Long Term Trastuzumab in Metastatic Setting of the Patients with HER2 Positive Breast Cancer
Received: 15-Dec-2015 / Accepted Date: 19-Jan-2016 / Published Date: 26-Jan-2016 DOI: 10.4172/2572-4118.1000103
Abstract
Empirically, trastuzumab has been continued in many patients with disease progression, mainly due to its favourable safety profile and the assumption that progression was due to resistance to the co-administered chemotherapeutic agent but not trastuzumab itself. Retrospective analyses provided some support for this treatment approach. Retrospectively were analyzed records of 11 patients with relapsing HER2 overexpressed breast cancer exposed to long-term trastuzumab therapy concurrently with multiple lines of chemotherapy or hormonotherapy according to the subsequent relapse of the disease. We evaluated the initial stage of the disease, site of relapse, median time to progression (TTP), median duration of response to first line therapy for metastatic, and duration and toxicity of long term trastuzumab. The starting points were the date of initial diagnosis of breast cancer and the date in which trastuzumab-based therapy started as a result of distant relapse of the disease. The dates of tumor and tumor progression were used to calculate median TTP. Eleven women with median age of 44.0 years (range 38-57) were included. 45.5% were pre-treated with trastuzumab-based therapy in adjuvant setting. Visceral metastases were identified in 7 patients (64%) and bone/soft tissue in 4 (36%). Median time to progression (TTP) was 43 months (range 13-115 months). Median duration of response to first line therapy concurrently with trastuzumab was 20 months (range 8-45 months). Median duration of trastuzumab therapy was 44 months (range 15-93 months). No unexpected toxic effects occurred. At a median follow-up of 37 months (range 15–93 months) from the start of rechallenge with trastuzumab-based first line therapy, 1 patient had died and 10 are still on trastuzumab therapy. Trastuzumab paired with a standard chemotherapy as starting treatment can also be continued alone, with subsequent chemotherapy or with hormone-blocking medications. Long-term trastuzumab-based therapy showed clinical benefit. In women at higher risk of recurrence and with no signs of a weak heart, long term trastuzumab offers far more benefits than risks
Keywords: Breast conserving surgery; Oncoplastic breast surgery; Wide local excision; Breast cancer; Cosmesis
Abbreviations
HER2: Human Epidermal Growth Factor Receptor 2; AKT: Protein Kinase B (PKB); LVEF: Left Ventricular Ejection Fraction; MBC: Metastatic Breast Cancer; TTP: Median Time to Progression; TTSP: Median Time to Second Progression Since the Initial Diagnosis; OS: Overall Survival; AJCC: American Joint Committee on Cancer Staging; HR: Hormonal Receptor
Introduction
Approximately 20% of breast cancer tumours show overexpression of human epidermal growth factor receptor 2 (HER2) protein, and HER2 overexpression has been frequently identified as a factor indicating poor prognosis [1,2]. Trastuzumab is a humanized monoclonal antibody that targets the extracellular domain of the HER2 protein. The antitumor functions of trastuzumab are associated with its ability to modulate signalling through the HER-2/neu receptor as well as initiate antibody-dependent cell-mediated cytotoxicity. Recent studies suggest that trastuzumab disrupts HER2/HER3 interactions, leading to down regulation of AKT signalling, which results in decreased cell proliferation [3]. Clinical studies have intensively investigated the potential therapeutic roles of this compound since the late 1990s. In particular, studies have shown that a combination of trastuzumab and conventional chemotherapy is significantly more effective at treating HER2-overexpressing metastatic breast cancers than chemotherapy alone [4]. In light of this finding, trastuzumab has come to be used in adjuvant or neo-adjuvant settings for operable HER2-overexpressing breast cancers [5-7].
Now, trastuzumab is an integral part of the therapy of HER2/ neu-positive breast cancer from early stage tumors to advanced stage disease. In the adjuvant setting, at any stage, the use of trastuzumab reduces recurrence by about 50% and increases overall survival by about 30% [8]. In women with metastatic HER2/neu-positive breast cancer, the use of trastuzumab may result in an improved prognosis compared with women with metastatic HER2/neu-negative breast cancer. A single institution study evaluating more than 2000 women with metastatic breast cancer demonstrated a 44% reduction in the risk for death among women with HER2/neu-positive disease who received trastuzumab compared with women with HER2/neu-negative breast cancer (P<0.0001, hazard ratio [HR]=0.56) [9].
