Review Article Open Access
Low-level Laser Therapy Promotes Decrease in Inflammatory Process in Obese Trained Rats
de Aquino AE Jr.1,2*, de Castro CA3,4, Ana da Silva K3, Carbinatto FM1, Anibal FF2, Duarte ACGO3,4, Bagnato VS1,2 and Parizotto NA2,51Optics Group from Institute of Physics of São Carlos (IFSC), University of São Paulo (USP), São Paulo, Brazil
2Biotechnology Post Graduation Program, Federal university of São Carlos (UFSCar), São Paulo, Brazil
3Physiological Science Post Graduation Program, Federal University of San Carlos, São Paulo, Brazil
4Laboratory of Nutrition and Metabolism Applied to Exercise, Physical Education and Motor Human Department, Federal University of San Carlos (UFSCar), São Paulo,Brazil
5Electrothermophototherapy Laboratory, Department of Physical Therapy, Federal University of San Carlos, São Paulo, Brazil
- *Corresponding Author:
- Antonio Eduardo de Aquino Jr., PhD
University of São Paulo, Institute of Physics of São Carlos (IFSC)
Trabalhador São Carlense Avenue
400, Pq Arnold Shimidt, São Carlos, São Paulo, Brazil
Tel: 55 (016) 3373 9810
E-mail: antonioaquino@ursa.ifsc.usp.br
Received date: Feb 02, 2016; Accepted date: Apr 09, 2016; Published date: April 25, 2016
Citation: de Aquino AE Jr., de Castro CA, Ana da Silva K, Carbinatto FM, Anibal FF, et al. (2016) Low-level Laser Therapy Promotes Decrease in Inflammatory Process in Obese Trained Rats. J Community Med Health 6:414. doi:10.4172/2161-0711.1000414
Copyright: © 2016 Junior AEA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Community Medicine & Health Education
Abstract
On present days, the obesity is present in 20% of the world. The combined use of low-level laser therapy and exercise may potentiate the effects promoted by exercise. The objective of this study was to investigate the conjugated effects of exercise and phototherapy on inflammatory markers. The 64 rats was used, which were divided into eight groups (n=8): normocaloric diet groups - sedentary normal diet; swimming trained normal diet; sedentary normal diet plus laser; swimming trained normal diet plus laser; and high-fat diet groups - sedentary highfat diet; swimming trained high-fat diet; sedentary high-fat diet plus laser; and trained high-fat diet plus laser . The rats were submitted to swimming training for 90 minutes/5 times per week for 8 weeks and low-level laser therapy (GA-Al-AS, 830 nm) at a dose of 4.7 J/point with a total energy of 9.4 J/animal, the point of application was gastrocnemius muscles after exercise. We observed alterations in inflammatory makers: reduction of IL-6 in the group sedentary normal diet versus swimming trained normal diet; and sedentary high-fat diet versus swimming trained high-fat diet; increase of IL-4 in the group sedentary normal diet versus swimming trained normal diet; reduction of TNF-α in the group sedentary normal diet versus and swimming trained normal diet; and increase sedentary high-fat plus versus sedentary high-fat diet plus laser; increase of IL-10 in the group sedentary normal diet versus swimming trained normal diet; and swimming trained high-fat diet versus trained high-fat diet plus laser; and reduction swimming trained normal diet versus swimming trained high-fat diet. All comparisons were significant to p<0.05. We concluded that this intervention promotes reduction of the inflammatory process. Thus, the reduction of the inflammatory process is extremely beneficial for the whole body.
Keywords
Exercise; Phototherapy; Obesity; Inflammatory factors; Rats
Introduction
The increase of obese people worldwide is a matter of global concern. This disease is qualified as the century epidemic known like subclinical chronic inflammatory disease, due to changes observed in several inflammatory markers. The 95% of cases of obesity are of exogenous origin. The exogenous obesity indicates that the excessive intake of calories and reduced daily physical activity have been the cause of many cases of obesity, increasing both in adults and in children [1].
