Research Article Open Access
Low Serum 25(OH)D Levels in Parkinson Disease; a Non Specific Marker of Neurodegeneration?
Olivola Enrica1, Cerroni Rocco1, Conti Marco1, Pierantozzi Mariangela1, Liguori Claudio2 and Stefani Alessandro1*
1Movement Disorders Centre, Department of Systems Medicine, University of Rome “Tor Vergata”, Italy
2Sleep Disorders Centre, Department of Systems Medicine, University of Rome “Tor Vergata”, Italy
- Corresponding Author:
- Alessandro Stefani
Department of Systems Medicine
University of Rome, “Tor Vergata”, Rome, Italy
Tel: +39-6-20903115
Fax : 39620903118
E-mail: stefani@uniroma2.it
Received date: October 19, 2015; Accepted date: December 07, 2015; Published date: December 14, 2015
Citation: Enrica O, Rocco C, Marco C, Mariangela P, Claudio L, et al. (2015) Low Serum 25(OH)D Levels in Parkinson’s Disease; a Non Specific Marker of Neurodegeneration? J Alzheimers Dis Parkinsonism 5:200. doi: 10.4172/2161-0460.1000200
Copyright: © 2015 Enrica O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Objective: Several investigations have recently inferred that a low serum vitamin D concentration may contribute to cognitive impairment in the elderly. Here, we assessed whether in a cohort of Parkinson’s disease (PD) patients attributable to any disease stage a deficit in serum 25-hydroxyvitamin D (25(OH) D) levels occurred as previously proposed and, more importantly, if it is correlated with cognitive impairment or disease duration. Methods: Our PD group (n=54) was compared to an age-matched control group (n= 30), but also to patients afflicted by Alzheimer’s disease (AD) (n= 17) and motor neuron disease (amyotrophic lateral sclerosis - ALS) (n=19). Results: Serum 25(OH)D levels > 30 ng/ml was found in 37% of PD patients (versus 76% in controls); in 34 out of 54 PD patients low 25(OH)D levels occurred (28,73 ± 20,22 ng/ml vs 44,52 ± 18,48 ng/ml in control group); however, no correlation with mini mental state examination (MMSE) or disease progression emerged in PD patients. AD cohort was characterized by a significantly lower vitamin D concentration (half manifesting severe deficiency below 10 ng/ml, in contrast with 18% amongst PD). To note, also in ALS, pathological low levels of vitamin D, comparable to PD, was found. Conclusion: Our findings highlight that vitamin D insufficiency or deficiency is shared by multiple neurodegenerative diseases, albeit a-specifically. In PD, 25(OH) D levels do not correlate with cognitive performance. On one hand, these data confirm the opportunity to restore vitamin D levels in a variety of neurological disorders; on the other, they suggest that, in PD, at contrast with AD, vitamin D insufficiency should not play a relevant role in influencing disease severity or unmasking an associated dementia.
Keywords
Parkinson’s disease; Vit D levels; Alzheimer’s disease; Motor neuron disease; Cognitive performance
Introduction
In recent years, several studies have been focused on the extraskeletal effects of vitamin D, which is intended as a steroid hormone, exerting cardiovascular, immunomodulatory, anti-tumoral effects in human body by regulating the expression of hundreds of genes through both genetic and epigenentic mechanisms [1-4]. Furthermore, growing evidence highlight the possible role of vitamin D in neuroprotection through neuronal calcium regulation, antioxidative pathway, immunomodulation and detoxification [5,6]. On the other hand, vitamin D deficiency may correlate with neuroinflammatoryautoimmune cascades, cerebrovascular lesions and, most recently, with neurodegenerative diseases [7-11].
In the context of a growing interest in biomarkers of neurodegenerative diseases [12,13] the correlation between vitamin D deficiency and neurodegeneration is becoming the real hot topic. Scientific literature in the last years emphasized the correlation between Alzheimer Disease (AD) and low levels of vitamin D. Recent meta-analyses confirmed that low serum vitamin D concentrations are associated with the impairment of specific cognitive domains, such as memory and executive functions [14,15]. Van der Schaft and colleagues conducted a systematic review including 25 cross-sectional and 6 prospective studies showing that 72% of the studies significantly exhibited either worse outcome on one or more cognitive function tests or a higher frequency of dementia if lower 25-hydroxyvitamin D (25(OH)D) levels or insufficient vitamin D intake occurred [16]. Llewellyn and coauthors showed an inverse relationship between serum 25(OH)D levels and cognitive impairment [17]. A large, prospective, population-based study conducted to examine the relationship of dementia and AD with vitamin D concentration confirmed that vitamin D deficiency is associated with a substantially increased risk of all-cause dementia and AD [18]. These data candidate vitamin D deficiency as a predictor factor for dementia.
