provide more reliable evidence on the abnormal GMV changes. Third, in previous studies, the duration of illness has been found to be associated with GMV reduction in both MDD (Frodl et al., 2008) and in HD patients (Goldstein & Volkow, 2011; Qiu et al., 2013; Yuan et al., 2010). Meanwhile, in the current study we found duration of illness to be statistically different between MDD and HD groups, thus, it is possible that the findings of this study may be partly influenced by the observed difference in the duration of illness. Therefore, future studies with well-matched groups that include control of the duration of illness might be helpful to ameliorate these findings.
To best of our knowledge, this is the first study to use unbiased VBM with whole brain analysis to compare the common brain regions for heroin-addicted individuals and patients with depression. The present study provides the preliminary findings that the similarities in symptomatic presentation between MDD and HD patients might be arising from the same neurobiological abnormalities in the PFC. The PFC is responsible for regulation of cognitive activities such as decision making, response, behavioral inhibition, planning and memory (Verdejo-Garcia et al., 2008; Wagner et al., 2008). Moreover, some neuroimaging studies provide initial evidence that reduced PFC perfusion may reflect on ongoing neurocognitive deficits, such as inhibitory control and decision-making (Wang et al., 2012; Yuan et al., 2009). This might imply that disruption to frontal cortex might be responsible for impaired decision making and behavioral inhibition observed in MDD and HD. However, it is not yet well understood whether or not the observed structural changes result from neuronal degeneration as consequence of heroin addiction or depressive symptoms. Thus, future longitudinal studies are needed to determine the neurodevelopment course of heroin and depression disorders.
In conclusion, consistent with this study, previous longitudinal and case-control studies found these two conditions to be associated with excessive GMV loss in the frontal lobe (Cotter, Pariante & Everall, 2001; Perlis et al., 2004; Wang et al., 2012). Thus, the common disruption in GMV frontal lobes, specifically, in left SFG, right lateral of MFG and MPFC which are responsible for emotional processing, motivation, working memory and decisions may be the neuroanatomical substrate for impaired motivational drive and behavioral control that characterize both: heroin-dependent subjects and patients with depression. Future studies are warranted to investigate the role of these regions in the development of similar symptoms in individuals with heroin addiction and major depressive disorder.
Acknowledgements
The authors acknowledge that Tumbwene E. Mwansisya and Zheng Wang are co-first authors. This Research was supported by grants from the National Natural Science Foundation of China (81071092 to ZL, 81171286 and 30971053 to ZM Xue), the National 973 Program of China (2011CB707802 to ZL).
References
- Abercrombie, H.C., Schaefer, S.M., Larson, C.L., Oakes, T.R., Rusch, B.D., Lindgren, K.A., et al. (1999). Regional metabolic rate before and after treatment in major depressive disorder. Psychophysiology, 36, S24-S24
- Ballmaier, M., Kumar, A., Thompson, P.M., Narr, K.L., Lavretsky, H., Estanol, L., et al. (2004). Localizing gray matter deficits in late-onset depression using computational cortical pattern matching methods. The American journal of psychiatry,161, 2091-2099.
- Berridge, C.W., & Waterhouse, B.D.(2003). The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain research. Brain research reviews,42, 33-84
- Bonson, K.R., Grant, S.J., Contoreggi, C.S., Links, J.M., Metcalfe, J., Weyl, H.L., et al.(2002). Neural systems and cue-induced cocaine craving. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology,26, 376-386
- Bora, E., Fornito, A., Pantelis, C., & Yucel, M.(2012). Gray matter abnormalities in Major Depressive Disorder: A meta-analysis of voxel based morphometry studies. Journal of affective disorders,138, 9-18
- Brady, K.T., & Sinha, R.(2005a). Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. The American journal of psychiatry,162, 1483-1493
- Brady, K.T., & Sinha, R.(2005b). Co-occurring mental and substance use disorders: The neurobiological effects of chronic stress. The American journal of psychiatry,162, 1483-1493
- Bremner, J.D., Vythilingam, M., Vermetten, E., Nazeer, A., Adil, J., Khan, S., et al.(2002). Reduced volume of orbitofrontal cortex in major depression. Biological psychiatry,51, 273-279