The presence of pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α occurs after trauma or pathogenic invasion of the organism. In the obesity situation, the levels of pro-inflammatory adipokines showed increase, like IL-6 [2,3]. IL-10 has been considered regulatory of associated production events that contribute to the balance between the 2 subgroups of lymphocytes: Th1 and Th2. The IL-4 is related to some chronic diseases associated with the presence of inhibitory effects of the inflammatory response, especially in chronic cases, in addition to characteristics of Th2 immune response.
The homeostasis subject is linked to the balance of the immune response against both pathogens, as in cases of pathophysiological, associated or not with factors such as obesity. Cytokines associated with cells and inflammatory mediators can modulate the inflammatory response profile in different models of inflammation and in part attenuate the symptoms that characterize this response.
The determination of the signs and symptoms involved in different inflammatory processes also depends on endocrine factors present and ingested over diet nutrients, which will contribute significantly to activation of the immune response. Therefore, understanding the immune response and the determination of the cytokine profiles are relevant factors that favor greater understanding of the inflammatory process during different therapeutic procedures.
However, excess of those cytokines characterize, many times, more severe frameworks and contribute to a worsening of symptoms in the immune response. The biological context and action of cytokines often favors the improvement or worsening of an inflammatory process. Therefore, it is important to evaluate the effects of therapeutic laser effects on some systemic cytokines and their anti-inflammatory effect within an organism with high calorie diet.
It is known that diet nutrients have great potential to modulate the production of cytokines. Currently, we seek tools that can effectively modulate the effects of overweight subjects, particularly the factors that contribute to decreased quality of life of these individuals. The literature shows the relation between subclinical inflammation and type-2 diabetes. This form, it is important to study non-invasive therapeutic tools that control the harmful effects of obesity.
The exercise goal has many features that can modulate the brain function and have wide effects on total brain health [4]. The important mechanism mediating these benefits of exercise on the brain is influence of the growth factors and cascades of this factor, common mechanism subjacent the systemic effects of exercise that might be related to inflammation. This fact can impair growth factor signaling both systematically as in the brain. The exercise ensures both successful brain function as help in homeostasis maintenance [4].
The binding between metabolism and immune response plays a main role in the progress of obesity-associated chronic diseases. The immune function affected by obesity involves impairment of both the innate and adaptive immune system [5]. This leads to increased risk of infections as well as chronic inflammation.
Thus, we have by hypothesis that Low Level Laser Therapy (LLLT), associated to aerobic training of moderate intensity may decrease inflammation status in obese animals, decreasing pro-inflammatory interleukins and increasing anti-inflammatory interleukins. The aim of this study was to investigate the effects of LLLT associated with moderate-intensity aerobic exercise on inflammatory status in obese rats.
Material and Methods
Animal care
All experiments were conducted according to the policy of Guide for care and use of laboratory animals (National Research Council 1998). Sixty male 90 day-old Wistar rats from the biotery of the Federal University of São Carlos (UFSCar), São Paulo, Brazil, were used.
The animals were kept in individuals polypropylene cages, at a constant temperature of 22°C ± 2°C, and cycle of 12 hours light/ darkness. During the all experiment period, the rats received standard rat chow (MP-77; Primor®, São Paulo, Brazil) and tap water ad libitum. The ethics committee on animal experimentation of the UFSCar, protocol n° 067/2010 approved the research.
Experimental groups
Sixty-four male Wistar rats (90 days old with weighted 317.00 ± 19.16 g) were used in this study. Before beginning the protocol, the groups [except for the normocaloric groups (N)] were fed ad libitum with the high-fat diet (H) for 3 weeks [6] for the development of dyslipidemia and obesity.
The animals were randomized according to diet into eight groups with eight rats each (n=08): normocaloric diet groups (N) - sedentary normal diet (SN); swimming trained normal diet (TN); sedentary normal diet plus laser (SNL); and swimming trained normal diet plus laser (TNL); and high-fat diet groups (H) - sedentary high-fat diet (SH);swimming trained high-fat diet (TH); sedentary high-fat diet plus laser (SHL), and trained high-fat diet plus laser (THL). Rats were kep tone per cage containing food and water ad libitum (8 weeks), on a 12: 12 h light/dark cycle at 23°C ± 1°C.
