Locally Advanced Anaplastic Thyroid Carcinoma with Long-Term Survival of More Than 7 Years after Combined Surgery Including Tracheal Resection and Radiotherapy: Case Report
Received: 15-May-2014 / Accepted Date: 11-Jun-2015 / Published Date: 16-Jun-2015 DOI: 10.4172/2161-119X.1000197
Abstract
We present a case of locally advanced Anaplastic Thyroid Carcinoma (ATC) with tracheal invasion in a 67-yearold elderly Chinese man who was treated with radical surgery encompassing total thyroidectomy, neck dissection and tracheal resection followed by adjuvant radiotherapy. Long-term disease-free survival is more than 7 years to date. A 10-year literature review of locally advanced ATC with long-term survival (more than 2 years) is also presented.
Keywords: Anaplastic thyroid carcinoma, Long term survival, Tracheal invasion
249682Introduction
Anaplastic thyroid carcinoma (ATC) accounts for about 2% of all thyroid carcinomas and is one of the most aggressive human malignancies [1-4]. In most series, mean survival time from diagnosis is 6 months regardless of treatment [5-13]. Peak incidence is usually more than 60 years of age and it occurs more commonly in females than males. Patients usually present with symptoms of extensive local invasion such as pain, dysphagia, hoarseness, respiratory distress and a rapidly enlarging neck mass.
We present a case of locally advanced ATC with trachea invasion in an elderly male that was treated with radical surgery and adjuvant radiotherapy with long-term disease-free survival of more than 7 years.
Case Report
A 67-year-old Chinese man first presented to us with a left neck mass of 2 months’ duration associated with hoarseness and compressive symptoms of 1 month.
He was a previous smoker and had a history of bilateral pulmonary silicosis, having previously worked in a granite quarry for more than 25 years. He has no family history of thyroid disease or previous exposure to irradiation.
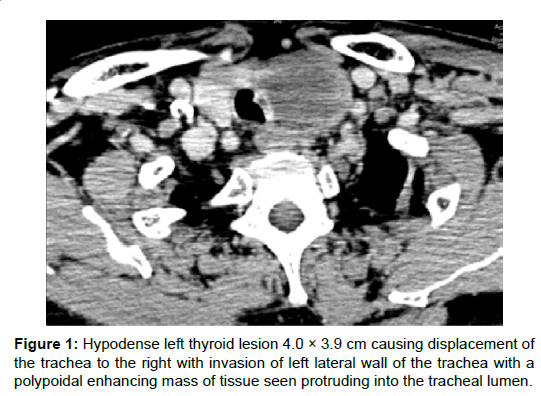
On examination, there was a hard 4 cm left thyroid mass that was fixed to the larynx and trachea. There were no palpable cervical nodes. Nasoendoscopy examination revealed left vocal cord paresis in the adducted position. Computed tomography (CT) revealed a left thyroid lesion with possible tracheal invasion (Figure 1). The left vocal fold was abducted. There was no evidence of metastatic cervical lymphadenopathy and systemic review was negative for distant metastasis. Fine-needle aspiration cytology (FNAC) showed features of a high-grade malignant tumour with necrosis favouring an anaplastic carcinoma of the thyroid. Thyroid function tests were normal.
He underwent elective total thyroidectomy, bilateral level 6 neck dissection, and tracheal resection with primary end-to-end anastomosis. There was frank tracheal invasion with gross tumour seen intra-luminally. Frozen section analysis showed a malignant highgrade neoplasm. Surgery was otherwise uneventful and he recovered well post-operatively with no complications. He was discharged well 12 days after surgery.
