Research Article Open Access
Long-term Results of Radiologically Guided Endoscopic Injection Sclerotherapy for Esophageal Variceal Bleeding: A Retrospective 30-year Survey
Hiroaki Iwase*, Masaaki Shimada, Noboru Hirashima, Masayuki Okeya, Nobumitsu Ryuge, Yuichi Kida, Masaya Esaki, Bunichiro Kato and Noboru Urata
Department of Gastroenterology, and Radiology, National Hospital Organization Nagoya Medical Centre, 4-1-1 Sannomaru, Naka-ku, Nagoya 460-0001, Japan
- *Corresponding Author:
- Hiroaki Iwase
Department of Gastroenterology
National Hospital Organization
Nagoya Medical Centre, 4-1-1 Sannomaru
Naka-ku, Nagoya 460-0001, Japan
Tel: +81 52 9511111
Fax: +81 52 9510664
E-mail: iwaseh@nnh.hosp.go.jp
Received date: April 22, 2014; Accepted date: November 11, 2014; Published date: November 17, 2014
Citation: Iwase H, Shimada M, Hirashima N, Okeya M, Ryuge N, et al. (2014) Long-term Results of Radiologically Guided Endoscopic Injection Sclerotherapy for Esophageal Variceal Bleeding: A Retrospective 30-year Survey. J Gastrointest Dig Syst 4:238. doi:10.4172/2161-069X.1000238
Copyright: © 2014 Iwase H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background: Endoscopic injection sclerotherapy (EIS) is one of the most commonly applied techniques in the treatment of patients with bleeding esophageal varices (EV). However, the role of EIS in the long-term management of patients with EV bleeding remains controversial. We conducted a retrospective 30-year survey of EIS in patients with EV bleeding.
Patients and Methods: Sclerosant with radiological contrast agent was endoscopically injected into the distal EV under fluoroscopic observation. The endpoint of injection was to fill the EV, including the supplying venous complex, which comprises the left and the short gastric veins.
Results: Of the 367 patients reviewed, 350 had liver cirrhosis and 17 had idiopathic portal hypertension. The Child-Pugh classification was A in 92 patients, B in 121, and C in 154. Fifty-seven patients had hepatocellular carcinoma (HCC) at the initial EIS. The primary successful hemostasis rate was 95.5%. The numbers of re-bleeding EV episodes were 61 at 0 to 1 year, 34 at 1 to 3 years, 21 at 3 to 6 years, 2 at 6 to 9 years, and 0 over 10 years from the initial EIS intervention. EV was eradicated in 87% of patients and re-bleeding EV was markedly reduced after eradication of EV. Complications were generally mild, serious events were rare after 2000, included renal failure (3.3%), liver failure (3.3%), and esophageal stricture, and shock (1.6%), esophageal ulcer bleeding (1.6%). The causes of death were established in 278 patients, included liver failure (51.8%), HCC (20.9%), bleeding EV (7.9%), and procedure-related mortality (4.3%). The median survival time for all patients was 3.3 years, while 1-, 10-, 20-, and 30-year cumulative survival rates were 72.8%, 7.8%, 3.2%, and 3.2%, respectively.
Conclusion: Our EIS treatment for bleeding EV was effective in reducing bleeding death over the long term. Improved survival requires amelioration of liver function and control of HCC.
Keywords
Survey; Sclerotherapy; Esophageal varices, Bleeding
Introduction
Endoscopic injection sclerotherapy (EIS), introduced in 1939 by Crafood and Frencker [1] has had a high success rate in controlling bleeding of esophageal varices (EV) and has become established worldwide as the recommended treatment in the management of patients with EV bleeding. In contrast, the EIS technique differs among users, leading to non-uniformity of results. In addition, its role in the long-term management of patients after bleeding EV remains controversial. However, follow-up in most previous reports has not exceeded 12 years [2-4].
We have previously reported an 11-year survey of safety and efficacy of radiologically guided EIS for EV [4]. At that time, we did not introduce endoscopic variceal ligation (EVL) in the treatment of bleeding EV. EVL has been undertaken for patients with active variceal bleeding or with severe underlying liver disease since 2000. This paper presents the documented results of a 30-year analysis of our technique, the results of determination of the efficacy, complications, and survival.
