Research Article Open Access
Longitudinal Changes in Clinical Epidemiology and Drug Sensitivity of Community-associated Methicillin-Resistant Staphylococcus Aureus in a Tertiary Hospital in Japan
| Jun Taniguchi1,2, Masao Yoshinaga1,3*, Yu Kucho3, Mayuko Watanabe1,4 and Chiharu Kushida1,5 | ||
| 1Infection Control Team, Kagoshima City, Kagoshima, Japan | ||
| 2Department of Pharmacy, Kagoshima City, Kagoshima, Japan | ||
| 3Department of Pediatrics, Kagoshima City, Kagoshima, Japan | ||
| 4Department of Nursing, Kagoshima City, Kagoshima, Japan | ||
| 5Department of Clinical Laboratory, National Hospital Organization Kagoshima Medical Center, Kagoshima City, Kagoshima, 892-0853, Japan | ||
| Corresponding Author : | Masao Yoshinaga National Hospital Organization Kagoshima Medical Center Shiroyama-cho 8-1, Kagoshima 892-0853, Japan Tel: +81-99-223-1151 Fax: +81-99-223-7918 E-mail: m-yoshi@biscuit.ocn.ne.jp |
|
| Received August 21, 2014; Accepted September 09, 2014; Published October 09, 2014 | ||
| Citation: Taniguchi J, Yoshinaga M, Kucho Y, Watanabe M, Kushida C (2014) Longitudinal Changes in Clinical Epidemiology and Drug Sensitivity of Communityassociated Methicillin-Resistant Staphylococcus aureus in a Tertiary Hospital in Japan. J Infect Dis Ther 2:172. doi: 10.4172/2332-0877.1000172 | ||
| Copyright: © 2014 Taniguchi J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Aim: The present study aimed to determine longitudinal changes in the background of patients with community acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) and those in the susceptibility of CA-MRSA to antibiotics in a tertiary center.
Methods: The characteristics of MRSA isolates and patients between October 2005 and December 2011 were determined. This period was divided into two: the first half was from October 2005 to December 2008 and the second half was from January 2009 to December 2011. Data of age and sex of patients, and date and site of isolation were obtained. Antimicrobial susceptibility was examined for each period. Multiplex polymerase chain reaction methods were used to determine Staphylococcal cassette chromosome mec types and MRSA typing.
Results: A total of 513 MRSA isolates were identified. Of 265 and 248 MRSA strains in the first and second halves of the study period, 51 (19%) and 50 (20%) strains, respectively, were CA-MRSA. The prevalence of CAMRSA was highest in patients aged in the 80s and CA-MRSA was mainly isolated from the nose/pharynx. Antimicrobial resistance increased from the 2005-2008 to 2009-2011 periods for erythromycin (p=0.03), levofloxacin (p=0.003), imipenem (p=0.01), fosfomycin (p=0.02), and minocycline (p<0.001), but not for gentamicin, clindamycin, rifampicin, and vancomycin.
Conclusions: CA-MRSA accounts for 20% of total MRSA isolates in Japan. Ages of subjects with CA-MRSA and isolation sites have changed from the emerging stage to recent years. Antibiotic resistance has significantly increased in a tertiary hospital in Japan.