The major toxicity associated with trastuzumab therapy is cardiac dysfunction. Most of the cardiac toxicity seen with treatment is limited to asymptomatic decreases in the left ventricular ejection fraction (LVEF); however, severe congestive heart failure will occur in approximately 4% of patients [10]. In cases where left ventricular dysfunction is asymptomatic, most studies have withheld treatment if the LVEF decreased by more than 15% of baseline or fell below 40%- 45% [11]. It should be noted that observed cardiac toxicity is almost always reversible with discontinuation of the drug, and the drug can often be restarted once left ventricular function has been restored.
However, despite trastuzumab’s promising usefulness in clinical settings, only a relatively small percentage of patients are reported to benefit from trastuzumab therapy alone, with response rates to trastuzumab as a single agent of approximately 20% [12]. In addition, even when trastuzumab therapy leads to temporary tumour shrinkage, clinical relapse is observed in virtually all metastatic patients. More effective treatment of HER2-overexpressing breast cancer requires a deeper understanding of the mechanisms of resistance to trastuzumab. The majority of trastuzumab-treated patients exhibit either de novo or acquired resistance to treatment or have early relapse [13]. In these patients, conventional chemotherapy, usually taxane based, is stopped and the patient is switched to a second-line treatment. The combination of lapatinib and capecitabine has been established as an additional HER2-directed treatment option for this group of patients. A significant prolongation of median time to disease progression (8.4 versus 4.4 months for capecitabine alone) could be achieved with this novel dual tyrosine kinase inhibitor of HER1 and HER2 [14].
In this article we present the results of treatment in patients with HER2 positive metastatic breast cancer (MBC) exposing to long term trastuzumab therapy (more than 2 years) using multiple lines of therapy concurrently with trastuzumab, balancing between aggressive systemic therapy and the quality of life and trying to identify factors that should be considered when selecting therapy for a patient with HER2 positive MBC that has progressed after first-line anti HER2 treatment.
Methods
We retrospectively analyzed records of 11 patients with relapsing HER2 overexpressed breast cancer exposed to long term trastuzumab therapy concurrently with multiple lines of chemotherapy or hormonotherapy according to the subsequent relapse of the disease in the period from July 2004 to January 2010. July 2004 was starting point because since then trastuzumab was available for the treatment of metastatic HER2 overexpressed breast cancer. At a cut-off point (June 2012), 10 patients were still alive. This analysis represents a retrospective audit of the standard treatment practice in our institution and was approved by The University Clinic of Radiotherapy and Oncology audit and Clinical Research Committee. All patients in this study have signed standard consent form, approved by Ministry of Health, for acceptance of treatment required and for use of their clinical data for educational and scientific purposes.
We evaluated the initial stage of the disease, site of relapse, median time to progression (TTP), median time to second progression since the initial diagnosis (TTSP), median duration of response to first line therapy for metastatic disease, overall survival (OS) and duration and toxicity of long term trastuzumab.
The starting points were the date of initial diagnosis of breast cancer and the date in which trastuzumab-based therapy started as a result of relapse of the disease. The dates of tumour relapse and tumour progression were used to calculate median TTP and median TTSP, whereas the date of death from any cause was used to calculate OS. Surviving patients were censored at the date of the cut-off point (June 2012).
Baseline characteristics of the patients at the time of initial treatment and at the time of rechallenge with trastuzumab because of metastatic disease are summarized in Table 1. All patients were women ≥ 18 years of age (median 44.0 years, range 38-57) with operable node-positive or node-negative, HER2 overexpressing histological confirmed breast adenocarcinoma. Patients had initial curative surgery (mastectomy or quadrantectomy including an axillary node dissection). Patients were staged according to the 2002 classification of the American Joint Committee on Cancer Staging (AJCC) [15].
| Characteristics | Number of patients (%) |
|---|---|
| Median age, years (range) | 44 (38–57) |
| HR* status (cut-off ≥10%) | |
| ER and or PgR positive | 4 (36) |
| ER and PgR negative | 7 (64) |
| Grading | |
| G2 | 6 (54) |
| G3 | 5 (46) |
| Stage at first diagnosis of breast cancer | |
| I/II | 8 (73) |
| IIIA/B | 2 (18) |
| IIIC | 1 (9) |
| Pattern of metastatic disease at the time of trastuzumab retreatment | |
| Bone/Soft tissue only | 4 (36) |
| Visceral | 7 (64) |
* HR, hormonal receptor
Table 1: Patient characteristics.