Patients and Methods
Patients
Between April 1982 and August 2013, a total of 411 patients with bleeding EV underwent EIS at the Department of Gastroenterology, National Hospital Organization, Nagoya Medical Centre. Among these 411 patients, 44 were excluded from this study because analysis of follow-up data was not possible. The remaining 367 patients were included for analyses. One hundred and eighty-two patients who underwent EIS within 24 hours of the bleeding EV were classified as Emergent treatment group, while 185 patients who were treated 24 hours after bleeding EV were classified as Elective treatment group.
Methods
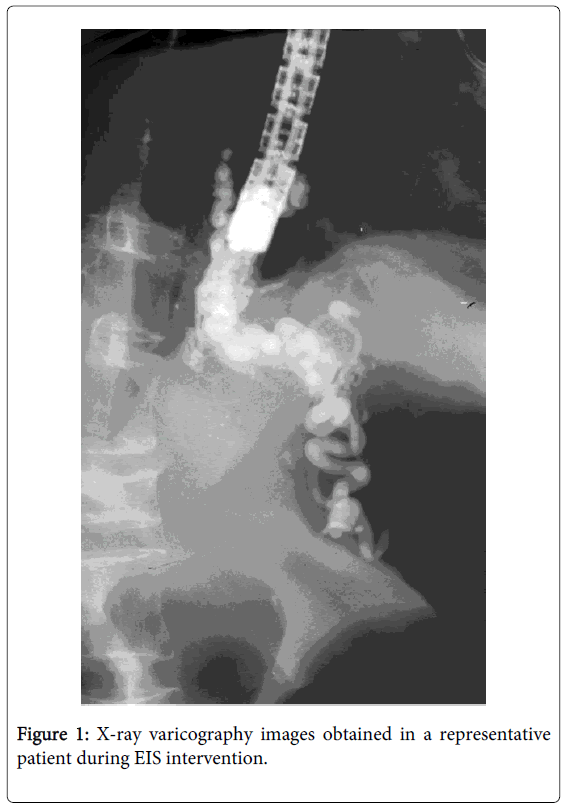
EIS was done with a forward-view fiberoptic endoscope and video endoscope (Olympus, Tokyo, Japan). As a sclerosant for EIS, a 2% sodium tetradecyl sulphate was used from 1982 to 1996, [4] and a 5% ethanolamine oleate (EOI) since 1997. After the balloon, which was attached to an endoscope was inflated to prevent the scleroant from flowing out of the varices, sclerosant with radiological contrast agent was endoscopically injected into the distal EV with the aid of a 23-gauge needle. Needle venepuncture was done carefully to avoid injection into the tunica muscularis. If the sclerosant leaked into the tissues, the injection at that site was immediately halted, and proceeded with injection into other varices. The flow of the sclerosant was monitored via a fluoroscope. The endpoint of the treatment was just to fill the EV including the supplying venous complex, which comprised the left and the short gastric veins with sclerosant (Figure 1). The amount of 5% EOI injected varied according to the status of EV, but the maximum dose was 20 ml at one EIS session.
On the day following the treatment, we assessed the side effects of treatment by conducting a blood biochemistry analysis, chest radiograph, and urine test. One week later, endoscopy was done to observe the EV. Additional EIS was undertaken to treat residual varices that were detected within 2 weeks. In patients with endoscopic eradication of EV, follow-up endoscopy was undertaken every 6 months. Whenever EV recurred, additional EIS was undertaken. Further, to eliminate the remaining EV and dilated veins with red color sign indicating possible haemorrage, a 25-gauge needle was used for intra-variceal injection of 5% EOI.
Since 2000, [5] EVL has been performed for patients with active bleeding EV by using selective banding method just for bleeding point, and severe underlying liver disease by using spiral banding method from the esophageal-gastric junction. Additional radiologically guided EIS treatment was undertaken for patients with persisting EV after EVL if they had an expected survival time of longer than 1 year.
Statistical analysis
Follow-up of patients was continued until death or up to 1 April 2014. Survival curves were plotted based on the Kaplan-Meier method and compared by Wilcoxon and log-rank tests with differences considered statistically significant at P<0.05.
