Magnitude of Time-Dependence of Beat-to-Beat Muscle Blood Flow Between Isometric Contraction and Relaxation During Repeated Knee Extensor Exercise at Incremental Workload
Received: 20-Nov-2017 / Accepted Date: 14-Dec-2017 / Published Date: 18-Dec-2017
Abstract
Blood flow (BF) due to muscle contractions during exercise providing information about increased oxygen supply and energy metabolism, which may be the underlying mechanism of exercise therapy for cardiovascular disease. The same mechanism also normalizes the central and peripheral hemodynamics in body. High time resolution Doppler ultrasound measurements have been reported to detect not only the beat-to-beat blood velocity profile in the nonmuscular contraction state (basal resting condition), but also alterations in blood velocity profile in exercising limb during muscle contractions. During sustained isometric (static) muscle contraction (IMC), the systole-diastolic blood velocity profile is not intermittently disturbed due to the absence of fluctuations of intramuscular pressures compared to isotonic (dynamic) muscle contractions. This offers the possibilities to evaluate the blood velocity and BF magnitude for each beat-to-beat cycle without the influence of rapid changes in intramuscular pressure. Our previous studies demonstrated that elevated intramuscular pressure during 10 s-sustained IMC may transiently restrict muscle hyperemia during steadystate repeated knee extensor exercise at 3 contractions per minute (10 s-IMC and 10 s-muscle relaxation) at 10%, 30%, 50% and 70% of maximum voluntary contraction. In contrast, the sudden increase in peak BF immediately after the end of a IMC steeply declines during the period of 10 s-muscle relaxation. However, there is a lack of information regarding the extent to which the beat-to-beat magnitude in BF during IMC and muscle relaxation, even if large differences are observed in the BF magnitude between IMC and relaxation phases. We provided that the time-dependent beat-to-beat BF magnitude may significantly (P<0.05) linearly correlate for both 10 s-IMC and 10 s-relaxation phase. Furthermore, the variation in the acceleration rate of time dependent changes in BF is small in individual subjects below 30% of maximum voluntary contraction both IMC and relaxation phase. The present commentary therefore discusses to what extent the time-dependence of beat-to-beat magnitude in BF may statistically correlate during repeated IMC and relaxations with supplementary consideration of isometric exercise induced BF changes and its evaluation.
Keywords: Exercising blood flow; Isometric muscle contraction and relaxation; Doppler ultrasound
Abbreviations
BF: Blood Flow; IMC: Isometric Muscle Contraction; MVC: Maximum Voluntary Contraction; CPM: Contractions Per Minute
Introduction
Exercise induced increased blood flow (BF) plays a key role in human health, as it increases the availability of oxygen and metabolites in skeletal muscles, which are also crucial for exercise tolerance and capacity [1-3]. During exercise, the increased limb oxygen uptake is directly proportional to the work performed [1,2]. The limb oxygen uptake is calculated as the product of exercising arterial BF and the arterio-venous oxygen difference to the exercising limb, therefore the determination of BF dynamics feeding the contracting muscles can contribute to understand the factors, limiting work capacity.
In resistance and/or endurance training, an exercise model with a prescription such as muscle contraction intensity, frequency and time interval plays a crucial role for alterations in the BF in exercised muscle [4-8]. Thus, the evaluation of the time course in BF magnitude, due to continuous repeated muscle contractions may potentially yield valuable information for the muscle circulation and/or metabolism following the exercise therapy.
Ultrasound Doppler devices can provide the measurement of high temporal resolution blood velocity. Pulsatile (oscillation) blood velocity profile in the conduit artery (in systole and diastole), may be detected at rest, synchronized with the heart beat and blood pressure [4]. Based on this technique, rapid changes in the blood velocity profiles in the conduit artery is measured with muscle contraction and/or muscle relaxation in different states of exercise, muscle contraction time/frequency and workload, and in relation to vasodilatation/vasoconstriction [9-12].
During rhythmic/dynamic muscle contractions, these oscillations are even more pronounced (disturbance of shaped systole-diastolic like profile), as they are also influenced by intramuscular pressure variations [9]. Therefore, the measurement of blood velocity should be done carefully to account for the influence of muscle contraction and relaxation duty cycles (changes in the muscle contraction force) inducing physiological blood velocity fluctuations (internal variability), as well as the alterations in the blood velocity (external variability) due to temporary slight changes in muscle strength, compared to the target intensity [6,9].