- Camchong, J., MacDonald, A.W., Bell, C., Mueller, B.A., & Lim, K.O.(2011). Altered Functional and Anatomical Connectivity in Schizophrenia. Schizophrenia bulletin,37, 640-650
- Cotter, D.R., Pariante, C.M.,&Everall, I.P., (2001). Glial cell abnormalities in major psychiatric disorders: The evidence and implications. Brain research bulletin,55, 585-595
- Darke, S., &Ross, J., (2002). Suicide among heroin users: rates, risk factors and methods. Addiction 97, 1383-1394
- Drevets, W.C., Bogers, W., & Raichle, M.E.(20020. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology,12, 527-544
- Drevets, W.C., Price, J.L., & Furey, M.L.(2008). Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure and Function,213, 93-118
- Drevets, W.C., Price, J.L., Simpson, J.R., Jr., Todd, R.D., Reich, T., Vannier, M., et al. (1997). Subgenual prefrontal cortex abnormalities in mood disorders. Nature, 386, 824-827
- du Boisgueheneuc, F., Levy, R., Volle, E., Seassau, M., Duffau, H., Kinkingnehun, S., et al. (2006). Functions of the left superior frontal gyrus in humans: a lesion study. Brain : a journal of neurology,129, 3315-3328
- Du, M.Y., Wu, Q.Z., Yue, Q., Li, J., Liao, Y., Kuang, W.H., et al. (2012). Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Progress in neuro-psychopharmacology & biological psychiatry,36, 11-16
- Farb, N.A., Anderson, A.K., Bloch, R.T., &Segal, Z.V., (2011a). Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biological psychiatry,70, 366-372
- Farb, N.A.S., Anderson, A.K., Bloch, R.T., &Segal, Z.V., (2011b). Mood-Linked Responses in Medial Prefrontal Cortex Predict Relapse in Patients with Recurrent Unipolar Depression. Biological psychiatry,70, 366-372
- Frodl, T., Koutsouleris, N., Bottlender, R., Born, C., Jager, M., Morgenthaler, M., et al. (2008). Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Molecular psychiatry,13, 1093-1101
- Garavan, H., &Hester, R., 2007. The role of cognitive control in cocaine dependence. NeuropsychologyReview, 17, 337-345
- Goldstein, R.Z., Leskovjan, A.C., Hoff, A.L., Hitzemann, R., Bashan, F., Khalsa, S.S., et al. (2004). Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia,42, 1447-1458
- Goldstein, R.Z.,& Volkow, N.D., (2011). Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Reviews Neuroscience,12, 652-669
- Hastings, R.S., Parsey, R.V., Oquendo, M.A., Arango, V.,& Mann, J.J.(2004). Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology,29, 952-959
- Kennedy, N., & Paykel, E.S.(2004). Residual symptoms at remission from depression: impact on long-term outcome. Journal of affective disorders,80, 135-144
- Koolschijn, P.C., van Haren, N.E., Lensvelt-Mulders, G.J., Hulshoff Pol, H.E.,& Kahn, R.S.(2009). Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Human brain mapping,30, 3719-3735
- Li, C.T., Lin, C.P., Chou, K.H., Chen, I.Y., Hsieh, J.C., Wu, C.L., et al. (2010). Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuro Image,50, 347-356
- Li, Q., Wang, Y., Zhang, Y., Li, W., Yang, W., Zhu, J., et al. (2012). Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: an event-related fMRI study. Brain research,1469, 63-72
- Lin, W.C., Chou, K.H., Chen, H.L., Huang, C.C., Lu, C.H., Li, S.H., et al. (2012). Structural deficits in the emotion circuit and cerebellum are associated with depression, anxiety and cognitive dysfunction in methadone maintenance patients: A voxel-based morphometric study. Psychiatry Research: Neuroimaging,201, 89-97
- Liu, H., Hao, Y., Kaneko, Y., Ouyang, X., Zhang, Y., Xu, L., Xue, Z., et al.(20090. Frontal and cingulate gray matter volume reduction in heroin dependence: optimized voxel-based morphometry. Psychiatry and Clinical Neurosciences,63, 563-568
- Liu, X., Matochik, J.A., Cadet, J.L., & London, E.D. (1998). Smaller volume of prefrontal lobe in polysubstance abusers: a magnetic resonance imaging study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology,18, 243-252
- MacQueen, G.M., Campbell, S., McEwen, B.S., Macdonald, K., Amano, S., Joffe, R.T., et al. (2003). Course of illness, hippocampal function, and hippocampal volume in major depression. Proceedings of the National Academy of Sciences of the United States of America,100, 1387-1392
- Maremmani, I., Pacini, M., Pani, P.P., Perugi, G., Deltito, J., Akiskal, H., (2007). The mental status of 1090 heroin addicts at entry into treatment: should depression be considered a 'dual diagnosis'? Annals of General Psychiatry, 6, 31