Results
Patients’ demographic variables
The median survival follow-up time was 2.8 years, range 0.2-30.1 years. The clinical features of the 367 study patients are presented in Table 1. There were 255 men with the median age 60 years, range, 19 to 87 years. Three hundred and fifty had liver cirrhosis (LC) and 17 had idiopathic portal hypertension (IPH). The Child-Pugh classification (C-P class) was A in 92 patients, B in 121, and C in 154. Hepatocellular carcinoma (HCC) was diagnosed at the time of the initial EIS in 57 patients.
| Emergency | Elective | Total | % | |
|---|---|---|---|---|
| No.of patients | 182 | 185 | 367 | |
| Median age (range,yr) | 60(19-83) | 60(29-87) | 60(19-87) | |
| Male/female | 139/43 | 116/69 | 255/112 | 69.5%/30.5% |
| Etiology of PHT Cirrhosis | 173 | 177 | 350 | 95.40% |
| Hepatitis B virus | 12 | 26 | 38 | 10.40% |
| Hepatitis C virus | 91 | 93 | 184 | 50.10% |
| Alcohol abuse | 23 | 21 | 44 | 12.00% |
| PBC | 3 | 12 | 15 | 4.10% |
| Unknown origin | 44 | 25 | 69 | 18.80% |
| IPH | 9 | 8 | 17 | 4.60% |
| Child-Pugh classification (A/B/C) |
30/48/104 | 62/73/50 | 92/121/154 | 25%/33%/42% |
| HCC Present | 57 | 41 | 98 | 26.70% |
| Absent | 125 | 144 | 269 | 73.30% |
Table 1: Demographic variables of the patients included in this investigation; PHT=Portal hypertension; PBC=primary biliary cirrhosis; IPH=idiopathic portal hypertension.
Hemostasis and re-bleeding rates
The primary successful hemostasis rate for bleeding EV was 95.5%. After the initial EIS treatment, EV was reduced to small or fine linear lesions and was eradicated in 87.5% of the patients with 2 to 3 additional EIS procedures. The numbers of re-bleeding EV episodes were 61 at 0 to 1 year, 34 at 1 to 3 years, 21 at 3 to 6 years, 2 at 6 to 9 years, and 0 at 10 years from the initial EIS treatment. Most often, re-bleeding occurred in incompletely eradicated EV at 0 to 3 years and in eradicated EV at 3 to 9 years. Re-bleeding in completely eradicated EV were from scanty recurrent EV and dilated veins with red color sign. Five patients with splenomegaly underwent splenectomy due to re-bleeding from the remaining EV that was incompletely eradicated after repeated EIS. Twenty-four patients with eradicated varices or small varices after repeated EIS developed GI haemorrhage including portal hypertensive gastropathy in 12 patients, gastric variceal bleeding in 6, rectal variceal bleeding in 3, duodenal variceal bleeding in 2, and bleeding from the dilated, reddish veins of the small intestine in 1. Peak GI haemorrhage occurred within 5 years after the initial EIS and the only 1 case of small intestinal bleeding occurred after 12 years.
Complications
The main complications were shallow ulcers and chest pain at the site of injection. All were mild and disappeared within 1 week. Serious complications were rare and are summarized for before and after 2000 in Table 2. They were reduced after 2000 when EVL was introduced. Thus esophageal stricture rate was 6.1% before 2000 and 2.5% after 2000, renal failure was 5.3% before and 3.3% after, esophageal ulcer bleeding was 4.9% before and 1.6% after, pleural effusion was 4.1% before and 0.8% after, shock was 5.3% before and 1.6% after, sepsis was 1.6% before and 0.0% after, pneumothorax was 0.8% before and 0.0% after, intramural hematoma was 0.8% before and 0.0% after, and cardiac tamponade was 0.4% before and 0.0% after.