This raises the issue of how to optimally determine exercise BF during steady-state repeated muscle contractions. In general, an optimal/valid BF in a non-exercise limb (for instance, basal resting state or non-contractile muscle) may exhibit minimum physiological BF variability using samplings of the cardiac beat-by-beat cycle [9,13]. However, during isotonic (dynamic) exercise, the muscle contractioninduced blood velocity profile may be largely influenced by the magnitude of intramuscular pressure variation and the superimposed influence of perfusion pressure variations [4,9-11].
Thus, the evaluation of time averaged-BF by the ‘net-muscle contraction-relaxation duty cycles’ may be required for rhythmic muscle exercise (high frequency muscle contractions, typically one muscle contraction per second, 60 contractions per minute, cpm) [4,9]. However, at lower contraction frequencies, typical of isometric muscle contractions (IMC), for instance 10-s IMC and 10-s muscle relaxation duty cycle (3 cpm), the blood velocity profile (shaped systole-diastolic profile) is not disturbed due to the lower intramuscular pressure variation during sustained IMC. This means that a stable beat-to-beat blood velocity profile is observed during IMC. In contrast, a rapid change in blood velocity increase is seen during muscle relaxation representing post muscle contraction hyperemic state [12,14,15].
However, during repeated IMC there is a lack of information for time-dependence of beat-to-beat magnitude regarding the extent to which the beat-to-beat magnitude in blood velocity/BF may statistically fit with correlation coefficients during IMC and muscle relaxation for the evaluation of sustained muscle contraction induced limb BF alterations at incremental exercise intensity.
The present commentary therefore highlights BF dynamics in relation to repeated voluntary IMC at a typical muscle contraction rate (10 s-IMC/10 s-muscle relaxation) with supplementary consideration of muscle contraction induced BF changes.
Methodology And Result
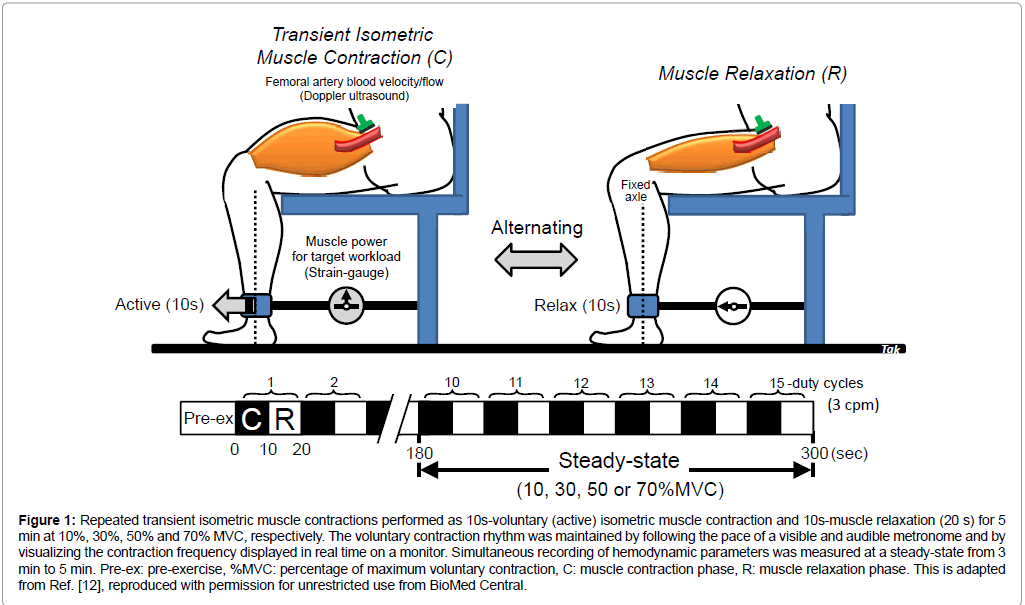
In one-legged, repeated isometric knee-extensor exercise performed by seven healthy male volunteers (mean age ± SD, 26.3 ± 7.6 years; mean height, 178.1 ± 7.2 cm; mean body weight, 71.1 ± 5.7 kg), the exercise is confined to the quadriceps muscle group (Figure 1). The muscle contraction rate for knee extensor muscle (mainly quadriceps muscle group) is 3 contractions per minute (10 s-on and 10 s-off) at 10%, 30%, 50% and 70% of maximum voluntary contraction (MVC). One legged-knee extensor exercise model allows stable measurements of femoral artery blood velocity using Doppler ultrasound [4-6].