Normocaloric diet
The experimental groups received the following alimentation: the normocaloric diet (N)—MP-77 standard rat chow diet provided in pellet form (Primor®, São Paulo, Brazil) containing 23 g of protein, 4 g of total fat, 49 g of carbohydrate, 7 g of ash, 5 g of fiber, and 6 g of vitamins per 100 g.
High-fat diet
The experimental groups received the standard rat chow diet (MP-77; Primor®, São Paulo, Brazil) in the pellet form, containing 23 g of protein, 4 g of total fat 49 g of carbohydrates, and 5 g of fiber per 100 g of diet.
The high-fat diet was composed of standard rat chow plus peanuts, milk chocolate, and sweet cookies in a proportion of 3:2:2:1. This diet contained 20 g of protein, 20 g of total fat, 48 g of carbohydrate, and 4 g of fiber per 100 g of diet.
The caloric density of the diets was measured with an adiabatic calorimeter (IKA-C400), and the values were: 5.12 kcal/g for the highfat diet and 4.07 kcal/g for the chow diet [7]. All components were ground and blended and offered to rats in the pellets form.
Swimming training
The swimming training program was conducted by previous described by Sene-Fiorese [8]. The training occurs in individual tanks with water, maintained at 28–32°C. The rats of the trained groups swam for 30, 60, and 90 min on the first, second, and third days to correct adaption.
The swimming time was then increased to 90 min/day, during 5 days/week. All rats receive the load (attached to the trunk by a jacket) of 3-5% of body mass to swim. The protocols were performed for 5 days/week during 8 weeks. This program is named to be of moderate intensity [8].
LLLT protocols
The LLLT parameters are shown in Table 1. It was irradiated transcutaneous way on the muscles of the rat’s paw (one point on quadriceps and other point on gastrocnemius). The quantity of energy density of laser irradiation and the anatomical points were chosen based on previous studies realized by our laboratory [9].
| Type | Ga-Al-As | Energy density | 1.66 J/cm2 |
|---|---|---|---|
| Wavelength | 830 nm (infrared) | Time | 47 s |
| Frequency | Continuous wave (CW) | Total energy | 9.4 J |
| Optical output | 100 mW | Power density | 35.36 W/cm2 |
| Spot diameter | 0.6 mm | Energy per point | 4.7 J/point |
Table 1: Low-level laser therapy (LLLT) parameters.
We decided not to use sham group because we have a positive control [8], a negative control (hypercaloric diet and not treated with LLLT), as well as positive (hypercaloric) and negative (normocaloric) controls for the factor diet, with or without laser intervention.
The laser was applied after the exercises because the advantage of using the stress induced to get the maximum of absorption and effects on metabolism. This protocol before was used in different papers from our laboratory [10].
Preparation of Samples
The animals were euthanized by decapitation at the end of 8 weeks of training and after a 24 h rest period. The collected blood was immediately centrifuged and frozen at -80°C. The gastrocnemius muscles (GAST) were immediately removed, weighted, and frozen at -20°C.
Measurement of cytokines
To determine serum cytokine levels were analyzed according to Machado et al. [11]. The serum samples were homogenized, briefly (Ultra-Turrax T8; IKA-Werke, Staufen, Germany) in 1.5 ml of RPMI medium, centrifuged at 1500 g, filtered, and stored at -70°C before analysis. Commercially available enzyme linked immunosorbent assay (ELISA) kits were used to measure IL-4, IL-6, IL-10 and TNF-α according to the manufacturer’s instructions (BD Pharmingen). Sensitivities were >10 pg/ml.
