Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Editorial Open Access
Lung Cancer Motion Management-Cinema at Its Best?
| Charles A Kunos*, Terry Kuykendall and Todd White | |
| Department of Radiation Oncology, Summa Health System, Akron, OH, USA | |
| Corresponding Author : | Charles Kunos Summa Comprehensive Cancer Institute Department of Radiation Oncology 161 North Forge Street, USA Tel: 330-375-3557 Fax: 330-375-3072 E-mail: kunosc@summahealth.org |
| Received December 11, 2013; Accepted December 12, 2013; Published December 19, 2013 | |
| Citation: Kunos CA, Kuykendall T, White T (2013) Lung Cancer Motion Management-Cinema at Its Best?. OMICS J Radiology 3:e124. doi: 10.4172/2167-7964.1000e124 | |
| Copyright: © 2013 Kunos CA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
| The field of radiation oncology has entertained either side-byside computing technology, overlay of diagnostic and planning image sets, or fusion of positron emission tomography (PET) and computed tomography (CT) data for the purpose of creating a single image data set for stereotactic radiosurgery planning [1]. 2-[18F]-fluoro-2-deoxy-Dglucose (18F-FDG) has starred among the radiotracer cast of characters because of its recognition and sequestration by the intracellular enzyme hexokinase. Once ensnared, 18F-FDG breakdown creates positrons detected by diagnostic scanners that will display different positron intensities according to brightness and color. Thresholded 18F-FDG intensity has guided radiation planning cancer target contouring (1), and perhaps, has strengthened a conceptual relationship between cancer target motion and quiet breathing during on-beam radiation treatment [2]. Here, we obtained approval from Summa Akron City Hospital (Akron, Ohio) to review a user-defined 18F-FDG PET setting threshold of 40% of the standard uptake value maximum in a series of irradiated stage III lung cancer patients (Table 1). |
| Five patients underwent stereotactic lung radiosurgery to a dose of 50 Gy in five every other day fractions. Three multiphase CT image data sets were acquired on each patient for radiosurgery planning: a freebreathing scan, a moderate inspiration breath-hold scan, and a moderate expiration breath-hold scan. During the CT scan (voltage 120 kVp, 350 mAs; 64-slice Toshiba Aquilon LB, Toshiba Medical Systems), patients rested comfortably in head-first supine treatment position with their arms supported over their head. 18F-FDG PET datasets were acquired on a mobile scanner (Siemens Biograph 6.0, Siemens Healthcare) after an intravenous administration of 13 to 17 mCi of 18F-FDG. Scans in a patient head-first supine position were done by institutional protocol, acquiring 18F-FDG signal between the orbitomeatal line to the upper thighs. Co-registration of multiphase CT and PET datasets were done by a certified medical dosimetrist and verified by a certified medical physicist in the Pinnacle 9.0 planning system (Philips Medical Systems, Cleveland, Ohio). Physician contours included separate volumes for free-breathing (FB-CTV), end-inspiration (INS-CTV), and endexpiration (EXP-CTV), each delineated on pre-set chest CT window settings. Post-imaging fusion of CTV contours depicted the ‘extremes’ of lung tumor target motion in the respiratory cycle, creating a single image-guided internal target volume (ITV). Radiation treatment planning was conducted on the free-breathing CT data set, with the ITV expanded all around by 5-millimeters to generate a planning tumor volume (PTV). |
| Table 1 reports the tumor volume on the free-breathing CT scan, the 40% 18F-FDG PET volume, and the PTV. A representative tumor quiet breathing hysteresis loop encapsulated by the 40% 18F-FDG PET contour (red line) is depicted in Figure 1. To account for respiratory motion with CT data alone, the FB-CTV, INS-CTV, and EXP-CTV data sets are all needed—all exposing a patient to small-scale radiation dose from the three-step image acquisition process. Even with small radiation doses from the CT scan, consequential DNA damage in cells can be manifested [3]. In pilot data presented here, the 40% 18F-FDG PET threshold mapped well tumor hysteresis during the respiratory cycle (Figure 1). |
| With further study, the 40% 18F-FDG PET threshold might emerge as a robust surrogate for lung tumor hysteresis during quiet breathing. This would provide the advantage of accurate respiratory motion management and of eliminating multiphase CT scans that expose patients to additional radiation dose. Determining 18F-FDG PET thresholds is important as next generation radiation treatment machines have the capability of on-the-fly motion management. |
| One Oscar-worthy radiosurgical platform, the Exac Trac Vero system (BrainLAB AG, Feldkirchen, Germany) utilizes a pivoting robotic O-ring gantry mounted with a 6 MV, 500 cGy/minute linear accelerator capable of rotating 360° around a patient during treatment, putatively lowering total radiation dose exposure to pass-through normal tissues [4]. Treatment beams on the Vero are shaped by a multi-leaf collimator comprised of 60 single-focused 5 mm leaves made of tungsten alloy capable of full over-center-travel. Vero has a minimum single treatment field size of 10×10 millimeters and a maximum treatment field size of 150×150 millimeters. What has the radiation oncology field abuzz is that the linear accelerator and multi-leaf collimator are contained within a Gimbal mount, allowing tracking of and treatment of mobile tumors. Further development of the Gimbal mount will expand the treatment field size to 230×230 millimeters. Accurate tracking of tumors during quiet respiration by Vero ultimately may allow for lung treatment fields to narrowly approximate PTV contours, adapt nearly in real-time with respiratory motion, and permit radiation dose intensification. Formal motion management studies and clinical trials involving the Exac Trac Vero system are eagerly awaited. |
References |
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
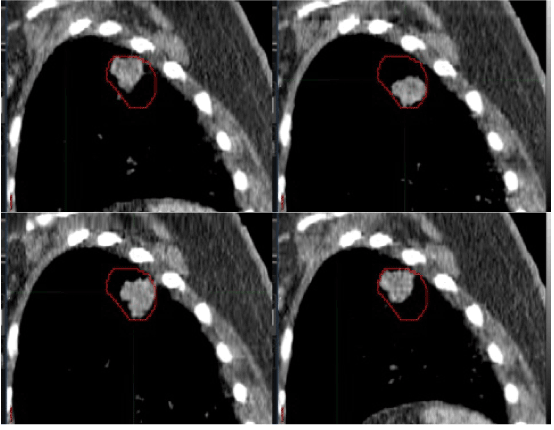 |
| Figure 1 |
Post your comment
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 13566
- [From(publication date):
January-2014 - Jul 16, 2025] - Breakdown by view type
- HTML page views : 8965
- PDF downloads : 4601