Figure 1: Repeated transient isometric muscle contractions performed as 10s-voluntary (active) isometric muscle contraction and 10s-muscle relaxation (20 s) for 5 min at 10%, 30%, 50% and 70% MVC, respectively. The voluntary contraction rhythm was maintained by following the pace of a visible and audible metronome and by visualizing the contraction frequency displayed in real time on a monitor. Simultaneous recording of hemodynamic parameters was measured at a steady-state from 3 min to 5 min. Pre-ex: pre-exercise, %MVC: percentage of maximum voluntary contraction, C: muscle contraction phase, R: muscle relaxation phase. This is adapted from [12], reproduced with permission for unrestricted use from BioMed Central.
The measurement of blood velocity in the femoral artery feeding the active thigh muscles using Doppler ultrasound, where BF is calculated by the product of blood velocity and cross-sectional area, has been validated and shown to produce accurate absolute values both at rest and during incremental leg exercise such as rhythmical/dynamic [4] or isometric (static) thigh muscle contractions [12]. The minimum value of the coefficients of variation (<5%) for the repeated blood velocity measurements represented the criteria for quality control of operator technique at rest as well as during exercise [9].
The measurement site of the femoral artery was distal to the inguinal ligament, but above the bifurcation into the branches of the superficial and deep femoral arteries. This location minimizes turbulence from the femoral bifurcation and the influence of blood velocity from the inguinal region. Furthermore, the changes of the vessel diameter of the conduit artery in the target area are mostly unaffected by muscle contractions and relaxations [4]. Therefore, changes in blood velocity may potentially correspond to changes in BF because BF is calculated by the product of mean blood velocity and the stable cross-sectional area (the square of the radius multiplied by π) in the artery. The parameters (blood velocity, blood pressure, voluntary muscle contraction power, and surface electromyography) were simultaneously recorded by beat- to-beat measurements at pre-exercise and during steady-state exercise of between 180 and 300 s of 15 repeated duty cycles at 10%, 30%, 50% and 70% MVC, respectively.
Statistical analysis in the BF kinetics between IMC and relaxation phase
Statistical analysis with a linear fitting regression correlation coefficient (r) and P value was examined for the time course in beat-to-beat measurements of BF (time from initial beat on the x axis, both BF on the y axis) during IMC and muscle relaxation phases, respectively (Microsoft Excel 2010). The acceleration rate of time-dependent changes in BF was also determined by the gradient of the regression line (Slope in Figure 2). Distribution of the acceleration rate of changes in BF between IMC and relaxation was represented by box and whisker plots.