- Markou, A., Kosten, T.R., & Koob, G.F.(1998). Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology,18, 135-174
- Miller, N.S., Klamen, D., & Flaherty, J.A.(1996). Prevalence of depression and alcohol and other drug dependence in addictions treatment populations. Journal of psychoactive drugs, 28, 111-124
- Nahas, Z., Anderson, B.S., Borckardt, J., Arana, A.B., George, M.S., Reeves, S.T., et al. (2010). Bilateral epidural prefrontal cortical stimulation for treatment-resistant depression. Biological psychiatry,67, 101-109
- Naravane, A., Lindo, J.F., Williams, L.A., Gardener, M.T., & Fletcher, C.K.(1997). Ascaris lumbricoides in the paranasal sinuses of a Jamaican adult. Transactions of the Royal Society of Tropical Medicine and Hygiene,91, 37
- Perlis, R.H., Miyahara, S., Marangell, L.B., Wisniewski, S.R., Ostacher, M., DelBello, M.P., et al.(2004). Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biological psychiatry,55, 875-881
- Qiu, Y.W., Jiang, G.H., Su, H.H., Lv, X.F., Tian, J.Z., Li, L.M., et al. (2013). The impulsivity behavior is correlated with prefrontal cortex gray matter volume reduction in heroin-dependent individuals. Neuroscience letters,538, 43-48
- Rajkowska, G., Miguel-Hidalgo, J.J., Wei, J., Dilley, G., Pittman, S.D., Meltzer, H.Y., et al. (1999). Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological psychiatry,45, 1085-1098
- Reid, A.G., Daglish, M.R., Kempton, M.J., Williams, T.M., Watson, B., Nutt, D.J., et al. (2008). Reduced thalamic grey matter volume in opioid dependence is influenced by degree of alcohol use: a voxel-based morphometry study. Journal of psychopharmacology,22, 7-10
- Rounsaville, B.J., Dolinsky, Z.S., Babor, T.F.,& Meyer, R.E. (1987). Psychopathology as a predictor of treatment outcome in alcoholics. Archives of general psychiatry,44, 505-513
- Sara, S.J.(2009). The locus coeruleus and noradrenergic modulation of cognition. Nature Reviews Neuroscience,10, 211-223
- Tanabe, J., Tregellas, J.R., Dalwani, M., Thompson, L., Owens, E., Crowley, T., et al. (2009). Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biological psychiatry, 65, 160-164
- Teesson, M., Havard, A., Fairbairn, S., Ross, J., Lynskey, M., Darke, S. (2005). Depression among entrants to treatment for heroin dependence in the Australian Treatment Outcome Study (ATOS): prevalence, correlates and treatment seeking. Drug and alcohol dependence,78, 309-315
- Trivedi, M.H., Hollander, E., Nutt, D.,& Blier, P.(2008). Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. Journal of Clinical Psychiatry,69, 246-258
- Upadhyay, J., Maleki, N., Potter, J., Elman, I., Rudrauf, D., Knudsen, J., et al. (2010). Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain : a journal of neurology,133, 2098-2114
- Vasic, N., Walter, H., Hose, A., & Wolf, R.C.(2008). Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: A voxel-based morphometry study. Journal of affective disorders,109, 107-116
- Verdejo-Garcia, A., Lawrence, A.J., & Clark, L.(2008). Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience and biobehavioral reviews,32, 777-810
- Verdejo-Garcia, A., Rivas-Perez, C., Lopez-Torrecillas, F.,& Perez-Garcia, M.(2006). Differential impact of severity of drug use on frontal behavioral symptoms. Addictive behaviors,31, 1373-1382
- Wagner, G., Koch, K., Schactitzabel, C., Reichenbach, J.R., Sauer, H., Schlosser, R.G.M. (2008). Enhanced rostral anterior cingulate cortex activation during cognitive control is related to orbitofrontal volume reduction in unipolar depression.Journal of Psychiatry&Neuroscience,33, 199-208
- Wang, X.Y., Li, B.J., Zhou, X.H., Liao, Y.H., Tang, J.S., Liu, T.Q., et al.(2012). Changes in brain gray matter in abstinent heroin addicts. Drug and alcohol dependence,126, 304-308
- Yuan, K., Qin, W., Dong, M., Liu, J., Sun, J., Liu, P., et al. (2010). Gray matter deficits and resting-state abnormalities in abstinent heroin-dependent individuals. Neuroscience letters,482, 101-105
- Yuan, Y., Zhu, Z.D., Shi, J.F., Zou, Z.L., Yuan, F., Liu, Y.J., et al. (2009). Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain and cognition,71, 223-228
- Zhang, X.C., Yao, S.Q., Zhu, X.Z., Wang, X., Zhu, X.L., Zhong, M.T.(2012). Gray matter volume abnormalities in individuals with cognitive vulnerability to depression: A voxel-based morphometry study. Journal of affective disorders,136, 443-452.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 11232
- [From(publication date):
March-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 10175
- PDF downloads : 1057