| Keywords | |
| CA-MRSA; Clinical epidemiology; Drug sensitivity | |
| Introduction | |
| Methicillin-resistant Staphylococcus aureus (MRSA) is one of the major microorganisms of health-care associated infections. Methicillin resistance in staphylococci is associated with acquisition of a large transmissible element known as staphylococcal cassette chromosome mec (SCCmec) [1]. MRSA, which is associated with health-care associated infection and has wide-ranging drug resistance, is termed hospital-associated MRSA (HA-MRSA). Three types of SCCmec elements (types I, II, and III) are mostly carried by HA-MRSA strains. | |
| Since the late 1990s, a different type of MRSA has emerged. This type is associated with skin and soft tissue infections and severe systemic infections, such as sepsis and necrotizing pneumonia, in healthy children or adolescents, and otherwise healthy individuals in the community [2]. This particular type is called communityacquired MRSA (CA-MRSA). CA-MRSA has smaller types of SCCmec than other types of MRSA, mainly type IV. CA-MRSA strains are increasingly implicated in health-care associated infections [3]. CAMRSA is usually more susceptible to some antibiotics than HA-MRSA, such as erythromycin, clindamycin, levofloxacin, and carbapenems [4]. Recently, antimicrobial resistance has steadily increased in certain lineages [5]. However, no data are available concerning longitudinal changes in clinical epidemiology and bacterial susceptibilities to drugs in one area. | |
| Therefore, the present study aimed to determine longitudinal changes in clinical epidemiology, including the background of patients and changes in the susceptibility of CA-MRSA to antibiotics, in a tertiary center for cardiovascular diseases, stroke, and cancer. | |
| Materials and Methods | |
| Bacterial isolates | |
| Stock of MRSA had been collected since October 2005 in our hospital. Characteristics of MRSA isolates between October 2005 and December 2011 were determined retrospectively. This period was divided into two: the first half of the study period was from October 2005 to December 2008 and the second half of the study period was from January 2009 to December 2011. MRSA was clinically screened by MRSA screening plates (E-MS97; Eiken, Tokyo, Japan), and finally determined when the mecA gene was present by polymerase chain reaction. Only one isolate based on the results of the genotyping of MRSA was selected from each patient. | |
| Characteristics of patients | |