Discussion
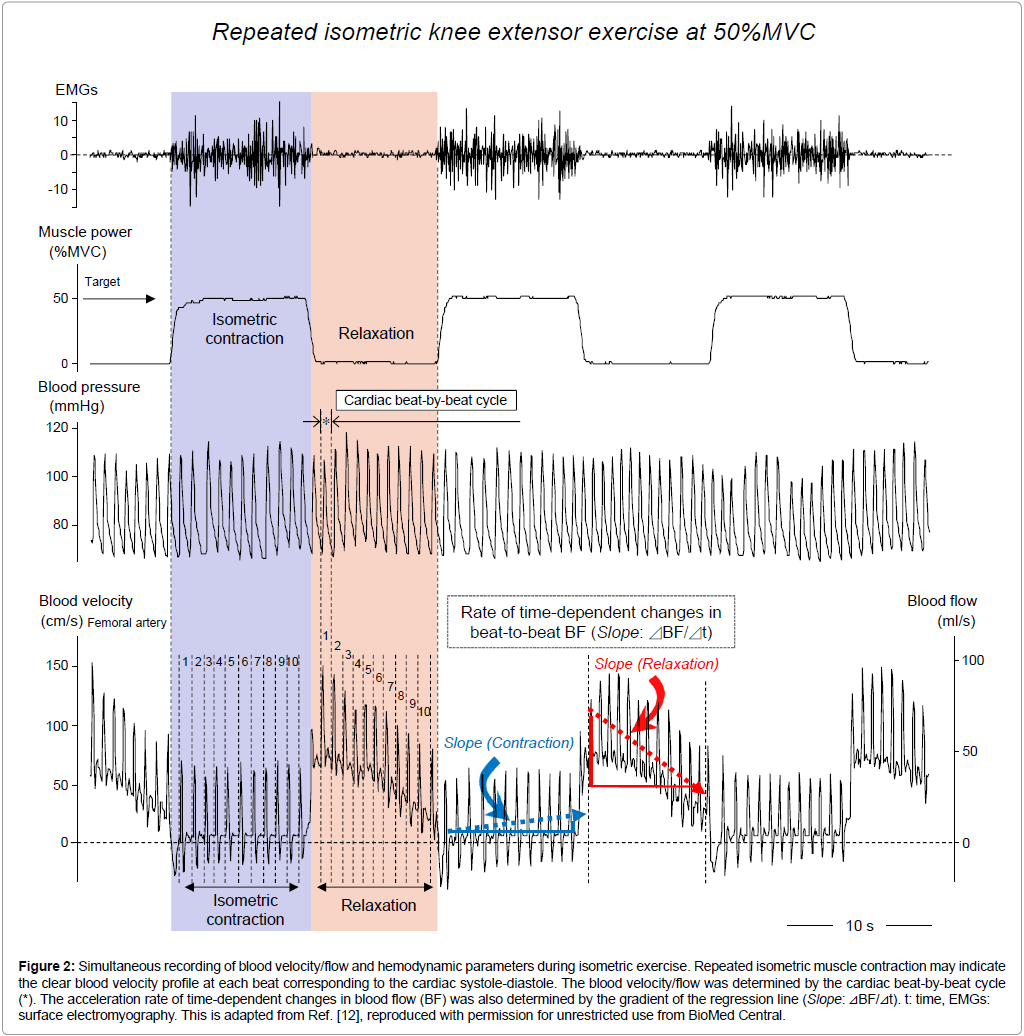
Our previous studies have demonstrated the difference in the time course of the magnitude of the blood velocity/flow between IMC and relaxation phases during steady-state isometric knee extensor exercise [12]. In isometric (static) exercise at 3 cpm (10 s-on and 10 s-off), the blood velocity profile presents a clear systolic and diastolic-like profile (blood pressure curve-like) in Figure 2. This is due to non-oscillation of the blood velocity profile (a non-disturbed blood velocity profile), lacking rhythmical vessel compression even under 10 s-sustained IMC. As shown in Figure 2, changes in beat-to-beat blood velocity/flow may be lower during 10 s-sustained IMC (the dotted arrow in blue color in Figure 2), although a gradual but non-significant BF increase was seen during 10 s-sustained IMC [12].
Figure 2: Simultaneous recording of blood velocity/flow and hemodynamic parameters during isometric exercise. Repeated isometric muscle contraction may indicate the clear blood velocity profile at each beat corresponding to the cardiac systole-diastole. The blood velocity/flow was determined by the cardiac beat-by-beat cycle (*). The acceleration rate of time-dependent changes in blood flow (BF) was also determined by the gradient of the regression line (Slope: ⊿BF/⊿t). t: time, EMGs: surface electromyography. This is adapted from [12], reproduced with permission for unrestricted use from BioMed Central.
In contrast, the variations in blood velocity/flow evaluated by beatto- beat cycle were significantly higher in 10 s-muscle relaxation than in 10 s-IMC at 10-70% MVC (see*, the comparison in net 1-10th beat-tobeat blood velocity/flow magnitude between IMC and relaxation phase in Figure 2 at 50% MVC). This result suggests that during 10 s-muscle relaxation the time course of the beat-to-beat blood velocity/flow magnitude may markedly change, due to the post-muscle contraction hyperemic response following the end of muscle contraction (the dotted arrow in red color in Figure 2). Thus, the magnitude of blood velocity/ flow and the difference in blood velocity/flow variations between IMC and relaxation may represent characteristics of exercise hyperemia. However, there is still a lack of specific information regarding the dynamics of time-dependence of beat-to-beat blood velocity/flow from the physiological aspect evaluated by the statically correlation.