| (1982-1999) | (2000-2013) | |||||
|---|---|---|---|---|---|---|
| Elective (n=121) | Emergency (n=124) | Total (n=245) | Elective (n=64) | Emergency (n=58) | Total (n=122) | |
| Esophageal stricture | 6(5.0%) | 9(7.3%) | 15(6.1%) | 1(1.6%) | 2(3.4%) | 3(2.5%) |
| Renal failure | 4(3.3%) | 9(7.3%) | 13(5.3%) | 2(3.1%) | 2(3.4%) | 4(3.3%) |
| Esophageal ulcer bleeding | 2(1.7%) | 9(7.3%) | 11(4.9%) | 1(1.6%) | 1(1.7%) | 2(1.6%) |
| Pleural effusion | 2(1.7%) | 8(6.5%) | 10(4.1%) | 1(1.7%) | 0(0.0%) | 1(0.8%) |
| Liver failure | 2(1.7%) | 8(6.5%) | 10(4.1%) | 2(3.1%) | 2(3.4%) | 4(3.3%) |
| Shock | 2(1.7%) | 11(8.9%) | 13(5.3%) | 0(0.0%) | 2(3.4%) | 2(1.6%) |
| Portal vein thrombosis | 1(0.8%) | 3(3.2%) | 4(1.6%) | 0(0.0%) | 1(1.7%) | 1(0.8%) |
| Sepsis | 1(0.8%) | 3(3.2%) | 4(1.6%) | 0(0.0%) | 0(0.0%) | 0(0.0%) |
| Pneumonia | 0(0.0%) | 3(3.2%) | 3(1.2%) | 0(0.0%) | 0(0.0%) | 0(0.0%) |
| Lung abscess | 0(0.0%) | 3(3.2%) | 3(1.2%) | 0(0.0%) | 1(1.7%) | 1(0.8%) |
| Pneumothorax | 0(0.0%) | 2(1.6%) | 2(0.8%) | 0(0.0%) | 0(0.0%) | 0(0.0%) |
| Cardiac tamponade | 0(0.0%) | 1(0.8%) | 1(0.4%) | 0(0.0%) | 0(0.0%) | 0(0.0%) |
| shshSock | 2(1.7%) | 11(8.9%) | 13(5.3%) | 0(0.0%) | 2(3.4%) | 2(3.4%) |
Table 2: List of serious complications
Causes of death
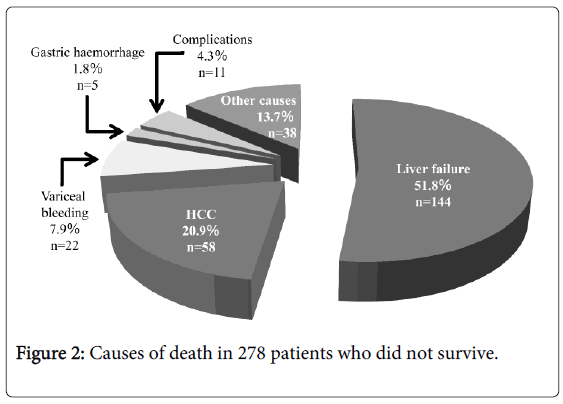
At the last follow-up day, 276 deaths were confirmed. Liver failure was the main cause of death in 143 cases (51.8%), followed by HCC in 58 cases (20.9%), bleeding EV in 22 cases (7.9%). Procedure-related mortality was found in 11 cases (4.0%), which included renal failure in 2 cases, portal vein thrombosis in 2, esophageal ulcer bleeding in 2, shock in 2, abscess in 2, and cardiac tamponade in 1. Gastric haemorrhage as cause of death was found in 5 cases (1.8%) included severe portal hypertensive gastropathy in 3 and gastric variceal bleeding in 2. Thirty-eight patients (13.7%) had miscellaneous causes of death (Figure 2).