The relationship between vitamin D and Parkinson Disease (PD) is less unequivocal. Previous studies have shown a strong correlation between motor dysfunction and vitamin D shortage (outdoor activity and total vitamin D intake are inversely associated with PD [19] but no conclusive evidences link cognitive dysfunctions and low vitamin D levels. A cross-sectional and longitudinal case-control study of 388 patients (mean Hoehn and Yahr stage of 2.1 ± 0.6) and 283 control subjects showed an increased frequency of vitamin D deficiency in PD patients compared to controls and a correlation of low 25(OH)D levels with higher total Unified Parkinson’s Disease rating Scale (UPDRS) scores at baseline and during follow up [20]. By the psycho-cognitive side, a longitudinal study suggested that higher plasma vitamin D level is associated with better cognition and better mood in PD patients without dementia [21].
The aim of our study was to verify if vitamin D deficiency is a peculiar element of PD, as already assessed in AD, or if it is a more general biomarker of senescence and neurodegenerative processes, as suggested by studies focused over others degenerative diseases like myotonic dystrophy type 1 [22] or multiple sclerosis (MS) [23,24]. To our purpose we compared a population of PD patients not only with healthy controls, but also with AD patients and amyiotrophic lateral sclerosis (ALS) patients. In PD patients, we also investigated whether an inverse relationship between serum 25(OH)D levels and mini mental state examination (MMSE) took place, as already demonstrated in AD patients.
Materials and Methods
One hundred twenty patients were included in the study: 54 with PD, 30 age-matched normal subjects and for comparative purposes a group of 17 patients with AD and 19 patients with ALS. Hepatic dysfunction, renal insufficiency and vitamin D supplementation were considered exclusion criteria. All subjects provided informed consent and underwent evaluation including a general neurological examination, laboratory tests and neuropsychological assessments MMSE. Serum 25(OH)D was measured by radioimmunoassay in all participants. All samples were collected during the same season. In PD group the disease severity was evaluated using the Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) and Hoehn and Yahr stage (H&Y). Vitamin D levels > 30 ng/ml were considered as normal, levels of 20–30 ng/ml represented insufficiency, whereas levels below 20 and 10 ng/ml represented respectively deficiency and severe deficiency [25,26].
Statistics
Results are expressed as mean ± SD or as number (percentages). Significant differences in demographic and vitamin D levels among groups were separately analyzed by performing Kruskal–Wallis test,then the Mann–Whitney U test was performed to compare significant Kruskal–Wallis results. Correlation coefficient (Spearman) was calculated to assess the relationship between variables. Statistical significance was defined as p values lower than 0.05.