| Data of age and sex of the patients, and date and site of isolation were obtained. Patients were classified as inpatient or outpatient groups. When the patient had a history of hospitalization, surgery, residency in a long-term care facility, and hemodialysis or peritoneal dialysis, the patient was classified as the inpatient group [6]. | |
| Prevalence of acquisition of CA-MRSA by age group | |
| The details of data of patients who were admitted to our hospital during the study period were available only for the second half of the study period from 2009 to 2011, because the computer system of the Medical Coding Division in our hospital was changed. During the second half of the study period, the number of admissions was counted as one for a patient when the patient was admitted multiple times to determine the prevalence of acquisition of MRSA in patients. | |
| Infection and colonization rates in the present study | |
| The presence of infection by MRSA was determined using the standard infection definitions [7]. MRSA colonization was assumed to be present in the absence of clinical signs and symptoms [8]. The infection or colonization rates in the present study were calculated as (number of inpatients with infection or colonization)/(number of admitted patients) during the study periods. | |
| Antimicrobial susceptibility testing | |
| Antimicrobial susceptibility was examined using the MicroScan WalkAway® System 40SI (Siemens Healthcare Diagnostics, West Sacramento, CA, USA). Panels used in the system were MicroScan® Pos BP Combo 41J (Siemens Healthcare Diagnostics) from January 2005 to October 2010 and MicroScan® Pos BP Combo 3.2B (Siemens Healthcare Diagnostics) from November 2010 and December 2012. Susceptibility to the following antimicrobial drugs was determined according to the product documents for Staphylococcus species: erythromycin ≤ 0.5 μg/mL, clindamycin ≤ 0.5 μg/mL, minocycline ≤ 4 μg/mL, levofloxacin ≤ 2 μg/mL, imipenem ≤ 4 μg/mL, gentamicin ≤ 4 μg/mL, fosfomycin ≤ 4 μg/mL, rifampicin ≤ 1 μg/mL, and vancomycin ≤ 2 μg/mL. | |
| Genotyping of MRSA | |
| Multiplex polymerase chain reaction methods were used to distinguish Staphylococcal cassette chromosome (SCC) mec types [9]. SCC types IV and V were classified as CA-MRSA. The presence or absence of Panton-Valentine genes (leuS/F and leuM) was determined [10]. Molecular typing of MRSA was performed to classify clinical isolates based on mec hypervariable region length polymorphisms [11]. The presence or absence of Staphylococcal enterotoxin genes A (sea), seb, sec, and see, and toxic shock syndrome toxin-1 gene (tst) was also used for molecular typing [12]. | |
