Research Article Open Access
Low-Frequency, Whole Body Vibration Induced Neurite Outgrowth by Pc12m3 Cells with Impaired Nerve Growth Factor-Induced Neurite Outgrowth
| Yoshihisa Koike1*, Reiko Tutida1, Yuko Hayashi1, Yoko Yamanishi1 and Yoshio Kano2 | |
| 1Department of Occupational Therapy, Faculty of Health and Welfare, Prefectural University of Hiroshima, Mihara City, Hiroshima 723-005, Japan | |
| 2Department of Occupational Therapy, School of Health Science, Kibi international University, 8 Iga-machi Takahashi City, Okayama 716-8508, Japan | |
| Corresponding Author : | Yoshihisa Koike Department of Occupational Therapy Faculty of Health and Welfare Prefectural University of Hiroshima, Mihara City Hiroshima 723-005, Japan Tel: +81-848-60-1212 E-mail: koike@pu-hiroshima.ac.jp |
| Received October 30, 2014; Accepted February 05, 2015; Published February 12, 2015 | |
| Citation: Koike Y, Tutida R, Hayashi Y, Yamanishi Y, Kano Y (2015) Low-Frequency, Whole Body Vibration Induced Neurite Outgrowth by Pc12m3 Cells with Impaired Nerve Growth Factor-Induced Neurite Outgrowth. J Nov Physiother 5:249. doi: 10.4172/2165-7025.1000249 | |
| Copyright: © 2015 Koike Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Novel Physiotherapies
Abstract
Purpose: To investigate the effects of very low-frequency whole body vibration (WBV) stimulation using PC12 m3 cells.
Methods: Mutant, drug-hypersensitive, PC12 m3 cells were obtained by means of continuous culture of neural PC12 cells and then stimulated by low-frequency vibration at frequencies of 7–13 Hz for 5–20 min with simultaneous exposure to nerve growth factor (NGF).
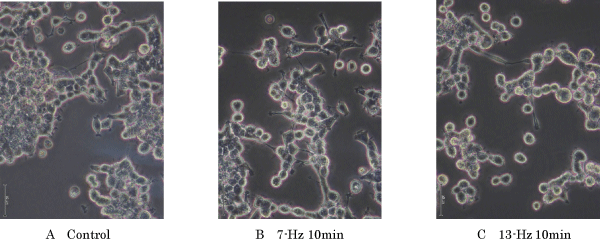
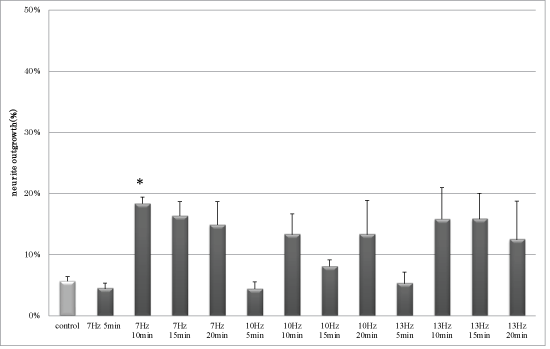
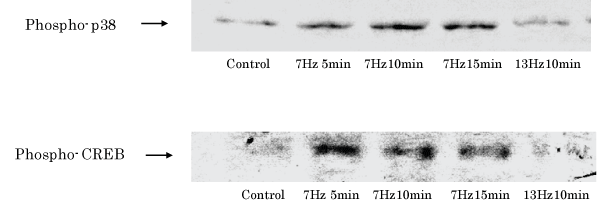
Results: The frequency of neurite outgrowth by PC12 m3 cells induced by 7-Hz vibratory stimulation was approximately 3-fold greater than that induced by NGF alone. Moreover, activated cyclic-AMP responsive element (CRE)-binding protein (CREB) expression was induced in PC12 m3 cells stimulated by 7-Hz vibration.
Conclusion: Low-frequency vibratory stimulation induces neurite outgrowth via a p38 mitogen-activated protein kinase/CREB signaling pathway in PC12 m3 cells. Together, these results suggest that WBV is an efficient method to stimulate neurite growth.
Figures at a glance
 |
 |
 |
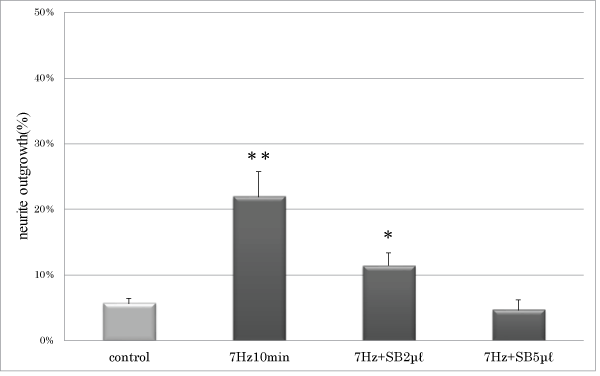 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Electrical stimulation
- High Intensity Exercise
- Muscle Movements
- Musculoskeletal Physical Therapy
- Musculoskeletal Physiotherapy
- Neurophysiotherapy
- Neuroplasticity
- Neuropsychiatric drugs
- Physical Activity
- Physical Fitness
- Physical Medicine
- Physical Therapy
- Precision Rehabilitation
- Scapular Mobilization
- Sleep Disorders
- Sports and Physical Activity
- Sports Physical Therapy
Recommended Journals
Article Tools
Article Usage
- Total views: 15057
- [From(publication date):
February-2015 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 10398
- PDF downloads : 4659