Initial Stage I disease was recorded in 3 (27%), Stage II in 5 (45%), and Stage III in 3 (27%) patients, respectively. Different tumour grades were classified and recorded according to the 2002 classification of the AJCC [15]. Tumour Grade 2 (G2) was recorded in 6 patients (54%) and Grade 3 (G3) in 5 patients (46%). Hormonal receptor (HR) status with cut-of ≥ 10% was positive in 5 patients (45%). HER2 overexpression was locally determined by CISH or immunohistochemistry(3+). Baseline LVEF was ≥ 55% using echocardiogram (ECHO) for all included patients. LVEF was measured in 3 monthly intervals during adjuvant chemotherapy. LVEF was also measured in 3 monthly intervals after rechallenge of trastuzumab because of the metastatic disease and the results were presented in 3 cut-off points (initial, at the time of rechallenge of trastuzumab, median, in the middle of the treatment period of each individual patient, and final, that was last measurement for each individual patient).
Previous adjuvant therapy is summarized in Table 2. Forty five percent of patients were pretreated with trastuzumab-based therapy in adjuvant setting. Adjuvant chemotherapy was applied in 10 patients followed by adjuvant hormonal therapy if hormonal receptors were positive (4 patients). One patient had received only adjuvant hormonotherapy. For most of the patients initial adjuvant chemotherapy consisted of antracyclin/taxane combination and if trastuzumab was a part of adjuvant treatment it was included in concurrent setting with taxane therapy. Initially, visceral metastases were identified in 7 patients (64%). Later on during the course of the disease, visceral metastases were identified in all 11 patients.
| Type and setting | Number of patients (%) |
|---|---|
| Adjuvant chemotherapy | 10/11 (91) |
| Anthracycline based | 3/10 (30) |
| Anthracyline and taxanee based | 7/10 (70) |
| Adjuvant trastuzumab | 5/11 (45.5) |
| Adjuvant endocrine therapy | 4/11 (36) |
| Tamoxifen | 2/4 (50) |
| Aromatase inhibitors | 2/4 (50) |
Table 2: Previous adjuvant therapy.
The details of trastuzumab-based first line therapy are summarized in Table 3. In 7 patients trastuzumab was combined with first line chemotherapy (taxane therapy in 6 patients and capecitabine in 1 patient). After 6 to 8 courses of concurrent taxane/trastuzumab therapy, trastuzumab was continued as monotherapy until progression of the disease, while in one patient after one year capecitabine/trastuzumab therapy and maintenance of stable disease treatment was continued with concurrent aromatase inhibitor/trastuzumab therapy (ER positive patient). First line hormonotherapy concurrently with trastuzumab was treatment of choice for 4 hormonal receptor positive patients. Subsequent lines of therapy (chemotherapy or hormonotherapy) were given according to the course of the disease concurrently with trastuzumab. Trastuzumab was continuously given beyond progression without interruption. Trastuzumab was temporally stopped in only 1 patient for a period of 2 months because of asymptomatic reversible decrease of LVEF. The median number of chemotherapeutic and hormonal lines for metastatic disease with trastuzumab was 4 (range, 2–8).
| Trastuzumab given with either chemotherapy or hormonal therapy | Number of patients (%) |
|---|---|
| Chemotherapy | |
| Taxane | 6 (55) |
| Capecitabine | 1 (9) |
| Hormonal therapy | |
| Aromatase inhibitor | 4 (36) |
| Median number of therapy lines (chemotherapy and hormonotherapy) | 4 (2-8) |
Table 3: Trastuzumab combination regimens as first systemic treatment.