Statistical analysis
All data were expressed as a mean and ± standard deviation. The Kolmogorov–Smirnov test was used to analyze the normality. For statistical evaluation of the metabolic parameters, a one-way ANOVA test with post hoc analysis (Tukey–Kramer multiple comparisons) was used between groups. Statistical analysis was performed using SPSS for Macintosh (version 20, SPSS Inc. Chicago, IL, USA). Statistical significance was set at p < 0.05 levels.
Results
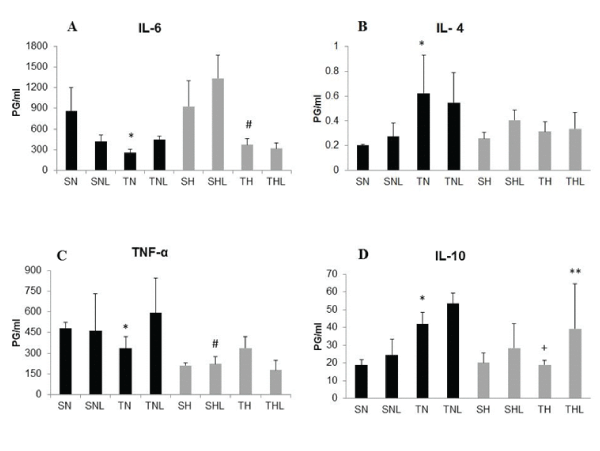
The Figure 1 demonstrated the cytokines levels in serum in this model. Figure 1A show decrease of IL-6 level in serum submitted of aerobic training feed with normal diet (TN) compared with sedentary normal rats (SN) (p < 0.001). A similar result was observed to obese trained group (TH) compared with sedentary obese rats (SH) (p < 0.001).
Figure 1: Circulating (serum) cytokine levels, A- IL 6; B- IL- 4, CIL- 10 and D-TNF-α. Cytokine levels were determined by ELISA analysis. Data are expressed as mean ± SD. Sedentary normal diet (SN); Swimming trained normal diet (TN); Sedentary normal diet plus laser (SNL); Swimming trained normal diet plus laser (TNL); Sedentary high-fat diet (SH); Swimming trained high-fat diet (TH); Sedentary high-fat diet plus laser (SHL); Trained high-fat diet plus laser (THL). The letter superscript allows the comparison between groups: * SN v SNL / SN v TN / SN v SH; # SH v SHL / SH v TH; +TN v TH / TN v TNL; ** TH v THL, represented statistical difference (p < 0.05).
To IL-4 level in serum, Figure 1B, we observed that the animals, which were submitted to aerobic training (TN) was bigger compared in relation sedentary rats, fed with normal diet (SN) (p < 0.001).
The aerobic training was able to decrease the TNF-α (Figure 1C) in normal group (TN) compared with sedentary normal group (SN) (p < 0.05). In the sedentary obese animals, the irradiation of LLLT (SHL) was able to increase the TNF-α (p < 0.05) compared with sedentary obese rats (SH).
In our study, we observed that, the normal animals that swam (TN) increase IL-10 levels in serum (p < 0.001) compared with animals that did not training (SN). For the animals that were obese (TH) the training was not capable to increase the IL-10 levels in serum, compared with normal trained animals (TN) (p < 0.001), however, the LLLT, associated with aerobic training on obese animals, was able to increase the IL-10 level in serum (p < 0.001). Although not statistically different, the LLLT was able to increase the values of this anti inflammatory cytokine to near normal values trained group (Figure 1D).
Discussion
In our study, we evaluated the efficiency of the moderate-intensity aerobic exercise and the LLLT in reducing possible serum interleukins in normal and obese trained rats. In previous study, we showed the obesity condition in experimental study [12]. Our results showed how the aerobic exercise by swimming and LLLT can be useful as a nonpharmacological treatment against obesity and decrease inflammatory cytokines production. The increased accumulation of lipids in the obesity can promote an inflammatory injury and feature as type 2 diabetes and cardiac diseases [13,14].