| Statistical Analysis | |
| Differences in the mean values and prevalence were examined using the Mann-Whitney U test and Fisher’s exact probability test, respectively. Statistical analysis was performed using IBM® SPSS® Statistics Version 21.0 (IBM Japan, Ltd., Tokyo, Japan). A two-tailed probability value of <0.05 was considered statistically significant. | |
| Results | |
| Number of isolates of CA-MRSA | |
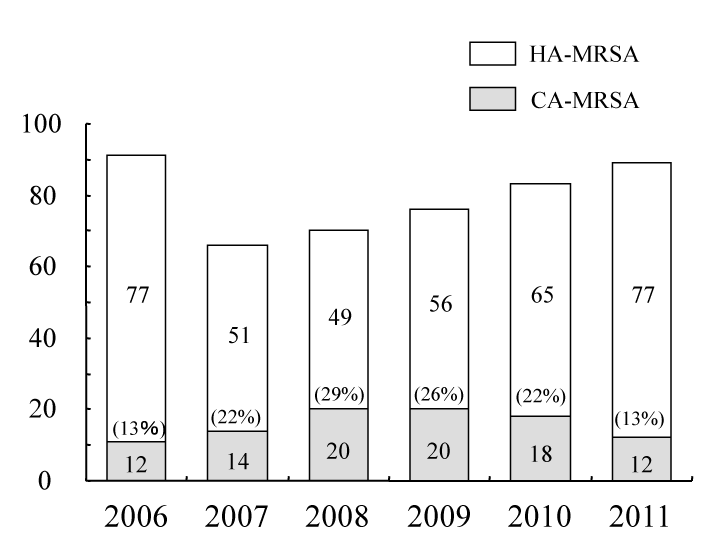
| A total of 513 MRSA isolates were identified in the study period. Of 265 and 248 MRSA strains in the first and second halves of the study period, 51 (19%) and 50 (20%) strains, respectively, were CA-MRSA. All of the CA-MRSA in the present study had type IV SCCmec. Figure 1 shows the prevalence of CA-MRSA among total MRSA between 2005 and 2011. The prevalence of CA-MRSA among total MRSA significantly increased from 2005 to 2008 (from 12/89 to 20/70, p=0.03) and decreased from 2008 to 2011 (from 20/70 to 12/89, p=0.03) (Figure 1). However, the prevalence of CA-MRSA was similar between the first and the second half of the study period (51/265 and 50/248, respectively, p=0.82). None of the CA-MRSA isolates had Panton-Valentine leukocidin genes. | |
| Characteristics of the patients | |
| The mean age of patients with CA-MRSA in the second half of the study period was significantly higher than that in the first half (p=0.03) (Table 1). The mean age in the CA-MRSA group was significantly lower than that in the HA-MRSA group in the first half of the study (p<0.001). However, this difference was not observed in the second half of the study period. The prevalence of female patients in the CA-MRSA group was higher than that in the HA-MRSA group in the first half of the study period (p=0.01). Isolation sites were not different between the first and second half of the study period in the CA-MRSA group. Five patients were outpatients in each of the first and second halves of the study period. Three of the five patients in the first half of the study period were infants (<1 year old). One of these three isolates was grown from a skin lesion and remaining two isolates were grown from the nose/throat. All five patients in the second half of the study period were adults (>50 years old) and all isolates were grown from the nose/throat. | |