Results
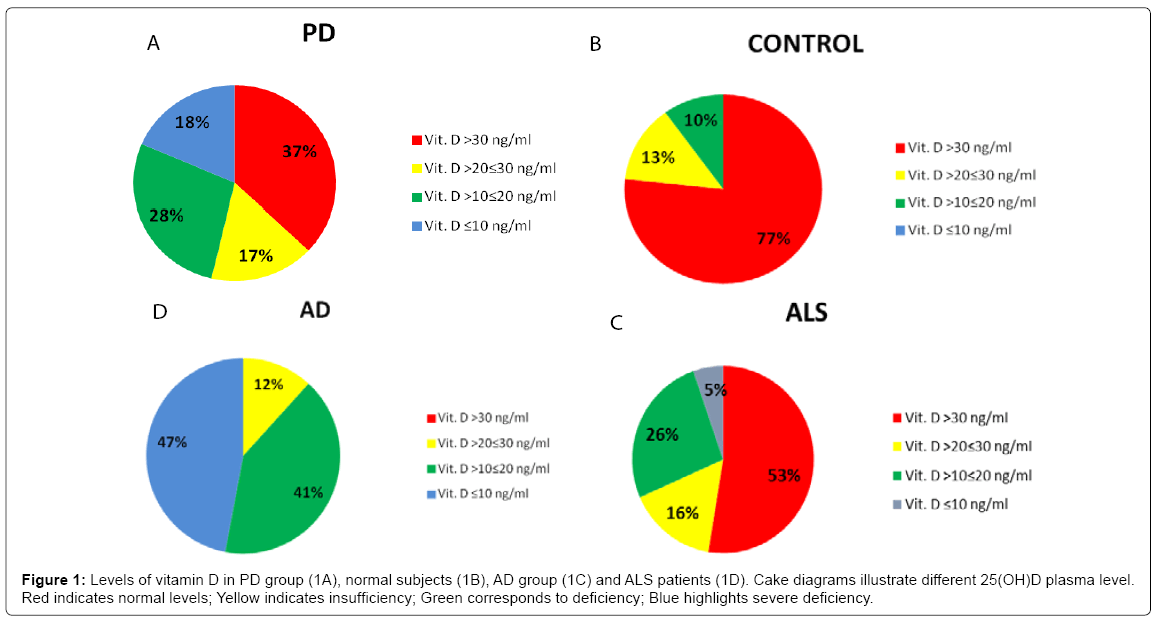
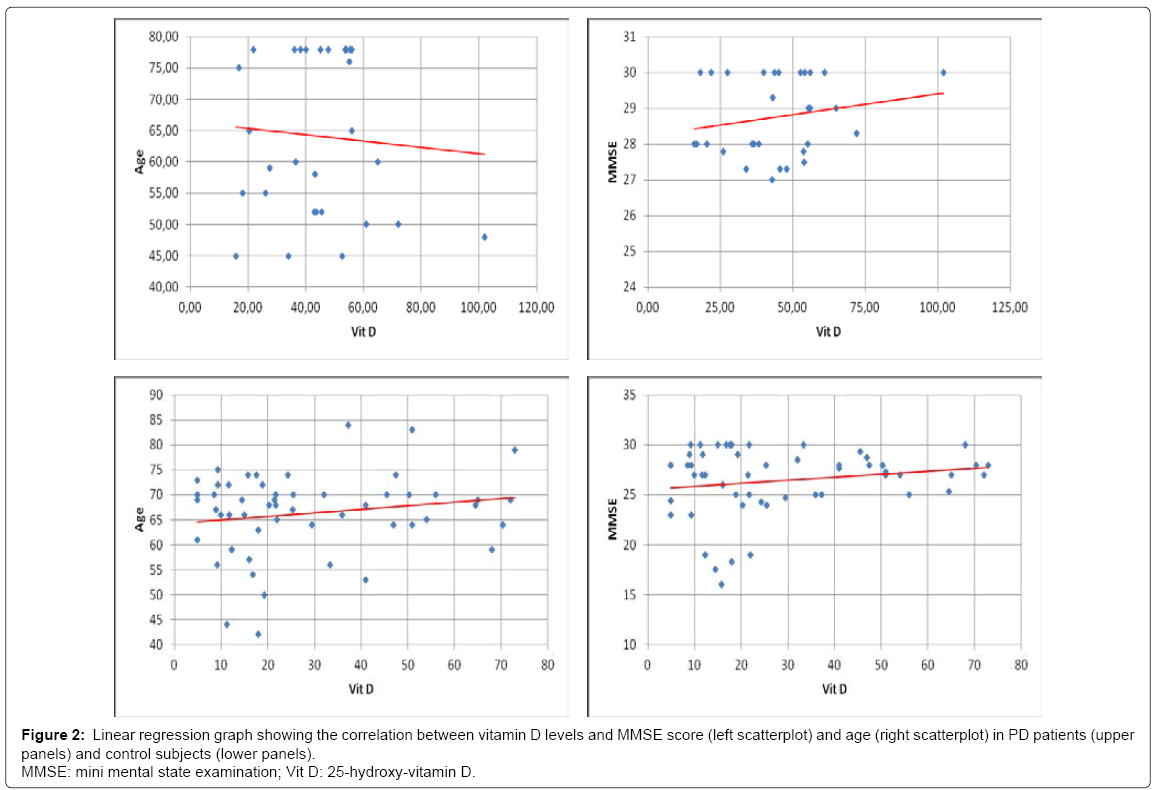
No significant differences in age and distribution of male/female ratio were observed comparing the studied groups (demographic data and serum 25(OH)D level are shown in (Table 1). The Kruskal- Wallis test showed significant differences in vitamin D levels among groups (p < 0,001). The PD group showed significant lower serum vitamin D levels compared to the controls (p < 0,001), with a mean value of 28,73 ± 20,22 ng/ml (vs 44,52 ± 18,48 ng/ml). Moreover, 34 PD patients (nearly 60%) showed levels of vitamin D below 30 ng/ml, in particular 9 patients out of the 34 exhibited levels of vitamin D between 20 and 30 ng/ml (insufficiency), 15 had levels between 10 and 20 ng/ ml (deficiency), whereas the remaining 10 showed vitamin D lower than 10 ng/ml (severe deficiency). Figures 1A and 1B illustrate cake diagrams of the vitamin D levels in the two groups. No difference in vitamin D levels was observed between male and female either in PD or control subjects; no correlation between age and vitamin D levels was observed in PD or control subjects (R=0,18 p=0,19 in PD; R=-0,07 p=0,70 in controls). Furthermore, in both groups vitamin D levels were not correlated with MMSE score (R=0,17 p=0,20 in PD; R=0,19 p=0,29 in control group) (Scatterplots are shown