Using Doppler ultrasound, to understand the time course in blood velocity/flow pattern between IMC and relaxation may offer insights for the evaluation of peripheral muscle BF during repeated IMC through physical and exercise therapy. Following the additional analysis using our previously-published data [12], the time-dependent beat-to-beat BF magnitude may significantly linearly correlate for both 10 s-IMC and 10 s-relaxation phase at incremental workloads (Table 1).
| 10 s isometric muscle contraction phase | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of subject | 10% MVC | 30% MVC | 50% MVC | 70% MVC | ||||||||||||
| Slope (ml/s) | Number of beat | r | P value | Slope (ml/s) | Number of beat | r | P value | Slope (ml/s) | Number of beat | r | P value | Slope (ml/s) | Number of beat | r | P value | |
| 1 | 22.7 | 10 | 0.976 | <0.001 | 35.6 | 9 | 0.995 | <0.001 | 15.9 | 9 | 0.961 | <0.001 | 14.7 | 9 | 0.92 | <0.001 |
| 2 | 9.2 | 10 | 0.85 | <0.01 | 21 | 10 | 0.961 | <0.001 | 14.6 | 9 | 0.943 | <0.001 | 11.5 | 9 | 0.973 | <0.001 |
| 3 | 43.6 | 7 | 0.962 | <0.001 | 63.2 | 8 | 0.819 | <0.05 | 34.9 | 8 | 0.833 | <0.05 | 78.8 | 9 | 0.956 | <0.001 |
| 4 | 19.6 | 9 | 0.666 | <0.05 | 33.4 | 9 | 0.902 | <0.001 | 44.9 | 9 | 0.904 | <0.001 | 34.1 | 11 | 0.876 | <0.001 |
| 5 | 7.6 | 9 | 0.58 | ns | 47.6 | 9 | 0.894 | <0.01 | 22 | 9 | 0.843 | <0.01 | 24.9 | 8 | 0.89 | <0.01 |
| 6 | 20.3 | 12 | 0.693 | <0.05 | 33.8 | 13 | 0.777 | <0.01 | 28.7 | 14 | 0.401 | ns | -28.9 | 12 | 0.55 | ns |
| 7 | 20.5 | 8 | 0.983 | <0.001 | 30.5 | 8 | 0.952 | <0.001 | 9.9 | 9 | 0.54 | ns | -2.5 | 9 | 0.345 | ns |
| 10 s muscle relaxation phase | ||||||||||||||||
| Number of subject | 10% MVC | 30% MVC | 50% MVC | 70% MVC | ||||||||||||
| Slope (-1xml/s) | Number of beat | r | P value | Slope (-1xml/s) | Number of beat | r | P value | Slope (-1xml/s) | Number of beat | r | P value | Slope (-1xml/s) | Number of beat | r | P value | |
| 1 | 93 | 10 | 0.99 | <0.001 | 140.4 | 10 | 0.991 | <0.001 | 200.2 | 12 | 0.975 | <0.001 | 195.3 | 12 | 0.98 | <0.001 |
| 2 | 83.3 | 10 | 0.973 | <0.001 | 196.6 | 11 | 0.994 | <0.001 | 263.7 | 11 | 0.97 | <0.001 | 215.1 | 9 | 0.972 | <0.001 |
| 3 | 88.3 | 8 | 0.983 | <0.001 | 201.7 | 9 | 0.987 | <0.001 | 331.6 | 10 | 0.974 | <0.001 | 182.9 | 10 | 0.988 | <0.001 |
| 4 | 81.4 | 8 | 0.988 | <0.001 | 207.8 | 10 | 0.951 | <0.001 | 270.5 | 10 | 0.931 | <0.001 | 99.5 | 12 | 0.778 | <0.01 |
| 5 | 58.8 | 9 | 0.957 | <0.001 | 253.2 | 9 | 0.992 | <0.001 | 208.2 | 10 | 0.999 | <0.001 | 288 | 9 | 0.997 | <0.001 |
| 6 | 99.5 | 13 | 0.988 | <0.001 | 186 | 14 | 0.964 | <0.001 | 60 | 14 | 0.902 | <0.001 | -107.8 | 14 | 0.813 | <0.001 |
| 7 | 64.2 | 8 | 0.984 | <0.001 | 220.4 | 8 | 0.995 | <0.001 | 102.3 | 9 | 0.933 | <0.001 | 127.2 | 10 | 0.948 | <0.001 |
Table 1: The acceleration rate of time-dependent changes in beat-to-beat exercising blood flow (BF) between isometric muscle contraction and relaxation phase. The acceleration rate of time-dependent changes in BF was also determined by the gradient of the regression line (Slope: ⊿BF/⊿t in Figure 2). Statistical analysis with a linear fitting regression correlation coefficient (r), and P value was examined for the time course in beat-to-beat measurements of BF (time from initial beat on the x axis, BF on the y axis) during isometric muscle contraction and muscle relaxation phases, respectively (Microsoft Excel 2010). A P value <0.05 was considered statistically significant.ns: not significance, %MVC: percentage of maximum voluntary contraction. This is our original unpublished data newly analyzed by the source [12], reproduced with permission for unrestricted use from BioMed Central.