Survival time
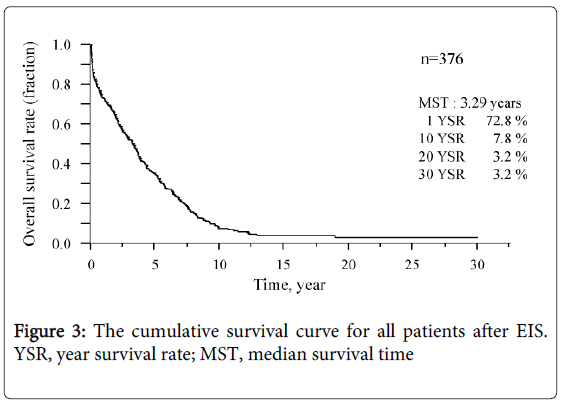
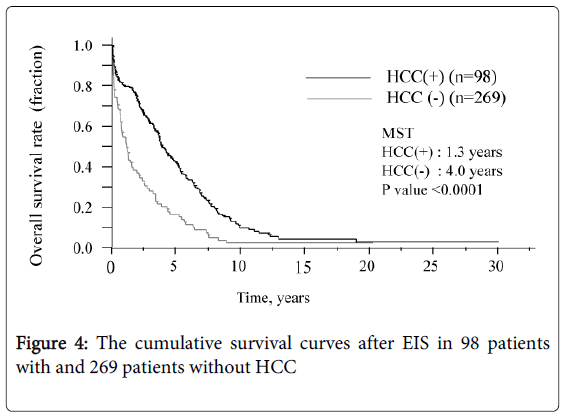
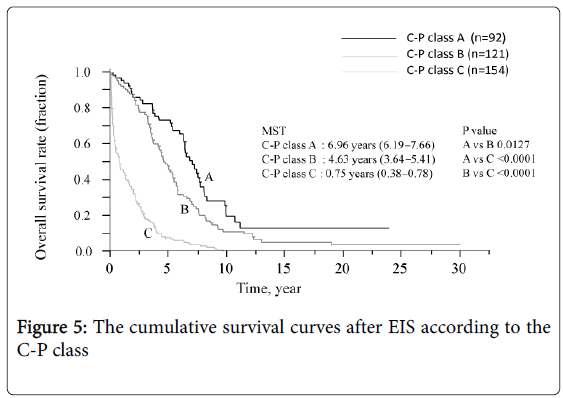
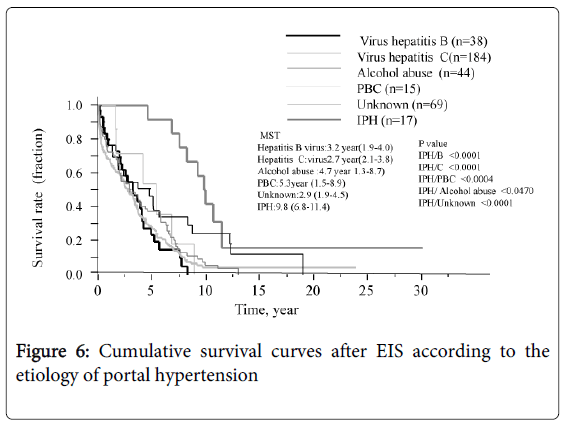
The survival curve for all patients is shown in Figure 3. The median survival time (MST) was 3.3 years and the survival rates were 72.8% (1 year), 7.8% (10 years), and 3.2% (20 and 30 years, each). Six of the 12 patients who underwent splenectomy before or after EIS survived for more than 10 years, and 3 of these 6 survived for more than 20 years. Figure 4 shows that survival was significantly shorter among subjects with HCC (MST = 1.1 years) than those without HCC (MST = 3.9 years, P<0.01). The patients in whom HCC was kept under control after EIS survived for more than 10 years. When classified according to the C-P class, the MST of class A, B, and C patients was 7.0 years, 4.6 years, and 0.8 years, respectively. The survival time of class C subjects was significantly shorter than that of other patients (Figure 5). Survival curves according to the etiology of portal hypertension are shown in Figure 6. There was no significant difference in MST among the liver cirrhosis groups: hepatitis B virus (3.2 years); hepatitis C virus (2.7 years); alcohol abuse, (4.7 years); PBC (5.3 years); and unknown causes (2.9 years). The patients in the IPH group had a significantly longer MST (9.8 years) than those with liver cirrhosis (Figure 3).
Discussion
Radiologically guided EIS with the combined use of a sclerosant and radiographic contrast agent is not a new technique, was reported previously as a strategy to insure intravariceal injection [6]. However, to our knowledge, there is no report in which the combination was injected just to fill the EV, including the supplying veins in Europe or in the USA. We have previously reported on the therapeutic efficiency of EIS in treating the supplying veins [4].
Similar data are reported by Takase et al. based on angiography [7]. Injecting the EV near the esophagogastric junction made it easier to fill the supply vein with an amount less than 20 ml of 5% EOI.5) Similarly, the endpoint of injection was achieved without limiting the amount of sclerosant to avoid toxicity [4,5,8]. In the present study, the successful primary hemostasis rate for EV bleeding was 95.5%. It was difficult to control active bleeding EV in the terminal stage LC or HCC as previously report [4], but the outcome was generally satisfactory and EIS was emerging as a life-saving procedure for patients with EV bleeding [8]. Re-bleeding episodes sometimes occurred from incompletely eradicated EV within 1 year after the initial EIS. Additional EIS treatment led to the complete eradication of EV including the supplying venous complex, which eliminated the risk of re-bleeding for a long time. A 25-gauge needle was convenient to stop small EV from recurring again in completely eradicated EV. Re-bleeding rates decreased over time with no occurrences in patients with longer than 10 years survival.