It is known that TNF-α [13,14] stimulates the production of IL-1 (Figure 1B) and IL-6 during inflammatory process and that these cytokines are of great importance to maintenance of inflammation [15] and interfere with the activation of macrophages [16], mainly peritoneal macrophages. This effect promoted a balance in proinflammatory cytokines, regulating the production of IL-6 (Figure 1A) and mainly TNF-α (Figure 1C), during the evaluation of the effects of photodynamic therapy in this model. Elevated levels of IL-6 in obese subjects significantly increased risk of severe cardiovascular disease, thereby increasing morbidity and mortality that involve the patients, mainly due to the progressive atherosclerosis [17]. Therefore, the control of these factors contributes significantly to improving the survival of the [13,14] individuals. Furthermore, it is known that increase in IL-6 can promote coronary acute onset of instability. Thus, the laser effect in decreasing TNF-α indirectly regulates the production of IL-6, contributing to reduce the risks associated with injuries promoted by atherosclerotic plaques.
The literature shows that not only the immune cells are able to produce and secrete cytokines, but other cell types present in the visceral and subcutaneous adipose tissue may also be substantial sources of cytokines (adipokines). Thus, the increase in this type of tissue may contribute significantly to the production of IL-1, IL-6 and TNF-α, contributing to harmful changes promoted by the presence of these cytokines [18], associated with changes in the function of the immune response [19].
Contrary, no significant differences in values of IL-4 (Figure 1B) were observed, suggesting that the laser did not stimulate Th2 response in these animals. This results have shown that photodynamic therapy when associated low intensity laser seems to regulate the levels of IL-10 (Figure 1D), especially in animals that received hypercaloric diet. These results suggest a compensatory effect for maintaining the immune balance in this experimental model, as that in animals treated with laser levels; Moreover, of IL-10 significantly increase the balance of Th1 versus Th2 in the immune response, independently of calorie intake. Some authors have shown that injection of IL-10 in the hypothalamus of obese animals was responsible for the improvement in insulin action and leptin, as these animals have been favored a smaller intake of food, satiating the hunger of these animals and indirectly contributing to the control of weight [20]. Thus, our results show that the use of laser can contribute directly and indirectly in weight control, favoring the individuals who use laser therapy increased serum IL-10. Consequently, this study contributes to better understanding of the effects of laser therapy on immune response in individuals with obesity.
This modulation of the immune response may be related to lower oxidative damage [21]. This form, it is suggested that biochemical modulation after application of LLLT is attributable to reduction in the extent of inflammatory responses.
Conclusion
The study showed a improvement of the inflammatory process when analyzed the association between LLLT and moderate-intensity aerobic exercise in obese rats, which is extremely beneficial for the organism.
Acknowledgement
This article was supported by Grants Institute of Physics of São Carlos (IFSC), University of São Paulo (USP, São Paulo, Brazil).
References
- Aquino AE, Sene-Fiorese M, Paolillo FR, Duarte FO, Oishi JC, et al. (2013) Low-level laser therapy (LLLT) combined with swimming training improved the lipid profile in rats fed with high-fat diet. Lasers Med. Sci 28: 1271–1280.
- Da Silva PL, De Mello MT, Cheik NC, Sanches PL, Correia FA, et al. (2012) Interdisciplinary therapy improves biomarkers profile and lung function in asthmatic obese adolescents. PediatrPulmonol 478-517.
- Pereira SS, Teixeira LG, Aguilar EC, Matoso RO, Soares FLP, et al. (2012) Differences in adipose tissue inflammation and oxidative status in C57BL/6 and ApoE-- mice fed high fat diet. AnimSci J 83: 549-555.
- Cotman CW, Berchtold NC, Christie LA (2007) Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30: 464-472.
- Kanneganti TD, Dixit VD (2012) Immunological complications of obesity. Nat Immunol 13: 707-712.
- Duarte FO,Sene-fiorese M, Cheik NC, Jr DA, Oishi JC, et al. (2012) Food restriction and refeeding induces changes in lipid pathways and fat deposition in the adipose and hepatic tissues in rats with diet-induced obesity. 7: 882-894.