Beat-to-beat dynamics during IMC phase
Regarding the phase of IMC, the time course in beat-to-beat BF dynamics showed a “positive” almost-linear correlation, featuring a slight increase except for 10% MVC in subject 5, and both 50% and 70% MVC in subjects 6 and 7 at all target workloads (Table 1). Interestingly, although some mechanical extravascular compression remains with increasing intramuscular pressure, the time course of beat-to-beat BF displayed slight vasodilation in time with no observed reduction of BF, as a function of mechanical arterial obstruction at incremental workload.
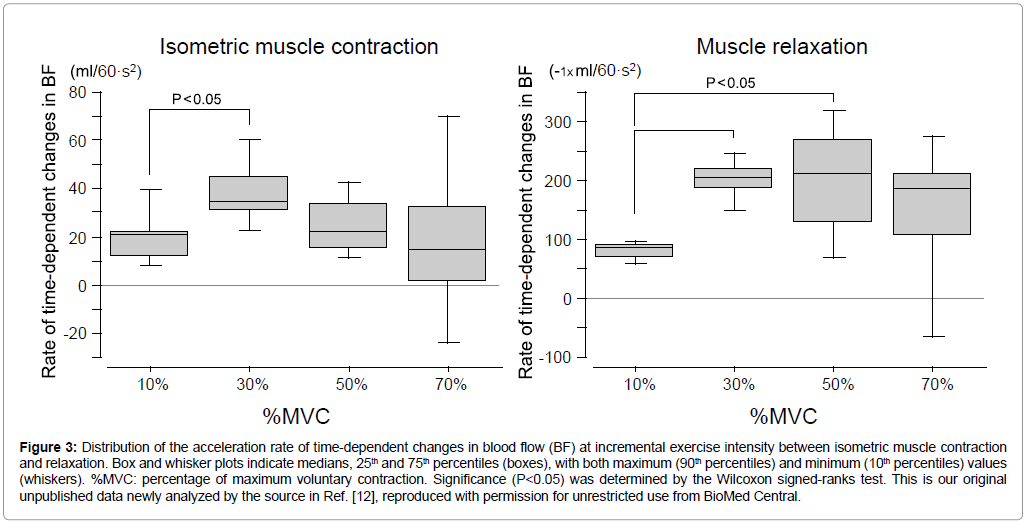
As seen in Figure 3, the range (distribution) for the increase rate of changes in BF between the 25th and 75th percentiles were small at both 10% (11.8-22.1 ml/60·s2) and 30% MVC (31.2-44.6 ml/60·s2) during IMC, which means the variation for the increase rate of BF was similar range in individual subjects below light exercise intensity. The significant difference in increase rate of changes in BF (the absolute value) might be represented between 10% and 30% MVC because of subject 5 with low value of correlation coefficient.