Serious complications have also been reported in association with EIS treatment including several unintended punctures that affected patients [2-5]. However, we found that overall, EIS with a reduced number of procedures was mostly safe and was well-tolerated. Radiologically guided EIS was used not only to obliterate the EV but also to avoid complications. Most of the complications of the EIS occurred in association with extra variceal injection. Thorough and minimal needle puncture was essential to avoid serious complications [5]. In particular, when pertained to esophageal ulcer bleeding, pleural effusion, lung abscess, pneumothorax, intramural hematoma, cardiac tamponade. Serious complications are more likely to occur at the initial stage and in active bleeding cases. It is thought that most, if not all serious complications can be avoided with good experience, skill, by using smaller gauge needles when introducing EVL in active bleeding cases. With these in mind, we have rarely experienced serious complication since 2000. In this study, 2 patients died, one due to renal failure and one due to portal vein thrombosis, both associated with intravariceal injection of sclerosant. More importantly, our technique involved gentle, and slow infusion to avoid unwanted systemic infusion under fluoroscopic observation [5,9]. The inflated balloon attached to the endoscope was another importnat feature in our study to prevent the scleroant from flowing out of varices.
EVL has been widely used because of the ease of carrying out the procedure and its minimal invasive effect on patients. EVL is an appropriate procedure for active EV bleeding and for patients whose condition is severe [5]. Following the introduction of EVL, serious complications related to endoscopic therapy have been decreasing. It is a simple local technique for variceal interruption without the need for treating the supplying veins of EV. When EVL was used alone, recurrence or worsening of EV over a short time period occurred in our previously study [10]. Therefore, since 2000 we have continued using our radiologically guided EIS for the patients with persisting EV after EVL. Hitherto, a detailed long-term follow-up data on the combination of EVL and EIS therapy has not been reported. We found interesting outcomes in the management of variceal bleeding with this combination therapy. Additionally, it appeared to be safe to combine EVL with EIS, and this combination could reduce the dose of sclerosant [5,10].
Extraesophagogastric variceal bleeding was found after EIS. The angiographic study by Takase, et al. showed that repeated EIS for EV might obliterate the coronary azygos system [6]. As a consequence of disruption of this system, the increased portal venous blood flow may induce a compensatory increase in flow through other post-systemic collaterals and produce varices in the rectum [11], duodenum, etc. [12,13]. Generally, EIS has been used in the treatment of these varices [11-13]. It was reported that patients treated by repeated EIS for EV must be diligently observed for development of extraesophagogastric varices [11-13]. However, occurrence of extraesophagogastric variceal bleeding in our study was rare and none was observed beyond 5 years after the initial EIS.
During the long observation period in this survey, the main cause of death in patients who underwent EIS for EV bleeding was liver failure, followed by HCC. The IPH group did not have any serious risk for HCC or for severe liver damage. Accordingly, their survival time was significantly longer than in patients with liver cirrhosis. The survival rate of patients with C-P class C was significantly shorter than those of class A or B. We previously reported that multivariable analysis of prognostic factors in the blood associated with severity of liver disease in patients with gastric variceal bleeding after EIS including serum total bilirubin (TB) level, prothrombin time (PT), and platelet (Plt) count were significantly associated with survival time[14]. Thrombocytopenia accompanied by hypersplenism was a common complication in patients with portal hypertension. Murata, et al. found that promotion of liver regeneration was related to Plt count and that thrombocytosis induced by splenectomy suppressed degeneration and improved regeneration of liver cells experimentally [15,16].
Additionally, Murata, et al. showed that the C-P class score significantly improved after splenectomy and serum levels of TB, PT, and Plt count similarly improved in patients with liver cirrhosis [17]. Therefore, in patients with EV bleeding survived by EIS, splenectomy might lead to long-term prognosis.