Results
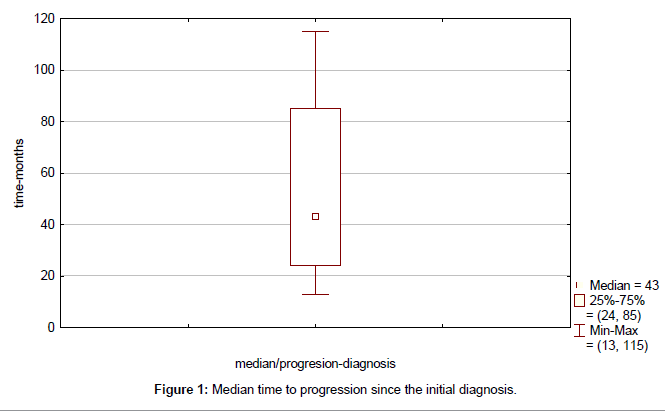
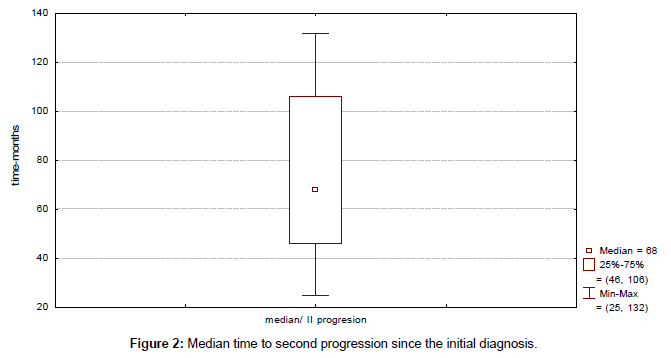
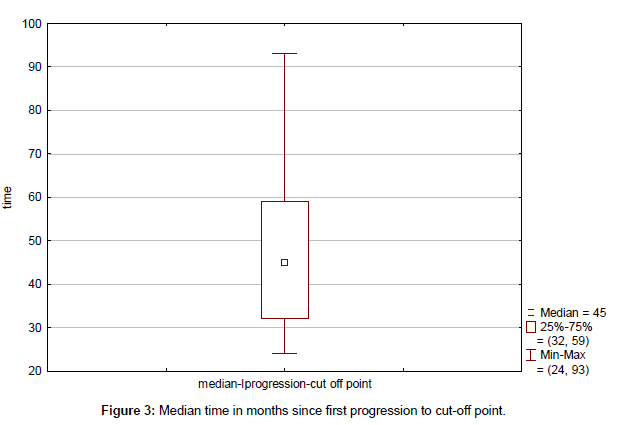
Clinical outcome is summarized in Table 4. Median time to progression since the initial diagnosis (TTP) was 43 months (range, 13-115 months) (Figure 1). Median duration of response to first line therapy concurrently with trastuzumab (median time from first to second progression) was 20 months (range, 8-45 months). Median time to second progression since the initial diagnosis (TTSP) was 68 months (range, 25-132 months) (Figure 2). Median time duration since first progression of the disease to cut-off point was 45 months (range, 24-93 months) (Figure 3). Overall median survival was not reached. Median duration of trastuzumab therapy was 44 months (range, 15-93 months).
| Results for all patients (N = 11) | Months (range) |
|---|---|
| Median time to progression | 43 (13–115) |
| Median duration of response | 20 (8–45) |
| Median time to second progression | 68 (25-132) |
| Median time from first progression to cut-off | 45 (32-59) |
| Median duration of trastuzumab therapy | 44 (15-93) |
Table 4: Results of trastuzumab-based therapy administered after progression.
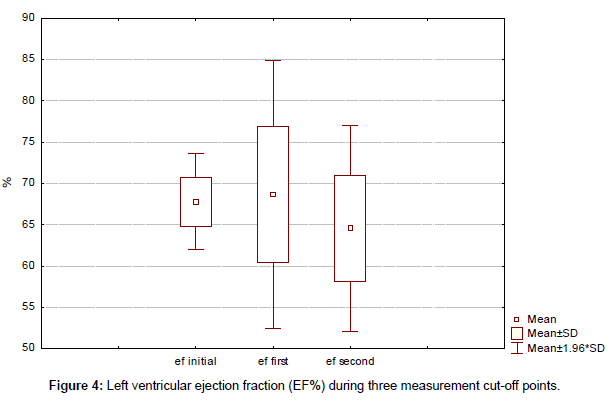
No unexpected toxic effects occurred. Trastuzumab was temporally stopped in only 1 patient for a period of 2 months. There was no statistically significant decrease of left ventricular ejection fraction (EF%) during three measurement cut-off points (initial, first, and second) with 67.8%, 68.6% and 64.5%, respectively (p<0.05) (Figure 4). At a median follow-up of 37 months (range, 15–93 months) from the start of rechallenge with trastuzumab-based first line therapy, 1 patient had died and 10 are still on trastuzumab therapy.
Discussion
In this retrospective study, the clinical outcome of 11 patients with HER2-positive metastatic breast cancer retreated with trastuzumabbased therapy beyond progression was evaluated.
