Research Article Open Access
Major Contributors to Nitrogen Gas Plasma Sterilization
| H Shintani* | |
| Faculty of Science and Engineering, Chuo University, Japan | |
| *Corresponding Author : | H Shintani Faculty of Science and Engineering Chuo University, 1-13-27, Kasuga Bunkyo, Tokyo 112-8551, Japan Tel: +81425922336 E-mail: shintani@mail.hinocatv.ne.jp |
| Received March 03, 2015; Accepted March 13, 2015; Published March 20, 2015 | |
| Citation: H Shintani (2015) Major Contributors to Nitrogen Gas Plasma Sterilization. Biochem Physiol 4:155. doi:10.4172/2168-9652.1000155 | |
| Copyright: © 2015 H Shintani. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Many papers have been published on gas plasma sterilization. Most have been conducted by engineers and physics researchers, so microbiological and chemical aspects are insufficient or inaccurate. Gas plasma sterilization research has significantly advanced since 2008 when biologists and chemists began contributing their expertise to the effort. However, the mechanism of sterilization by gas plasma has not yet been elucidated. Based on their life spans and other characteristics, metastables and/or photons can speculated to be the most likely candidates contributing to the mechanism of gas plasma sterilization. OH and/or NO radicals may be minor contributors due to significant short period of life. Spore death can be explained by the hydration of dipicolinic acid (DPA) in the spore core. The energy of metastables and/or photons can cause the formation of pin holes in spores that allow water to penetrate into the core and hydrate the DPA. Hydrated DPA transfers to the spore surface. DPA in the spore surface was collected by extraction with water and enriched by solid phase extraction. Eluted material was vaporized, condensed, and analyzed by the reverse phase C-18 HPLC. Elution from the C-18 column was carried out with acetonitrile/water (1/4, v/v, pH 5) and detected at 235 nm and by mass spectrometry (MS). Based on a comparison of the retention time and MS fragmentation pattern with that of standard DPA, the spore surface particles were confirmed to be composed of DPA. The hydration process occurred within the spore and did not cause any structural change within the spore. Therefore the structure of spores remained almost unchanged after sterilization.
| Keywords |
| Gas plasma sterilization; Metastables; Photon; Dipicolinic acid; Hydration; HPLC; Mass spectrometry |
| Introduction |
| Many papers on gas plasma sterilization have been published (Shintani et al. [1]; Sakudo and Shintani [2]; Shintani, et al. [3]; Deng et al. [4]; Ono et al. [5]; Guerra et al. [6]; Teramoto et al. [7]; Rossi et al. [8]; Rossi and Kylian [9]). Most papers and books reporting on gas plasma sterilization were by physical researchers (Deng et al. [4]; Ono et al. [5]; Guerra et al. [6]; Teramoto et al. [7]; Rossi et al. [8]; Rossi and Kylian [9]). However, research on gas plasma sterilization methods and the mechanisms of gas plasma sterilization requires a combined knowledge of chemistry, engineering, and microbiology. The combined efforts of specialists in these fields to analyze the effectiveness and mechanism of gas plasma sterilization have contributed to improvements in the technology. |
| The present study aims to increase our understanding of the mechanisms of sterilization by nitrogen gas plasma. Progress towards an understanding of the mechanism of gas plasma sterilization is approaching a final stage because of the research contributions of microbiologists and chemists to those of the engineering researchers. |
| It should be noted that current information regarding the mechanism of sterilization by gas plasma is limited to sterilization using nitrogen gas plasma. The mechanisms of other gas plasmas such as oxygen have not yet been determined. For example, different gas plasmas cause quite different effects on spores: oxygen gas plasma causes significant shrinkage, whereas nitrogen, argon, or helium gases do not (Rossi et al. [8]). Therefore, the mechanism of gas plasma sterilization by each type of gas plasma must be individually investigated. Right now only the mechanism of nitrogen gas plasma sterilization can be speculated and this speculation may be correct. There is evidence that hydration of dipicolinic acid (DPA), which is stored within the core of the endospore, is the cause of spore death (Young and Setlow [10]; Yaohua [11]; Reineke et al. [12]; Zhang et al. [13]; Silva et al. [14]; Setlow et al. [15]; Magge et al. [16]; Paidhungat et al. [17]; Slieman and Nicholson [18]). Following exposure to gas plasma, we have found small particles on spore surfaces by scanning electron microscopy (SEM). We speculate that these small particles may be hydrated dipicolinc acid that migrated from the spore core. In order to investigate whether the small particles are composed of DPA or not, we analyzed them by HPLC with UV and MS detection. |