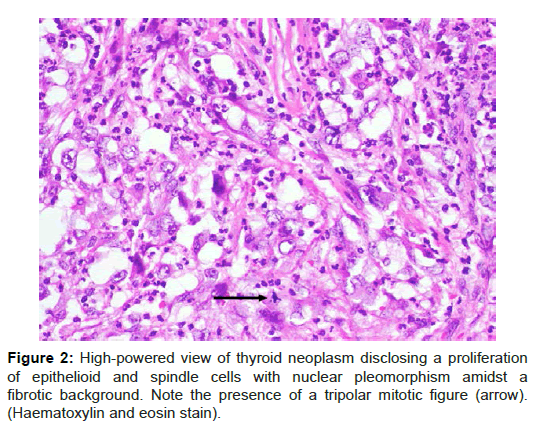
Final histology showed anaplastic carcinoma of the isthmus and left hemithyroid measuring 5 × 3.7 × 2.5 cm. It was a high-grade neoplasm composed of epithelioid and spindle cells with marked anisonucleosis. Necrosis and mitotic activity including tripolar mitotic figures were present (Figure 2). The tumour had infiltrated posteriorly into the adjacent trachea reaching the connective tissue below the epithelial lining of the trachea and was 2 mm from the closest tracheal margin. No follicular or papillary component was noted despite extensive sampling. On immunohistochemistry, the tumour was positive for cytokeratin AE1/3 but not CD31. A total of 11 level 6 lymph nodes were negative for tumour.
He subsequently underwent adjuvant intensity-modulated radiation therapy (IMRT) of 66Gy over 33 fractions. He has since been on regular follow-up and to date has been disease-free for more than 7 years. Post-treatment CT scans has showed no evidence of local recurrence or distant metastasis. He is on thyroxine replacement.
Discussion
ATC is one of the most lethal human cancers and to date, the management remains challenging and controversial. Based on the American Thyroid Guidelines on ATC published in 2012 [14], in patients with extra-thyroidal invasion, an en bloc resection should be considered if grossly negative margins (R1 resection) could be achieved.
There have been many studies looking at various prognostic factors affecting survival. Kebebew et al. [5] studied a cohort of 516 patients with ATC wherein multivariate analysis showed that although most patients with ATC had an extremely poor prognosis, patients less than 60 years old with intra-thyroidal tumours survived longer. Surgical resection with external beam radiotherapy was associated with lower cause-specific mortality.
Other studies have variably shown that younger age, tumour size less than 6 cm, localized disease, female gender, and tumour resectability are independent predictors of lower cause-specific mortality [8,15-17]. In particular, complete surgical resection appears to be an important determinant of survival. Haigh et al. [18] reported that the primary factor associated with survival was potential curative surgery. In their study, neither tumour size nor age influenced survival. Kobayashi et al. [19] also observed that complete tumour resection achieved better prognosis and that age did not significantly impact survival.
In contrast to the above findings, Sugitani et al. [9] performed a retrospective analysis of 44 patients with ATC and devised a novel prognostic index (PI) based on four prognostic factors to select patients for aggressive multimodal treatment. The features were the presence of acute symptoms, large tumour size (>5 cm), distant metastasis, and leukocytosis (white blood cell count >10,000/mm3), but notably did not include complete surgical resection. The presence of acute symptoms and large tumour size probably reflect rapid disease progression. Smaller tumour size may correlate with resectability. Patients with distant metastasis inevitably do poorly and the presence of leukocytosis likely represent the late stage of specific subtypes of ATC secreting granulocyte colony-stimulating factor (G-CSF) or related cytokines. The PI is calculated by totaling the number of unfavourable prognostic factors a given patient possessed: 0 to 4. The study showed that patients whose PI was ≤ 1 had a 62% survival rate at 6 months. No patients whose PI was ≥ 3 survived more than 6 months and all patients whose PI was 4 died within 3 months. The authors also noted that the mean PI of the patients treated by multimodal therapy was 0.6 (either 0 or 1), whereas the PI of those who were not was 2.3, and hence proposed that multimodal treatment be advocated for PI of ≤1 while aggressive treatment is avoided when PI is ≥ 3.
Orita et al. [20] recently published the prospective application of this PI in the treatment strategy of 74 patients with ATC. 6-month survival rates for PI ≤ 1 and PI ≥ 3 were 72% and 12%, respectively. Both groups (P1 ≤ 1 and ≥ 3) demonstrated significantly better diseasespecific survival as compared to the previous study above. Within each group, the survival rates did not differ between stages. The authors thus concluded that the PI is valid for anticipating prognosis and aiding timely decisions on treatment policy for ATC.