- Speretta GFF, Rosante MC, Duarte FO, Leite RD, Lino ADDS, et al. (2012) The effects of exercise modalities on adiposity in obese rats. Clinics (Sao Paulo) 67: 1469-1477.
- Sene-fiorese M, Duarte FO, Scarmagnani FRR, Cheik NC, Manzoni MSJ, et al. (2008) Efficiency of Intermittent Exercise on Adiposity and Fatty Liver in Rats Fed With High-fat Diet pp: 1-6.
- Syn WK, Yang L, Chiang DJ, Qian Y, Jung Y, et al. (2009) Genetic differences in oxidative stress and inflammatory responses to diet-induced obesity do not alter liver fibrosis in mice. Liver Int 29: 1262-1272.
- de Brito Vieira WH, Ferraresi C, Perez SEA, Baldissera V,Parizotto NA (2012) Effects of low-level laser therapy (808nm) on isokinetic muscle performance of young women submitted to endurance training: a randomized controlled clinical trial. Lasers Med Sci 27: 497-504.
- Machado ER, Ueta MT, de Gonçalves-Pires MDRF, de Oliveira JBA, Faccioli LH, et al. (2003) Strongyloidesvenezuelensis alkaline extract for the diagnosis of human strongyloidiasis by enzyme-linked immunosorbent assay. MemInstOswaldo Cruz 98: 849-851.
- Masquio DCL, De Piano A, Sanches PL, Corgosinho FC, Campos RMS, et al. (2013) The effect of weight loss magnitude on pro-/anti-inflammatory adipokines and carotid intima-media thickness in obese adolescents engaged in interdisciplinary weight loss therapy. ClinEndocrinol (Oxf) 79: 55-64.
- Wellen KE, Hotamisligil GS (2003) Obesity-induced inflammatory changes in adipose tissue. 112: 1785-1788.
- Hung MC, Ong SH, Ee JL, Chung M, Hong S, et al. (2011) The Influence of Obesity on Postoperative Inflammatory Cytokine Levelspp: 2370-2378.
- Trayhurn P, Wood IS (2004) Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92: 347.
- Oliver E, McGillicuddy F,Phillips C, Toomey S, Roche HM (2010) The role of inflammation and macrophage accumulation in the development of obesity-induced type 2 diabetes mellitus and the possible therapeutic effects of long-chain n-3 PUFA. ProcNutrSoc 69: 232.
- Mangge H, Almer G, Schmidt A,Gasser R, Fuchs D (2010) Inflammation , Adiponectin , Obesity and Cardiovascular Risk pp: 4511-4520.
- Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, et al. (2005) Adiponectin induces TNF-α and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. BiochemBiophys Res Commun 335: 1254-1263.
- Packard RRS, Lichtman AH, Libby P (2009) Innate and adaptive immunity in atherosclerosis. SeminImmunopathol 31: 5-22.
- Ropelle ER, Flores MB, Cintra DE, Rocha GZ, Pauli JR, et al. (2010) IL-6 and IL-10 Anti-Inflammatory Activity Links Exercise to Hypothalamic Insulin and Leptin Sensitivity through IKKβ and ER Stress Inhibition. PLoSBiol 8: e1000465.
- Silveira PCL, Silva LA, Freitas TP, Latini A, Pinho RA (2011) Effects of low-power laser irradiation (LPLI) at different wavelengths and doses on oxidative stress and fibrogenesis parameters in an animal model of wound healing. Lasers Med Sci 26: 125-131.
Relevant Topics
- Addiction
- Adolescence
- Children Care
- Communicable Diseases
- Community Occupational Medicine
- Disorders and Treatments
- Education
- Infections
- Mental Health Education
- Mortality Rate
- Nutrition Education
- Occupational Therapy Education
- Population Health
- Prevalence
- Sexual Violence
- Social & Preventive Medicine
- Women's Healthcare
Recommended Journals
Article Tools
Article Usage
- Total views: 11010
- [From(publication date):
April-2016 - Apr 11, 2025] - Breakdown by view type
- HTML page views : 10146
- PDF downloads : 864