| Experimental |
| Low pressure nitrogen gas plasma apparatus |
| The nitrogen gas plasma sterilization chamber that we used is described in Shintani et al. [1]. The low pressure nitrogen gas plasma apparatus can be used at around 60 oC under half atmospheric pressure with a 40 to 150 mm gap between the cathode and anode. The sterility assurance level (SAL) of 100 from an initial population of 106 CFU (Colony Forming Unit), which represents a 6 log reduction, was achieved in 7 min, indicating that the D value (decimal reduction value, time or dose to decrease one log) was 1.2 min. |
| Sterilization process of microorganisms |
| Sterility assurance was confirmed by using the biological indicator (BI) Geobacillus stearothermophilus ATCC 7953 (Shintani et al. [1]; Sakudo and Shintani [2]). 106 CFU/carrier of the BI were inoculated onto a surface modified SUS (stainless steel) by gas plasma and 1X 106 CFU were evenly inoculated and distributed onto the modified SUS surface at the nm level to avoid formation of clumps. Clump-free BI was commercially available from Merck Co. Ltd. (Tokyo, Japan). |
| HPLC and automated solid phase extraction (SPE) conditions, Detection by UV and MS |
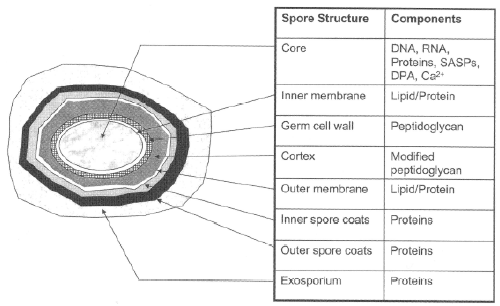
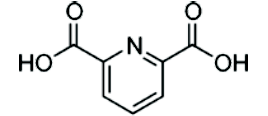
| Sterilized spores were extracted with water. Extraction with water was used to distinguish between the DPA on the surface and in the core (Figure 1). The solubility of DPA in water is only 1%; therefore, more than 100 sterilized BIs were necessary to collect sufficient material for analysis. The collected water including sterilized spores was adjusted with acetic acid to pH 5 to suppress the ionization of the carboxylic acid of DPA (Figure 2, http://commons.wikimedia.org/ wiki/File:Dipicolinic_acid.png) and to allow adherence to C-18 solid phase extraction (SPE) cartridges. The pH-adjusted water was applied to a C-18 SPE column and elution was carried out with acetonitrile. The acetonitrile was evaporated, condensed and re-dissolved into the mobile phase (acetonitrile/water, 1/4, v/v, pH 5) for HPLC analysis. Samples (10 μL) were applied onto a CapcellpackR C18 column (4.6 X 250 mm) from Shiseido. The flow rate of HPLC was 1 mL/min. and chemicals were detected at 235 nm and by mass spectrometry. |
| Results and Discussion |
| As shown in http://www.astp.com/plasma-equipment/applications, several types of gas plasma sterilization factors are expected to be involved in the sterilization of bacterial spores and microorganisms. These species include atoms, molecules, positively and negatively charged ions, photons, electrons, free radicals, and metastables, as well as UV and VUV. Among these, the contributions of UV and VUV to the sterilization process were shown to be minimal (Deng et al. [4]). According to Deng’s data, UV resulted in a decline of less than one log over 10 min, whereas other sterilization factors cause a 4 log reduction in 10 min, indicating that UV and VUV do not significantly contribute to sterilization (Deng et al. [4]). Most gas plasma researchers currently agree that UV and VUV do not make significant contributions to sterilization. |
| Free radicals, especially OH, NO or OONO radicals, are attractive candidates to contribute to the sterilization mechanism due to their high oxidation-reduction potential (Table 1), but the life period of OH or NO radicals is too short (~a few μs, Table 2). Life period of OONO radical have a few s and several sorts of biological ability, so OONO still remains one of candidates of sterilization factor. The flight distance during the life period of OH or NO radicals compared with N2 metastables is too short (Table 3); therefore, OH and NO radicals may contribute to sterilization as minor factors. Photons have a long life of 1018 years, so flight distances will be infinite. Metastables and photons have an energy, neutral and long flight distances; therefore, they may be major candidates for the sterilization process. From the excited state down to the ground state, metastables emit energy, and the resulting energy attacks the surface layer of the spores or microorganisms, producing pin holes on their surfaces. |
| Ions, electrons or charged molecules shown in http://www.astp. com/plasma-equipment/applications become trapped in the outer layers of the gram negative or gram positive cell envelope or spore outer layers (McDonnell [19]) and cannot penetrate into the interior. Therefore, charged factors are not considered to be major contributors to the mechanism of sterilization. |