Empirically, trastuzumab has been continued in many patients with disease progression, mainly due to its favourable safety profile and the assumption that progression was due to resistance to the co-administered chemotherapeutic agent but not trastuzumab itself [16]. Retrospective analyses provided some support for this treatment approach, at a weak level of evidence [17-20]. These findings have recently been confirmed by the German Breast Group 26/Breast International Group (BIG) 03-05 study, a randomized, controlled trial of trastuzumab treatment in combination with capecitabine continued beyond progression [21].
Median duration of trastuzumab therapy for our group was 44 months (range, 15-93 months). At a median follow-up of 37 months (range, 15–93 months) from the start of rechallenge with trastuzumab-based first line therapy, 1 patient had died and 10 are still on trastuzumab therapy. No unexpected toxic effects occurred. Overall median survival was not reached. Trastuzumab was temporally stopped in only 1 patient for a period of 2 months. There was no statistically significant decrease of left ventricular ejection fraction (EF%) during three measurement cut-off points (initial, first, and second) with 67.8%, 68.6% and 64.5% respectively (p < 0.05).
The main goals in treating patients with MBC are to maintain quality of life, prolong survival, and minimize toxicity. Because there is no commonly accepted standard of care in these situations, individualized treatment is based on consideration of multiple factors, making treatment selection particularly challenging in clinical practice. One must consider the hormone receptor status of the tumour, the HER2 status, the patient’s performance status, exposure to prior therapies (in particular the prior adjuvant therapy), and perhaps resistance mechanisms that may have emerged as a result of selection pressure from prior treatment.
In 7 of our retrospectively analyzed patients trastuzumab was combined with first line chemotherapy (taxane therapy 6 patients and capecitabine 1 patient). After 6 to 8 courses of concurrent taxane/ trastuzumab therapy, trastuzumab was continued as monotherapy until progression of the disease, while in one patient after one year capecitabine/trastuzumab therapy and maintenance of stable disease treatment was continued with concurrent aromatase inhibitor/ trastuzumab therapy (ER positive patient). First line hormonotherapy concurrently with trastuzumab was treatment of choice for 4 hormonal receptor positive patients. Subsequent lines of therapy (chemotherapy or hormonotherapy) were given according to the course of the disease concurrently with trastuzumab.
There is little agreement about the most appropriate treatment strategy beyond the first line. One could consider sequential endocrine manipulation. Combination chemotherapy once again may be particularly important in symptomatic patients perhaps, but there is greater toxicity compared with sequential mono chemotherapy. Sequential single-agent chemotherapy is perhaps the mainstay of therapy after patients become hormone refractory. Response may be more modest with single-agent chemotherapy, but the regimens could be better tolerated and on balance, associated with fewer toxicities and greater preservation of quality of life.
Studies continue to address the question of whether to continue trastuzumab during subsequent lines of chemotherapy following recurrence after first-line trastuzumab therapy. A systematic review of observational analyses of this question revealed that responses to a second line of trastuzumab therapy were lower than first responses but still promising (respective ORR of 33% and 59%, and respective clinical benefits of 62% and 83%) [22]. In another retrospective study, patients who continued trastuzumab therapy beyond disease progression (administered concurrently with one to two additional lines of chemotherapy) exhibited better response rates than those who halted trastuzumab (35% versus 16%, respectively). No significant differences in OS were observed [23]. In another study, however, patients who continue trastuzumab therapy following development of brain metastases experienced longer disease-free survival compared with those who receive no further trastuzumab therapy after being diagnosed with brain metastases [24].
The results from the prospective, observational German study [25] showed that survival from progression was significantly longer among 261 patients continuing trastuzumab treatment beyond disease progression (TBP: median 22.1 months). In addition to TBP, a positive endocrine receptor status, a longer relapse-free interval, no visceral metastasis, no concomitant chemotherapy during first-line treatment, and first-line response were independently significant prognostic variables for longer survival on multivariate analysis. The positive effect of trastuzumab continuation retained statistical significance in a multivariate model.
In the observational LHORA study conducted in 57 centres in France [26] 160 patients with HER2+ metastatic breast cancer who had long response to first-line trastuzumab therapy were evaluated. The PFS (progression free survival) in this population was over 6 years and an acceptable safety profile was noted, only 2% of patients had cardiac event related to the drug. No trastuzumab-related deaths were observed. The median duration of first-line trastuzumab was 4.5 years (0.8-12.1).