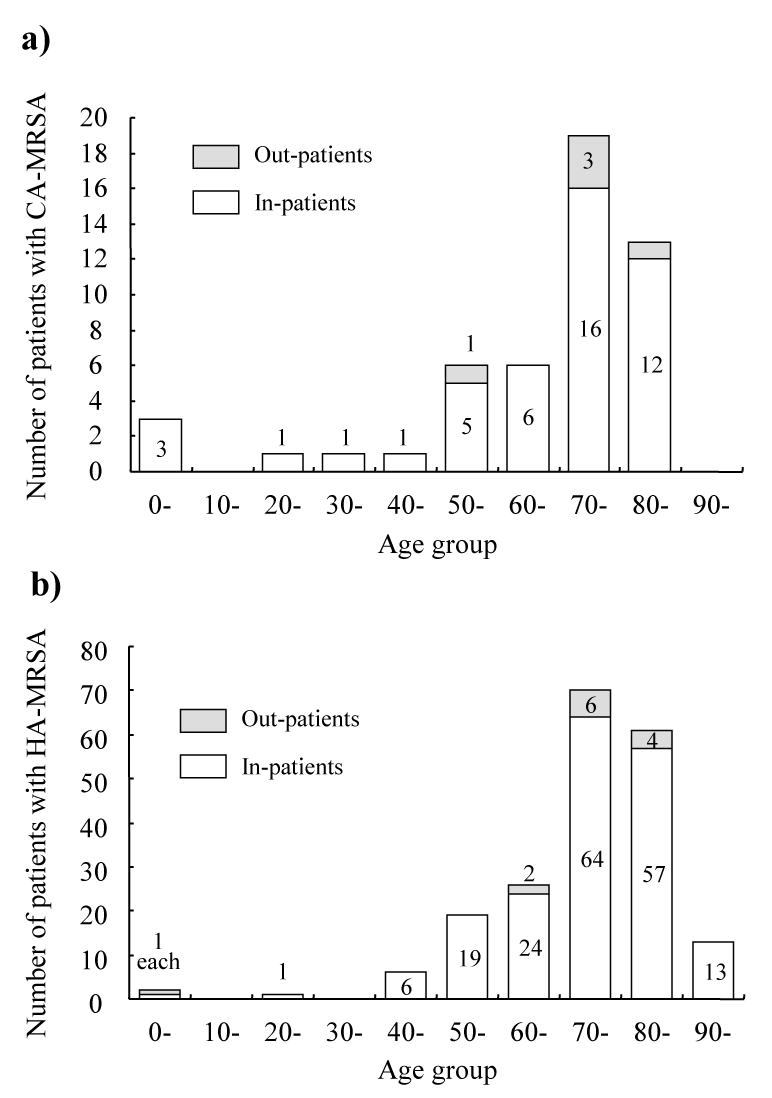
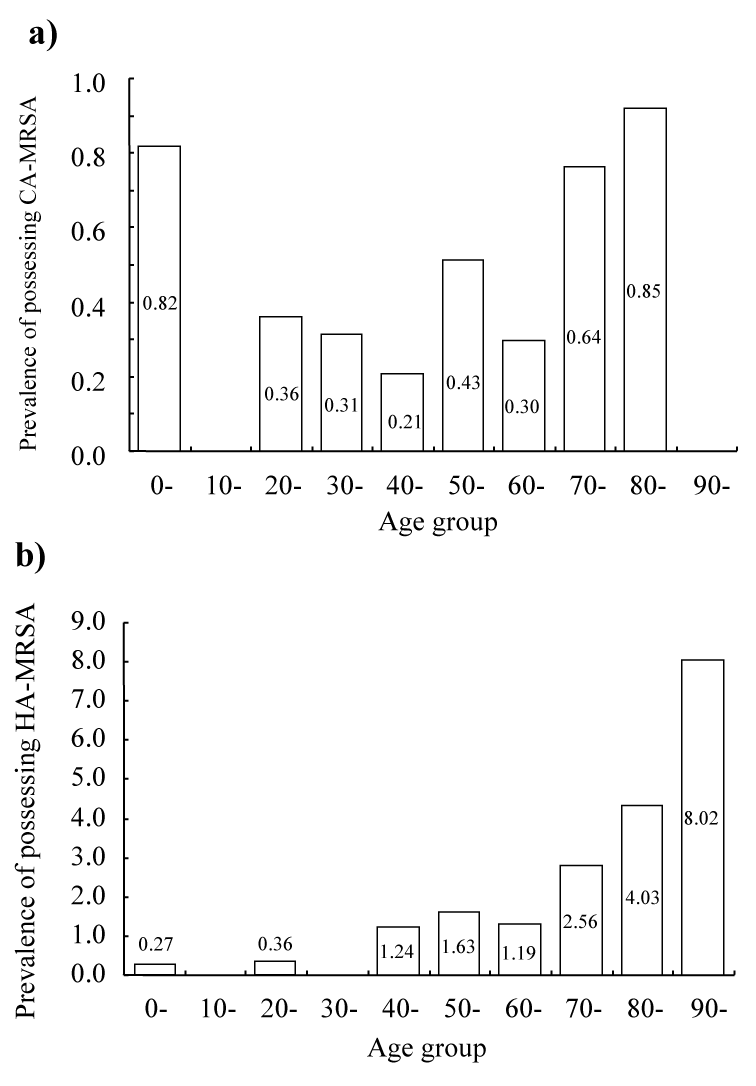
| The number of patients having CA-MRSA (Figure 2a) and HAMRSA (Figure 2b) by age groups at a 10-year-interval were determined in the second half of the study period. Because some patients had admitted more than once, one admission was counted from one subject. The prevalence of possessing CA-MRSA (Figure 3a) and HAMRSA (Figure 3b) among admitted patients for each age group was highest in the 80s and 90s age groups, respectively. The prevalence of possessing CA-MRSA and HA-MRSA was second highest in the 0-9 age group and in the 80th age group, respectively. | |
| Infection and colonization rates in the present study | |
| Nosocomial infection rate of CA-MRSA and HA-MRSA in the first half periods were 0.05% and 0.38%, respectively (Table 2). The rates were not significantly changed between the first and second periods. | |
| Comparison of drug susceptibility | |
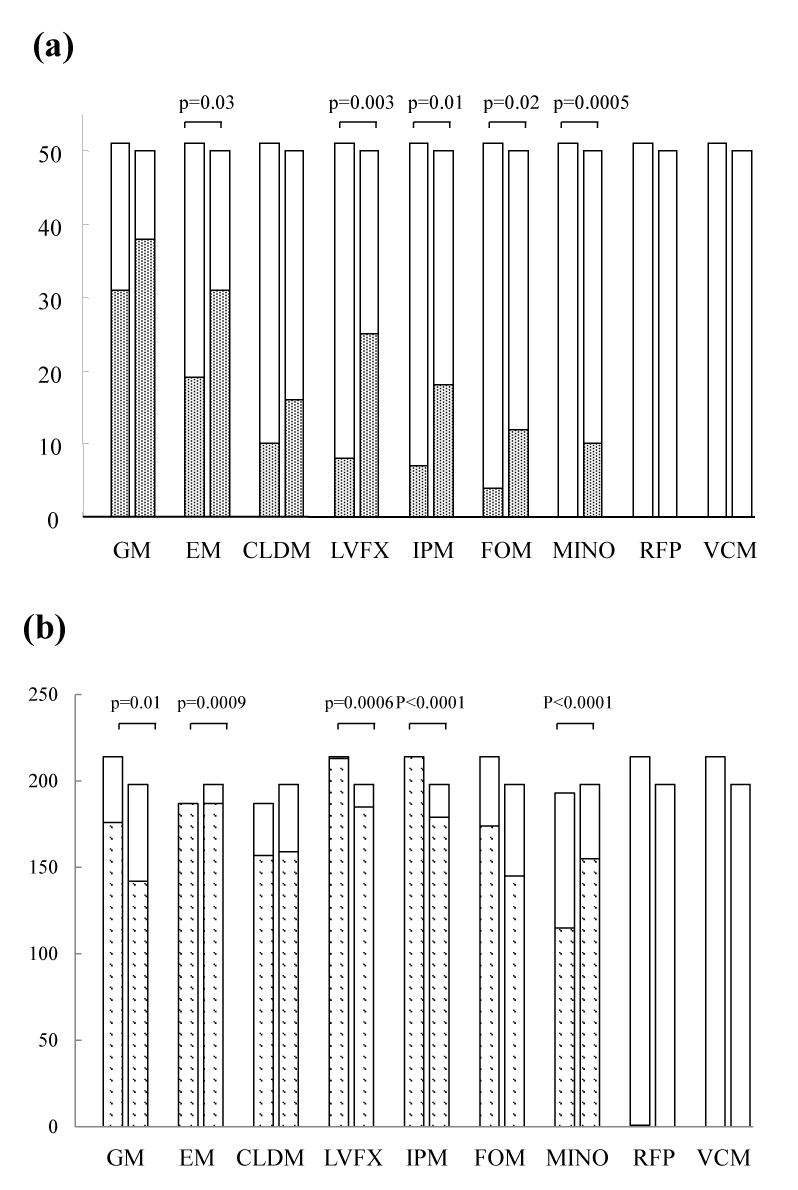
| Resistance of CA-MRSA to erythromycin (p=0.03), levofloxacin (p=0.003), imipenem (p=0.01), fosfomycin (p=0.02), and minocycline (p<0.001) significantly increased from the first half to the second half of the study period (Figure 4a). Susceptibility to clindamycin and gentamicin was not significantly different between the two halves of the study period. All CA-MRSA isolates were sensitive to rifampicin and vancomycin. The percentages of antimicrobial resistance of CA-MRSA with type IV SCCmec to antibiotics in the literature and data of the first half of the study period in the present study were shown in Table 3. | |
| Concerning HA-MRSA, resistance to minocycline (p<0.001) significantly increased from the first half to the second half of the study period (Figure 4b). | |
| Discussion | |
| The present study showed that CA-MRSA accounts for 20% of total MRSA isolates in Japan, and those ages of subjects with CA-MRSA and isolation sites have changed from the emerging stage to recent years. Antibiotic resistance has significantly increased in a tertiary hospital in Japan. | |
| Isolation of CA-MRSA during the study period was highest in 2008. However, the proportion of CA-MRSA to HA-MRSA was similar between the first (19%) and second (20%) halves of the study period. Yanagihara et al. reported that the percentage of SCCmec IV was 20% among a total of 857 cases of MRSA isolated in a nationwide study in Japan conducted from 2008 to 2009 [13]. Motoshima et al. also reported a similar