in Figure 2). In addition, there were no significant difference in vitamin D levels dividing PD patients for age (below and above 70 years), gender, MMSE score and disease severity as measured by Hoehn & Yahr (H&Y) stages: no significant difference in levels of vitamin D were found comparing PD patients with H&Y < 2 (n=14) and H&Y ≥2 (n=40) and comparing patients with MMSE ≤ 20/30 (n=5) and MMSE >20/30 (n=49) (Table 2).
| PD | CTR | AD | ALS | |
| n | 54 | 30 | 17 | 19 |
| Male/Female | 30/24 | 16/14 | 7/10 | 11/8 |
| Age (Years) | 66,29 ± 8,21 | 64,17 ± 12,92 | 69,82 ± 5,20 | 63,95 ± 5,27 |
| Vit D (ng/ml) | 28,73 ± 20,22 | 44,52 ± 18,48 | 12,13 ± 4,77 | 31,37 ± 4,55 |
Table 1: Demographic data and vitamin D levels of studied groups. Values are expressed as means ± standard deviation or numbers.
| Vit D ng/ml, mean ± SD (range) | p value | |
| H&Y < 2 (n=14) | 33,53 ± 20,89 (4,9-65) |
0,35 |
| H&Y ≥2 (n=40) | 27,05 ± 19,98 (4,9-73) |
|
| Age < 70 y (n=34) | 29,01 ± 20,76 (4,9-72) |
0,9 |
| Age ≥ 70 y (n=20) | 28,24 ± 19,78 (4,9-73) |
|
| MMSE ≤ 20/30 (n=5) | 16,52 ± 3,69 (12,33-22,00) |
0,29 |
| MMSE > 20/30 (n=49) | 29,97 ± 20,81 (4,9-73) |
|
| Male (n=30) | 31,91 ± 21,67 (4,9-73) |
0,22 |
| Female (n=24) | 24,74 ± 17,9 (4,9-65) |
Table 2: Vitamin D levels in PD patients divided for disease severity, age, MMSE score and sex.