In conclusion, in this investigation, radiologically guided EIS for EV bleeding appeared to be a safe procedure and provided good hemostasis with a low rate of recurrence, even over the long term. Our impression is that EVL followed by EIS should be recommended for active EV bleeding and serious complications should be avoided in patients with severe underlying liver disease. Further, for EIS treatment to be effective in reducing bleeding EV death and improve survival time, requires better liver function and control of HCC complications.
Acknowledgments
No external fund was used for this study. The authors thank Dr A R Saniabadi, Department of Pharmacology, Hamamatsu University School of Medicine for reviewing the manuscript.
References
- Crafford C, Frenckner P (1939) New surgical treatment of varicose veins of the oesophagus. ActaOtolaryngol 27: 422-429.
- Paquet KJ, Lazar A, Rambach W (1991) Long term varicealsclerotherapy: is endoscopic sclerosis a unique therapeutic approach and a true alternative to surgery? HPB Surg 4: 11-25.
- Van Hootegem P, Van Besien K, Broeckaert L, Rutgeerts P, Fevery J (1988) Endoscopic sclerotherapy of esophageal varices. Long-term follow-up, recurrence, and survival. J ClinGastroenterol 10: 368-372.
- Iwase H, Suga S, Shimada M, Yamada H, Horiuchi Y, et al. (1996) Eleven-year survey of safety and efficacy of endoscopic injection sclerotherapy using 2% sodium tetradecyl sulfate and contrast medium. J ClinGastroenterol 22: 58-65.
- Iwase H (2014). Techniques of endoscopic therapy for esophageal varices. GastroenterolEndsc (Japanese) 56: 1574-1588.
- Grobe JL, Kozarek RA, Sanowski RA, LeGrand J, Kovac A (1984) Venography during endoscopic injection sclerotherapy of esophageal varices. GastrointestEndosc 30: 6-8.
- Takase Y, Shibuya S, Chikamori F, Orii K, Iwasaki Y (1990) Recurrence factors studied by percutaneous transhepaticportography before and after endoscopic sclerotherapy for esophageal varices. Hepatology 11: 348-352.
- Iwase H, Morise K, Kawase T, Horiuchi Y (1994) Endoscopic injection sclerotherapy for esophageal varices during pregnancy. J ClinGastroenterol 18: 80-83.
- Yamaga H, Hashizume M, Kitano S, Higashi H, Yoshino I, et al. (1989) Acute renal failure after endoscopic injection sclerotherapy: a report of two cases. Endoscopy 21: 43.
- Iwase H, Kusugami K (1997) Symposium on endoscopic hemostasis in gastric diseases. 2. Choice of endoscopic therapy for esophagogastricvarices: aiming for safer and more effective therapy. Intern Med 36: 128-129.
- Iwase H, Kyogane K, Suga S, Morise K (1994) Endoscopic ultrasonography with color Doppler function in the diagnosis of rectal variceal bleeding. J ClinGastroenterol 19: 227-230.
- Barbish AW, Ehrinpreis MN (1993) Successful endoscopic injection sclerotherapy of a bleeding duodenal varix. Am J Gastroenterol 88: 90-92.
- Labenz J, Börsch G (1993) Successful endoscopic hemostasis of duodenal variceal bleeding with histoacryl. Endoscopy 25: 194.
- Iwase H, Shimada M, Tsuzuki T, Hirashima N (2011) Long-term results of endoscopic obliteration with cyanoacrylate glue for gastric fundal variceal bleeding: a 17-year experience. JJPN 17: 137-144.
- Murata S, Hashimoto I, Nakano Y, Myronovych A, watanabe M, et al (2008) Single administration of thrombopoietin prevents progression of liver fibrosis and promotes liver regeneration after partial hepatectomy in cirrhotic rats. Ann Surg 248: 821-828.
- Murata S, Ohkohchi N, Matsuo R, Ikeda O, Myronovych A, et al. (2007) Platelets promote liver regeneration in early period after hepatectomy in mice. World J Surg 31: 808-816.
- Murata K, Ito K, Yoneda K, Shiraki K, Sakurai H, et al. (2008) Splenectomy improves liver function in patients with liver cirrhosis. Hepatogastroenterology 55: 1407-1411.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14394
- [From(publication date):
November-2014 - Jul 14, 2025] - Breakdown by view type
- HTML page views : 9824
- PDF downloads : 4570