The data that we have presented demonstrate that HER2 does remain a valid target after progression with prior trastuzumab treatment. This validates the clinical practice of continuing HER2 inhibition after disease progression and suggests that further manipulation of HER2 with new therapies may provide additional benefit to our patients.
Our results suggest that the continuation of anti-HER2 treatment is associated with improved clinical outcome in HER2-positive metastatic breast cancer patients progressing to first line anti HER2 based therapy.
However, this study was retrospective and the results obtained could have been the consequence of more favourable clinical characteristics of patients treated with trastuzumab-based therapy after progression.
The number of HER2-targeted therapies continues to grow and some drugs are in clinical development in trastuzumab-pretreated patients, alone or in combination with other agents.
With neratinib, an oral irreversible pan-ErbB receptor tyrosine kinase inhibitor, an objective response rate of 24% was reported, with diarrhoea of grade 3–4 in 30% of cases, requiring dose reduction in 29% of patients [27].
Pertuzumab, a monoclonal antibody directed against the highly conserved dimerization domain of HER2 that inhibits HER2 homo- and heterodimerization, was evaluated in combination with trastuzumab. Pertuzumab is approved for use in combination with the first HER2 therapy ever launched, trastuzumab (Herceptin, Genentech/Roche), and with the chemotherapy agent docetaxel. In the CLEOPATRA trial, the addition of pertuzumab significantly increased median survival, compared with trastuzumab plus docetaxel (56.5 versus 40.8 months) [28,29]. When tested as a single agent early in its development, pertuzumab showed only modest antitumor activity; however, it demonstrated synergy when used with trastuzumab. Although both products are humanized monoclonal antibodies that stimulate antibody-dependent cell-mediated cytotoxicity, they bind at different points on HER2 and have slightly different mechanisms of action and together provide a more comprehensive blockade of HER2 signalling resulting with greater antitumor activity.
T-DM1 is an antibody–drug conjugate consisting of the antibody trastuzumab (Herceptin) and linked to the cytotoxin mertansine (DM1). It incorporates the antitumor activities of trastuzumab and the HER2-targeted delivery of DM1. Results from the phase III EMILIA trial showed that trastuzumab emtansine improved outcome in patients with confirmed HER2-positive locally advanced or metastatic breast cancer. The trial enrolled 991 patients and randomized 495 to receive trastuzumab emtansine and 496 to the capecitabine/lapatinib arm [30].
In the MARIANNE trial, 1095 women were given either T-DM1 alone or in combination with pertuzumab compared with Herceptin plus either docetaxel or paclitaxel. The primary endpoints of MARIANNE study were PFS and the incidence of adverse events, while secondary endpoints included overall survival, response rates and duration of response. The study showed similar PFS rates in all three groups, thus meeting its noninferiority goals. However, the PFS in the T-DM1 arms did not meet the superiority threshold.
Conclusion
Trastuzumab paired with a standard chemotherapy as starting treatment can also be continued alone, with subsequent chemotherapy or with hormone-blocking medications, such as an aromatase inhibitor or tamoxifen. In spite of the fact that it was a small and heterogeneous study group where statistical analysis does not have enough credibility and it can be difficult to draw some reliable conclusions, long term trastuzumab-based therapy showed clinical benefit (CB) and 50% of patients survive more than 122 months. In women at higher risk of recurrence and with no signs of a weak heart, long term trastuzumab offers far more benefits than risks. In the future, randomized data may answer whether continuation of trastuzumab beyond progression is a viable treatment choice compared with other available options.
References
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, et al. (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244: 707-712.
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, et al. (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235: 177-182.
- Wallasch C, Weiss FU, Niederfellner G, Jallal B, Issing W, et al. (1995) Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J 14: 4267-4275.
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, et al. (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783-792.
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, et al. (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353: 1659-1672.
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, et al. (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353: 1673-1684.
- Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, et al. (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J ClinOncol 23: 3676-3685.
- Bedard PL, Piccart-Gebhart MJ (2008) Current paradigms for the use of HER2-targeted therapy in early-stage breast cancer. Clin Breast Cancer 8 Suppl 4: S157-165.
- Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH (2010) Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J ClinOncol 28: 92-98.