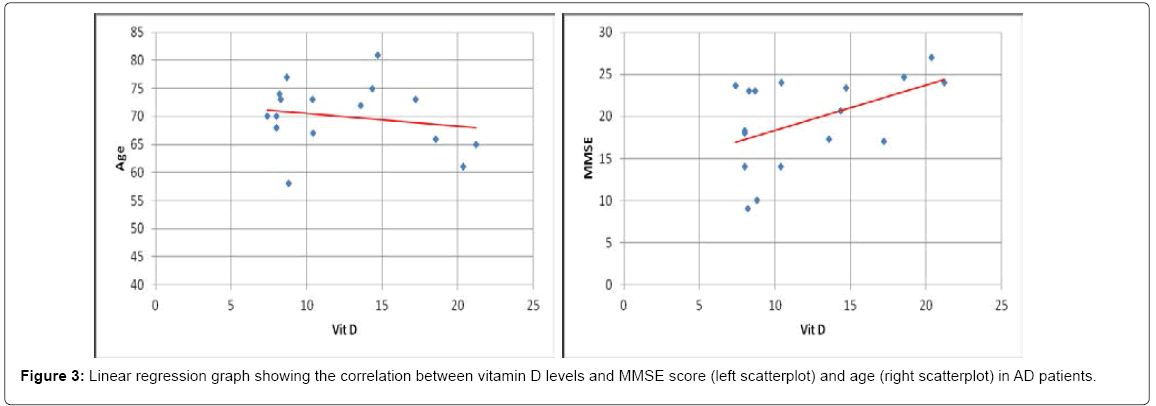
For comparative purposes a group of 17 patients with AD was also examined. In AD patients vitamin D levels showed a significant reduction (p < 0,001) compared to controls. Nearly half of the AD patients (47%) had levels of vitamin D lower than10 ng/ml (severe deficiency) (Figure 1C). In fact, as expected, difference in vitamin D levels between AD and PD group was significant (p < 0,001). Also in AD group vitamin D levels did not correlated with age (R=-0,19 p=0,47). The small cohort hampered to find a strong correlation with MMSE (yet, R=0,48 p= 0,052) (Scatterplots are shown in Figure 3). Finally, we took advantage of a coeval population of patients afflicted by ALS. Also this group showed vitamin D levels significantly lower than controls (p < 0,05); however, about half of the ALS patients (53%) had normal levels of vitamin D (>30 ng/ml) and only 5% (one patient) showed vitamin D lower than 10 ng/ml (severe deficiency) (Figure 1D). To note, no significant difference was found comparing ALS with PD group (p=0,25).
Discussion
Our study emphasized that most PD patients manifested a low serum 25(OH)D levels. However, this finding was tempered by two evidence: first, the absence of correlation with MMSE or disease staging; second, also patients afflicted by motor neuron disease exhibited similar deficiency
On the other hand, our study highlights once more that AD patients manifest a clearcut vitamin D deficiency; such an impairment is significantly more pronounced than other populations affected by different neurodegenerative disorders. 47 % of the AD cohort showed indeed vitamin D levels < 10 ng/ml, indicative of severe deficiency; whilst both PD and ALS, albeit characterized by pathological serum levels, in the majority of cases showed levels > 20 ng/ml. In PD, for instance, only 18% manifest severe deficiency and in ALS group only 5%. On the other hand, the significant low level in PD compared to controls suggests that vitamin D represents a relevant metabolic biomarker. Low vitamin D is frequently found in PD. Similar findings were described efficaciously in previous studies [20,27,28]. However, at odds with other contributions [29,30], we failed to detect correlations between vitamin D levels and PD symptom severity, as measured by both H&Y and UPDRS part III. In one of the larger studies conducted on PD patients, it was found a 18% of vitamin D deficiency and 47% of vitamin D insufficiency (compared to 7 and 40% in control, respectively). To note, they described a relation between “worse UPDRS scores” and specificially low 25(OH)D3 as well as low total 25(OH)D levels (yet, the latter p = 0.02). However, similar to our findings,“associations with the HY scale failed to reach statistical significance” [20].
A limitation of our study relates to the evidence that only 5 PD patients exhibited MMSE < 20. Nevertheless, the presented cognitive performance are a reliable sample as it occurs in a natural, observational population of an unbiased clinical practice. If, on the other hand, we recruit a cohort characterised by a larger prevalence of a severe cognitive decline, chances for an opposite bias - the inclusion of PD dementia or dementia with Lewy bodies - would be higher.
Despite the fact that our PD cohort, as implied by the observational nature of the study, did not show a high prevalence of dementia, patients MMSE disclosed variable cognitive perfomance; this allowed to verify the possible correlation between serum 25(OH) D levels and cognitive scores; albeit in PD patients with mild cognitive impairment a tendency towards a lower vitamin D appeared, it did not reach significance.
Of course PD patients may suffer from a profound change of their daily activities (low exposure to sunlight, limited outdoor activities). Yet, since most of the control populations is represented by spouses, likely to share an analogous pattern of outdoor/indoor activities, the role of diurnal habits seems modest. Besides, also the notion that vitamin D levels failed to correlate with disease staging appears in apparent contradiction with the hypothesis claiming that severe motor impairment, as far as it collides with functional independence, imposes institutionalization and thus reduced sun exposure. The opportunity to extend the analysis of larger cohort is mandatory (expecially if a larger cohort of patients will allow a comparison between de novo and advanced ones).