proportion of CA-MRSA during 2002-2007, with 20.2% of 155 MRSA isolates in 2002-2003, 20.9% of 164 isolates in 2004-2005, and 21.5% of 162 isolates in 2006-2007 [14]. These data suggest that the proportion of CA-MRSA to total MRSA isolates among patients in recent years is approximately 20% in Japan. The proportion of MRSA outside Japan was similar in USA (20%) [15], Canada (23%) [8], and Asian countries (25.5%) [16] in recent reports. | |
| We think that the ages of patients with CA-MRSA and isolation sites have changed from the emerging stage to recent years. In the emerging stage, CA-MRSA is associated with skin and soft-tissue infections and severe systemic infections, such as sepsis and necrotizing pneumonia, in healthy children or adolescents, and otherwise healthy individuals in the community [2]. In the present study, the highest prevalence of acquisition of CA-MRSA was in patients aged in their 80s who had suffered from cardiovascular diseases, stroke, and cancer, but not healthy children and adolescents (Figure 3a). Isolation sites in the first and second halves of the study period were mainly nasopharyngeal and respiratory. These data indicate that isolation or the presence of CAMRSA implies health-care associated infections in a tertiary center, even in local areas. | |
| The current study showed that antibiotic resistance has increased from the first to the second half periods. Table 2 shows the percentages of antimicrobial resistance of CA-MRSA with type IV SCCmec to antibiotics in the literature and data of the first half of the study period in the present study. These data showed that a regional difference was present in antibiotic susceptibility for CA-MRSA. | |
| There were some limitations in the present study. A multilocus sequence typing was not performed; the typing is needed for global epidemiology of CA-MRSA [1]. Secondary, mechanism of the resistance of each drug was not performed. Determination of the presence or absence of a resistance gene to each drug or performing conjugative transfer experiments [17,18] may be required to clarify the acquisition of drug resistance of CA-MRSA. Finally, we did not show the reason(s) why antibiotic resistance has increased from the first to the second half periods in the present study; further studies are needed to clarify the reason(s). | |
References
- Mediavilla JR, Chen L, Mathema B, Kreiswirth BN (2012) Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA).CurrOpinMicrobiol 15: 588-595.