Figure 3: Distribution of the acceleration rate of time-dependent changes in blood flow (BF) at incremental exercise intensity between isometric muscle contraction and relaxation. Box and whisker plots indicate medians, 25th and 75th percentiles (boxes), with both maximum (90th percentiles) and minimum (10th percentiles) values (whiskers). %MVC: percentage of maximum voluntary contraction. Significance (P<0.05) was determined by the Wilcoxon signed-ranks test. This is our original unpublished data newly analyzed by the source [12], reproduced with permission for unrestricted use from BioMed Central.
Regarding moderate exercise intensity the increase rate of changes in BF was large at both 50% MVC (14.9-33.4 ml/60·s2) and 70% MVC (1.0-31.8 ml/60·s2), however medians is almost similar (range, 14.7-33.8 ml/60·s2) among 10% and 70% MVC during IMC.
Beat-to-beat dynamics during muscle relaxation phase
Conversely, a close relationship in beat-to-beat BF during muscle relaxation phase with ‘negative’ linear fitting was evident among subjects. High correlation coefficients with P values below 0.001 (except for subject 4 at 70% MVC in P<0.01 and subject 6 at 70% MVC representing positive linear correlation) were determined in beat-to-beat BF magnitude for the linear fitting correlation during 10 s muscle relaxation (Table 1). At least, for the repeated isometric exercise at steady-state presented in this study, this correlation suggests that a momentary peak BF (maximum vasodilation at the first beat corresponding to ‘1’ during relaxation phase as in Figure 2) immediately after the end of muscle contraction (corresponding to the start of muscle relaxation) will regularly/steeply decline to the pre-exercise state until next muscle contraction, although this was not seen in subject 6 at 70% MVC (Table 1). The distribution for the decline rate of changes in BF between 25th percentile and 75th percentiles is very small; at both 10% (68.5-91.9 ml/60·s2) and 30% MVC (188.6-217.2 ml/60·s2) during muscle relaxation compared to both 50% and 70% MVC, which means the variation in the decline rate of BF is small in individual subjects below 30% MVC. Furthermore, the decline rate of changes in BF was significant (P<0.05) higher both 30% and 50% MVC than 10% MVC, however the medians is similar range (182.9-208.2 ml/60·s2) among 30% and 70% MVC during muscle relaxation.
For moderate exercise intensity at 50% and 70% MVC as shown in Figure 3, the range for the rate of changes in BF was large both IMC and relaxation of which may be related to the individual difference for the release of vasoactive substances with muscle contraction against the muscle contraction intensity [16].
Conclusion
Since there is a large difference in single blood velocity/flow value between first and last beats in muscle relaxation phase, which may represent the time course in beat-to-beat BF kinetics being steeply decline in linear manner; the short temporal time averaged-BF at least below 10 s may potentially be over or under-estimated in the muscle relaxation phase compared to IMC phase at relatively moderate exercise intensity with a typical muscle contraction rate (10 s-IMC/10 s-muscle relaxation). Therefore, the beat-to-beat BF magnitude describing the linear fitting correlation may contribute to the supplementary consideration for the estimation of time-averaged BF value at the target point and/or comprehensive BF between the IMC and relaxation phases.
The exercise-induced muscle hyperemia has been controlled by an inter-play between both “feedback” and “feedforward” vascular control which include central mechanisms (neural and hormonal factors) [17- 19], as well as local mechanisms involving the myogenic activity [20], and mediators derived from the endothelium [21], muscle fibers [22] and/or muscle mechanical factors [12]. Therefore, the characteristics in the beat-to-beat BF magnitude between IMC and relaxation would be one of helpful information for the cardiovascular adjustment during repeated exercise and its evaluation in relation to the integrated applied physiology and/or the physical exercise therapy.
Acknowledgements
The authors acknowledge the long-term support of the late professor emeritus Bengt Saltin, the staff of the Copenhagen Muscle Research Centre, and the volunteers who participated in the studies. The study was supported by the Danish National Research Foundation Grant 504-14, as well as the “Excellent Young Researchers Overseas Visit Program” in Scientific Research (No. 21-8285) 2010 and Scientific Research (C) general (No. 15K01730) from MEXT and JSPS (T. Osada).
References
- Andersen P, Saltin B (1985) Maximal perfusion of skeletal muscle in man. J Physiol 366: 233-249.
- Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B (1985) Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol 59: 1647-1653.
- Blomstrand E, RÃ¥degran G, Saltin B (1997) Maximum rate of oxygen uptake by human skeletal muscle in relation to maximal activities of enzymes in the Krebs cycle. J Physiol 501: 455-460.