One of the striking result here presented regard the low vitamin D levels encountered in the population affected by motor neuron disease. Only few previous studies showed similar results [31,32]. In addition, vitamin D deficiency has been also documented in other neurodegenerative diseases, as recently documented in a study conducted by Terracciano et al., focusing myotonic dystrophy type 1 [22].
In this context, if the lack of vitamin D represents a general marker of senescence or a marker associated with neurodegeneration is not fully understood. There is strong evidence that vitamin D is involved in neuroprotection through various mechanisms: up-regulation of neurotrophic factors, antioxidative mechanisms, neuronal calcium regulation, nerve growth factor modulation [33-37], regulation of the toxicity of reactive oxygen species [38] and through immunomodulation and vasoprotection [7,10]. However, it is also possible that lower vitamin D levels could be a common factor in diseases that share neuro-inflammatory dysfunction as observed in MS. In accord with the association between MS and low vitamin D hematic concentration, observational studies suggest that adequate vitamin D nutrition affect the course of disease [39]; healthy whites with serum levels of 25(OH) D of 100 nmol/L or greater have a 50% reduced risk of developing MS as compared to those with levels below 75 nmol/L. Among MS patients, low 25(OH)D levels early in the disease course are a strong risk factor for long term MS MRI activity and clinical progression [23]. Noticeably, MS has acquired recently the status of neurodegenerative disease.
Conclusion
Our study confirmed the evidence of a non-specific vitamin D deficiency in patients with PD, as well as in ALS patients, considered as a comparison cohort. Our study may support the hypothesis that PD predisposes to vitamin D deficiency (conceivably by limiting outdoor activities), whilst the role of vitamin D deficiency as active factor on disease progression is more than doubtful. The similar pattern of vitamin D concentration in ALS group (and the more dramatic deficiency in AD) is strongly evocative in that sense. In conclusion, although vitamin D supplementation has to be considered in different neurodegenerative conditions, at least in terms of metabolic rebalancing, its potential efficacy as a preventive strategy, in PD, is far from established.
References
- Sebaaly A, Bachour F, Bayoud W, Adib G , Bedran F, et al. (2015) The ExtraskeletalActions Of Vitamin D--Myths And Facts. J Med Liban 63: 87-93.
- Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, et al. (2010) Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med 152: 307-314.
- Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, O’Sullivan MJ, et al. (2006) Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 354: 684-696.
- Manson JE, Mayne ST, Clinton SK (2011) Vitamin D and prevention of cancer--ready for prime time?.N Engl J Med 364: 1385-1387.
- Lin AM, Chen KB, Chao PL (2005) Antioxidative effect of vitamin D3 on zinc-induced oxidative stress in CNS. Ann N Y Acad Sci 1053: 319-329.
- Chen KB, Lin AM, Chiu TH (2003) Systemic vitamin D3 attenuated oxidative injuries in the locus coeruleus of rat brain. Ann N Y Acad Sci 993: 313-324.
- Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, et al. (2013) Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun Rev 12: 976-989.
- Peterson CA, Heffernan ME (2008) Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond) 5:10.
- Buell JS, Dawson-Hughes B, Scott TM, Weiner DE, Dallal GE, et al. (2010) 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology 74: 18-26.
- Chung PW, Park KY, Kim JM, Shin DW, Park MS, et al. (2015) 25-hydroxyvitamin D status is associated with chronic cerebral small vessel disease. Stroke 46: 248-251.
- Kubis AM, Piwowar A (2015) The new insight on the regulatory role of the vitamin D3 in metabolic pathways characteristic for cancerogenesis and neurodegenerative diseases. Ageing Res Rev 24: 126-137.
- Stefani A, Brusa L, Olivola E, Pierantozzi M, Martorana A (2012) CSF and clinical hallmarks of subcortical dementias: focus on DLB and PDD. J Neural Transm 119: 861-875.
- Stefani A, Olivola E, Stampanoni Bassi M, Pisani V, Imbriani P, et al. (2013) Strength and weaknesses of cerebrospinal fluid biomarkers in Alzheimer's disease and possible detection of overlaps with frailty process. CNS Neurol Disord Drug Targets 12: 538-546.
- Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, et al. (2012) Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology 79: 1397-1405.
- Annweiler C, Llewellyn DJ, Beauchet O (2013) Low serum vitamin D concentrations in Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis 33: 659-674.
- van der Schaft J, Koek HL, Dijkstra E, Verhaar HJ, van der Schouw YT, et al. (2013) The association between vitamin D and cognition: a systematic review. Ageing Res Rev 12: 1013-1023.
- Llewellyn DJ, Langa KM, Lang IA (2009) Serum 25-hydroxyvitamin D concentration and cognitive impairment. J Geriatr Psychiatry Neurol 22: 188-195.
- Littlejohns TJ, Henley WE1, Lang IA, Annweiler C1, Beauchet O, et al. (2014) Vitamin D and the risk of dementia and Alzheimer disease. Neurology 83: 920-928.
- Zhu D, Liu GY, Lv Z, Wen SR, Bi S, et al (2014) Inverse associations of outdoor activity and vitamin D intake with the risk of Parkinson’s disease. J Zhejiang Univ Sci B 15: 923-927.
- Ding H, Dhima K, Lockhart KC, Locascio JJ, Hoesing AN, et al. (2013) Unrecognized vitamin D3 deficiency is common in Parkinson disease: Harvard Biomarker Study. Neurology 81: 1531-1537.
- Peterson AL, Murchison C, Zabetian C, Leverenz JB, Watson GS, et al. (2013) Memory, mood, and vitamin D in persons with Parkinson's disease. J Parkinsons Dis 3: 547-555.
- Terracciano C, Rastelli E, Morello M, Celi M, Bucci E, et al. (2013) Vitamin D deficiency in myotonic dystrophy type 1. J Neurol 260: 2330-2334.
- Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A (2006) Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 296: 2832-2838.
- Huang J, Xie ZF (2012) Polymorphisms in the vitamin D receptor gene and multiple sclerosis risk: a meta-analysis of case-control studies. J Neurol Sci 313: 79-85.
- Hamilton B (2010) Vitamin D and human skeletal muscle. Scand J Med Sci Sports 20: 182-190.
- Holick MF (2007) Vitamin D deficiency. N Engl J Med 357: 266-281.
- Newmark HL, Newmark J (2007) Vitamin D and Parkinson's disease--a hypothesis. Mov Disord 22: 461-468.
- Evatt ML, Delong MR, Khazai N, Rosen A, Triche S, et al. (2008) Prevalence of vitamin d insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol 65: 1348-1352.
- Moghaddasi M, Mamarabadi M, Aghaii M (2013) Serum 25-hydroxyvitamin D3 concentration in Iranian patients with Parkinson's disease. Iran J Neurol 12: 56-59.
- Peterson AL, Mancini M, Horak FB (2013) The relationship between balance control and vitamin D in Parkinson's disease-a pilot study. Mov Disord 28: 1133-1137.
- Camu W, Tremblier B, Plassot C, Alphandery S, Salsac C, et al. (2014) Vitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosis. Neurobiol Aging 35: 1198-1205.
- Karam C, Scelsa SN (2011) Can vitamin D delay the progression of ALS? Med Hypotheses 76: 643-645.
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ (2005) Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 29: 21-30.
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D, et al. (2002) Vitamin D is a potent secosteroid hormone with diverse biological functions that include protection against neuronal damage. Trends Endocrinol Metab 13: 100-105.
- Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, et al. (2001) Vitamin D confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci 21: 98-108.
- Chabas JF, Alluin O, Rao G, Garcia S, Lavaut MN, et al. (2008) Vitamin D2 potentiates axon regeneration. J Neurotrauma 25: 1247-1256.
- Brown J, Bianco JI, McGrath JJ, Eyles DW (2003) 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett 343: 139-143.
- Garcion E, Sindji L, Montero-Menei C, Andre C, Brachet P, et al. (1998) Expression of inducible nitric oxide synthase during rat brain inflammation: regulation by 1,25-dihydroxyvitamin D3. Glia 22: 282-294.
- Munger KL, Ascherio A (2011) Prevention and treatment of MS: studying the effects of vitamin D. Mult Scler 17: 1405-1411.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 10104
- [From(publication date):
December-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9312
- PDF downloads : 792