- Kawaguchiya M, Urushibara N, Kuwahara O, Ito M, Mise K, et al. (2011) Molecular characteristics of community-acquired methicillin-resistant Staphylococcus aureus in Hokkaido, northern main island of Japan: identification of sequence types 6 and 59 Panton-Valentine leucocidin-positive community-acquired methicillin-resistant Staphylococcus aureus.Microb Drug Resist 17: 241-250.
- Otter JA, French GL (2011) Community-associated meticillin-resistant Staphylococcus aureus strains as a cause of healthcare-associated infection.J Hosp Infect 79: 189-193.
- Takano T, Higuchi W, Yamamoto T (2009) Superior in vitro activity of carbapenems over anti-methicillin-resistant Staphylococcus aureus (MRSA) and some related antimicrobial agents for community-acquired MRSA but not for hospital-acquired MRSA. J Infect Chemother 15:54-57.
- McDougal LK, Fosheim GE, Nicholson A, Bulens SN, Limbago BM, et al. (2010) Emergence of resistance among USA300 methicillin-resistant Staphylococcus aureus isolates causing invasive disease in the United States.Antimicrob Agents Chemother 54: 3804-3811.
- Maree CL, Daum RS, Boyle-Vavra S, Matayoshi K, Miller LG (2007) Community-associated methicillin-resistant Staphylococcus aureus isolates causing healthcare-associated infections. Emerg Infect Dis 13:236-242.
- Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting.Am J Infect Control 36: 309-332.
- Simor AE, Gilbert NL, Gravel D, Mulvey MR, Bryce E, et al. (2010) Methicillin-resistant Staphylococcus aureus colonization or infection in Canada: National Surveillance and Changing Epidemiology, 1995-2007.Infect Control HospEpidemiol 31: 348-356.
- Oliveira DC, de Lencastre H (2002) Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus.Antimicrob Agents Chemother 46: 2155-2161.
- Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, et al. (2002) Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun 70:631-641.
- Nishi J, Miyanohara H, Nakajima T, Kitajima I, Yoshinaga M, et al. (1995) Molecular typing of the methicillin resistance determinant [mec] of clinical strains of Staphylococcus based on mechypervariable region length polymorphisms. J Lab Clin Med 126:29-35.
- Mehrotra M, Wang G, Johnson WM (2000) Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J ClinMicrobiol 38:1032-1035.
- Yamamoto T, Takano T, Higuchi W, Iwao Y, Singur O, et al. (2012) Comparative genomics and drug resistance of a geographic variant of ST239 methicillin-resistant Staphylococcus aureus emerged in Russia.PLoS One 7: e29187.
- Yamamoto T, Nishiyama A, Takano T, Yabe S, Higuchi W, et al. (2010) Community-acquired methicillin-resistant Staphylococcus aureus: community transmission, pathogenesis, and drug resistance. J Infect Chemother 16:225-254.
- Farr AM, Aden B, Weiss D, Nash D, Marx MA (2012) Trends in hospitalization for community-associated methicillin-resistant Staphylococcus aureus in New York City, 1997-2006: data from New York State's Statewide Planning and Research Cooperative System. Infect Control HospEpidemiol 33:725-731.
- Simor AE, Gilbert NL, Gravel D, Mulvey MR, Bryce E, et al. (2010) Methicillin-resistant Staphylococcus aureus colonization or infection in Canada: National Surveillance and Changing Epidemiology, 1995-2007.Infect Control HospEpidemiol 31: 348-356.
- Song JH, Hsueh PR, Chung DR, Ko KS, Kang CI, et al. (2011) Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study.J AntimicrobChemother 66: 1061-1069.
- Shittu AO, Udo EE, Lin J (2009) Phenotypic and molecular characterization of Staphylococcus aureus isolates expressing low- and high-level mupirocin resistance in Nigeria and South Africa.BMC Infect Dis 9: 10.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
|||
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 13656
- [From(publication date):
December-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 9102
- PDF downloads : 4554