- RÃ¥degran G (1997) Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol 83: 1383-1388.
- Osada T, RÃ¥degran G (2002) Femoral artery inflow in relation to external and total work rate at different knee extensor contraction rates. J Appl Physiol 92: 1325-1330.
- Osada T, RÃ¥degran G (2009) Femoral artery blood flow and its relationship to spontaneous fluctuations in rhythmic thigh muscle workload. Clin Physiol Funct Imaging 29: 277-292.
- Roberg RA, Icenogle MV, Hudson TL, Greene ER (1997) Temporal inhomogeneity in brachial artery blood flow duringforearm exercise. Med Sci Sports Exerc 29: 1021-1027.
- MacDonald MJ, Shoemaker JK, Tschakovsky ME, Hughson RL (1998) Alveolar oxygen uptake and femoral artery blood flow dynamics in upright and supine leg exercise in humans. J Appl Physiol 85: 1622-1628.
- Osada T (2004) Muscle contraction-induced limb blood flow variability during dynamic knee extensor. Med Sci Sports Exerc 36: 1149-1158.
- Osada T, RÃ¥degran G (2005) Alterations in the rheological flow profile in conduit femoral artery during rhythmic thigh muscle contractions in humans. Jpn J Physiol 55: 19-28.
- Osada T, RÃ¥degran G (2006) Alterations in the blood velocity profile influence the blood flow response during muscle contractions and relaxations. J Physiol Sci 56: 195-203.
- Osada T, Mortensen SP, RÃ¥degran G (2015) Mechanical compression during repeated sustained isometric muscle contractions and hyperemic recovery in healthy young males. J Physiol Anthropol 34: 36.
- Osada T, RÃ¥degran G (2006) Differences in exercising limb blood flow variability between cardiac and muscle contraction cycle related analysis during dynamic knee extensor. J Sports Med Phys Fitness 46: 590-597.
- Wesche J (1986) The time course and magnitude of blood flow changes in the human quadriceps muscles following isometric contraction. J Physiol 377: 445-462.
- Osada T, RÃ¥degran G (2016) Difference in muscle blood flow fluctuations between dynamic and static thigh muscle contractions: How to evaluate exercise blood flow by Doppler ultrasound. Phys Med Rehabil Res 1: 1-7.
- Joyner MJ, Dietz NM (1997) Nitric oxide and vasodilation in human limbs. J Appl Physiol 83: 1785-1796.
- Ishii K, Liang N, Oue A, Hirasawa A, Sato K, et al. (2012) Central command contributes to increased blood flow in the noncontracting muscle at the start of one-legged dynamic exercise in humans. J Appl Physiol 112: 1961-1974.
- Hedman A, Andersson PE, Reneland R, Lithell HO (2001) Insulin-mediated changes in leg blood flow are coupled to capillary density in skeletal muscle in healthy 70-year-old men. Metabolism 50: 1078-1082.
- Ciccone MM, Scicchitano P, Cortese F, Gesualdo M, Zito A, et al. (2013) Modulation of vascular tone control under isometric muscular stress: role of estrogen receptors. Vascul Pharmacol 58: 127-133.
- Kruse NT, Silette CR, Scheuermann BW (2016) Influence of passive stretch on muscle blood flow, oxygenation and central cardiovascular responses in healthy young males. Am J Physiol Heart Circ Physiol 310: 1210-1221.
- Hearon CM Jr, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA (2016) Endothelium-dependent vasodilatory signalling modulates α1-adrenergic vasoconstriction in contracting skeletal muscle of humans. J Physiol 594: 7435-7453.
- Lash JM (1996) Regulation of skeletal muscle blood flow during contractions. Proc Soc Exp Biol Med 211: 218-235.
Citation: Osada T, Ueno R, Rådegran G (2017) Magnitude of Time-Dependence of Beat-to-Beat Muscle Blood Flow Between Isometric Contraction and Relaxation During Repeated Knee Extensor Exercise at Incremental Workload. J Card Pulm Rehabil 1: 119.
Copyright: ©2017 Osada T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 6778
- [From(publication date): 0-2017 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 5624
- PDF downloads: 1154