| Among metastables, the lifetime of singlet oxygen is 7s and that of nitrogen metastables is 2s (Table 2). We can observe the production of N2 metastables by applying pulsed-amperometric discharge and we measured the lifetime of N2 metastables as a few s (experimentally 2 s, Table 2) (Ono et al. [5]; Guerra et al. [6]; Teramoto et al. [7]). During the return of the excited N2 metastables down to the ground state, energy is emitted and is useful for disruption of spores and bacterial cells. We can therefore hypothesize that N2 or O2 metastables and photons may be the most likely candidates to inactivate bacterial spores and vegetative cells. |
| However, we have a problem. Bacterial spore death is considered to be caused by hydration of DPA (Figures 1 and 2) (Young and Setlow [10]; Yaohua [11]; Reineke et al. [12]; Zhang et al. [13]; Silva et al. [14]; Setlow et al. [15]; Magge et al. [16]; Paidhungat et al. [17]; Slieman and Nicholson [18]), so how can we connect N2 metastables to the hydration of DPA? We observed dead spores with scanning electron microscopy (SEM) and found that dead spores had tiny white particles on their surfaces. We proposed that the white particles may be hydrated and dried dipicolinic acid, so we carefully collected white surface particles with water. This is because if organic solvent is used in place of water as an extraction solvent, interior DPA may also be recovered together with the surface particles. |
| HPLC analysis of DPA, detection by UV and MS |
| Water containing the dissolved white particles was adjusted to pH 5 with acetic acid to decrease the dissociation of the carboxylic acid of DPA, and was applied to a C-18 SPE cartridge. Elution was carried out with acetonitrile. The automated BenchmateR SPE method was used. Sample recovery using automated SPE is reproducible due to the constant pressure exerted compared with manual SPE. The acetonitrile was evaporated and the sample was re-dissolved in a mobile phase of acetonitrile/water that had been adjusted to pH 5 with acetic acid (1/4, v/v, pH 5). Ten μL was injected onto an HPLC equipped with a CapcellpackR C-18 column (4.6 X 250 mm, Shiseido Co., Tokyo, Japan) and detection was carried out at 235 nm and with MS. The flow rate was 1 mL/min. We confirmed that DPA from within the spore core was not eluted during extraction with water, so any DPA detected must be from the surface. The retention time and MS fragmentation of the target compound coincided with those of standard DPA; therefore, the surface substance was confirmed to be DPA, and suggests that release of DPA from the core contributed to spore death. Peaks with m/z of 167, 123, 105, 79 are typical of DPA fragmentation, and are consistent with previously reported data (Goodacre et al. [20]; Srivastava et al. [21]). Other researchers have reported that spore death resulted from the inactivation of catalase or leakage of DPA (Roth et al. [22]; Lerouge et al. [23]; Han et al. [24]; Kvam et al. [25]), but unlike our study, the sterilization process being examined in their studies used UV-C or VUV. Our studies indicate that spore death during nitrogen gas plasma sterilization is caused by DPA hydration (Roth et al. [22]). We showed that core DPA was hydrated with the penetrated water presumably as a result of pinholes formed by N2 metastables and photons. This allowed core DPA to be transported from the core to the surface of the spore. Water for hydration was from the interior of the spore (Table 4) as well as from surrounding spore. One question that remains is how the spore died while maintaining an intact structure (Rossi et al. [8]). The killing process must occur within the spore because the outer appearance of dead spores is almost identical to that of viable spores (Shintani et al. [1]; Sakudo and Shintani [2]; Deng et al. [4]). |
| Conclusion |
| The energy of the N2 metastables and photons introduced pinholes into the surface of the spores. As a result, DPA in the spore core was hydrated with water from within and surrounding the spore. The hydrated DPA was transported to the surface layer and remained and dried as white particles. This is proposed to be the mechanism of spore death by metastables and photons. The outwardly visible structure of the spore remained unchanged after death because the killing process mainly occurred within the core. |
| DPA at the spore surface was analyzed by ion-suppression reversed phase C-18 combined with detection by UV spectroscopy at 235 nm and MS. The mobile phase was acetonitrile/water (1/4, v/v) at pH 5 |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 13965
- [From(publication date):
June-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 9425
- PDF downloads : 4540