- Fedenko K, Cortazar P, Keegan P, Pazdur R. (2009) Trastuzumabcardiotoxicity. FDA review of four adjuvant breast cancer clinical trials leading to trastuzumab marketing approval. J ClinOncol 27: 115-120.
- Chien AJ, Rugo HS (2010) The cardiac safety of trastuzumab in the treatment of breast cancer. Expert Opin Drug Saf 9: 335-346.
- Hudis CA (2007) Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 357: 39-51.
- Spector NL, Blackwell KL (2009) Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J ClinOncol 27: 5838-5847.
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, et al. (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355: 2733-2743.
- Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, et al. (2002)AJCC Cancer Staging Manual. (6th edn), Springer-Verlag, New York.
- Tripathy D, Slamon DJ, Cobleigh M, Arnold A, Saleh M, et al. (2004) Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J ClinOncol 22: 1063-1070.
- Gelmon KA, Mackey J, Verma S, Gertler SZ, Bangemann N, et al. (2004) Use of trastuzumab beyond disease progression: observations from a retrospective review of case histories. Clin Breast Cancer 5: 52-58.
- GarcÃa-Sáenz JA, MartÃn M, Puente J, López-Tarruella S, Casado A, et al. (2005) Trastuzumab associated with successive cytotoxic therapies beyond disease progression in metastatic breast cancer. Clin Breast Cancer 6: 325-329.
- Bartsch R, Wenzel C, Hussian D, Pluschnig U, Sevelda U, et al. (2006) Analysis of trastuzumab and chemotherapy in advanced breast cancer after the failure of at least one earlier combination: an observational study. BMC Cancer 6: 63.
- Bartsch R, Wenzel C, Altorjai G, Pluschnig U, Rudas M, et al. (2007) Capecitabine and trastuzumab in heavily pretreated metastatic breast cancer. J ClinOncol 25: 3853-3858.
- vonMinckwitz G, du Bois A, Schmidt M, Maass N, Cufer T et al. (2009) Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German Breast Group 26/Breast International Group 03-05 Study. J ClinOncol 27: 1999-2006.
- Fabi A, Metro G, Ferretti G, Giannarelli D, Di Cosimo S, et al. (2008) Do HER-2 positive metastatic breast cancer patients benefit from the use of trastuzumab beyond disease progression? A mono-institutional experience and systematic review of observational studies. Breast 17: 499-505.
- Cancello G, Montagna E, D'Agostino D, Giuliano M, Giordano A, et al. (2008) Continuing trastuzumab beyond disease progression: outcomes analysis in patients with metastatic breast cancer. Breast Cancer Res 10: R60.
- Park IH, Ro J, Lee KS, Nam BH, Kwon Y, et al. (2009) Trastuzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol 20: 56-62.
- Jackisch C, Welslau M, Schoenegg W, Selbasch J, Harich H-D, et al. (2014)Impact of trastuzumab treatment beyond disease progression for advanced/metastatic breast cancer on survival Results from a prospective, observational study in Germany. The Breast Vol23: 603-608.
- Spano JP, Beuzeboc P, Coeffic D, Arnould L, Lortholary A, et al. (2015) Long term HER2+ metastatic breast cancer survivors treated by trastuzumab: Results from the French cohort study LHORA. Breast 24: 376-383.
- Burstein HJ1, Sun Y, Dirix LY, Jiang Z, Paridaens R, et al. (2010) Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J ClinOncol 28: 1301-1307.
- Baselga J, Cortés J, Kim SB, Im SA, Hegg R, et al. (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366: 109-119.
- Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, et al. (2012) Confirmatory overall survival analysis of CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study with pertuzumab, trastuzumab, and docetaxel in patients with HER2-positive first-line MBC. Cancer Res 72: P5-18-26.
- Verma S, Miles D, Gianni L et al. (2012) Updated overall survival results from EMILIA, a phase 3 study of trastuzumabemtansine (T-DM1) vscapecitabine (X) and lapatinib (L) in HER2-positive locally advanced or metastatic breast cancer (MBC). ESMO Congress.
Citation: Smichkoska S, Lazarova E (2016) Long Term Trastuzumab in Metastatic Setting of the Patients with HER2 Positive Breast Cancer. J Blood Lymph 1:103. DOI: 10.4172/2572-4118.1000103
Copyright: © 2016 Smichkoska S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12404
- [From(publication date): 3-2016 - Nov 09, 2024]
- Breakdown by view type
- HTML page views: 11675
- PDF downloads: 729