For our patient, long-term survival with no evidence of disease after 7 years is unusual, especially considering the presence of tracheal invasion. However, based on the above PI, he has a PI of 1 for tumour size, which indicated that he would have benefitted from the multimodal treatment he received.
In the past, there has been anecdotal evidence of cases of ATC with long-term survival. Since the mid-1980s, a group of poorly differentiated thyroid cancers (PDTC) has been recognized and considered to be tumours of biological aggressiveness intermediate between the more indolent well-differentiated thyroid carcinomas and ATC [21]. Historically, the distinction between poorly differentiated thyroid cancer (PDTC) and ATC has always been difficult. In our patient, there was no question that the tumour was an ATC.
We carried out a literature review of all ATC cases with survival greater than 2 years. Prior to review, we identified several features thought to influence survival as discussed above, namely age, tumour size, extent of tumour spread, adequacy of surgical resection, histopathology and neoadjuvant/adjuvant therapy. We restricted our review to papers published after January 1990. Exclusion criteria included papers not published in peer-reviewed journals as well as any case series in which the prognostic factors for the longest surviving cases were not specified. Medical subject headings and main keywords used were: ‘undifferentiated’, ‘anaplastic’ and ‘thyroid’, with variants of the main keywords also applied.
The initial review yielded a total of 37 articles, 10 case reports and 21 case series. 13 case series were further excluded due to insufficient data. We further excluded 3 case reports and 1 case series with only intra-thyroidal ATC tumours (T4a) for ease of comparison. A total of 7 case reports and 7 case series remained for our review.
A total of 22 cases of locally advanced ATC (T4b) with long-term survival of more than 2 years were compiled from the remaining 14 articles (Table 1). The length of survival varied from more than 2 to 12 years. The age at diagnosis ranged from 26 to 85 years. 4 cases [22- 24] were diagnosed with ATC incidentally after surgery for presumed benign thyroid disease. Of cases that were known, most did not present with acute symptoms, usually that of a rapidly enlarging neck mass. Only 3 cases [22,25,26] had evidence of tracheal invasion while 1 case [27] had tumour extending to the cervical esophagus.
All cases received multi-modality treatment with most cases being treated with radical surgery followed by either concurrent chemoradiation or radiotherapy or radioactive iodine ablation. Only 3 cases [27-29] received neoadjuvant chemoradiation followed by surgery. The extent of thyroidectomy performed also differed and ranged from lobectomy to total thyroidectomy. All patients with clinically or radiologically positive cervical lymph nodes underwent neck dissections. Surgical resection included debulking surgery, macroscopically complete resections and microscopically complete resections. In general, most studies concluded that although the prognosis of most patients with ATC continues to be poor, complete resection combined with adjuvant chemotherapy and radiotherapy resulted in better survival.
| SN | Journal | Age/ Gender |
Presentation | Extent of surgery | Positive margins | Neoadjuvant / Adjuvant Therapy | Tumour size (cm) | Formal Histopathology | Presence of extrathyroidal spread & location | TMN Stage | Length of survival |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Kanaseki et al.[25] | 52/F | Enlarging anterior neck mass with vocal cord palsy | TT with tracheal wall resection Interval upper mediastinal LN dissection |
No | Adjuvant CTX followed by CRT | 9.0 × 6.5 × 3.0 | ATC with tracheal invasion Positive superior mediastinal LN | Yes Tracheal wall and mediastinal LN | Stage IVB T4bN1bM0 |
>2 years |
| 2 | Shinohara et al. [27] | 53/F | Anterior neck pain with left thyroid nodule | Total pharyngo–laryngo–esophagectomy with bilateral neck and upper mediastinum LN dissection | No | Neoadjuvant CTX followed by RT Tumour regrowth on MRI after RT, proceeded with surgery | 4.1 | Tumour replaced by granulation tissue and necrosis, no cancer cells (ATC on initial fine-needle aspiration cytology) |
Yes Cervical esophagus | Stage IVB T4bN0MO |
>2 years |
| 3 | Kurukahvecioglu et al. [22] | 35/F | Rapidly growing right thyroid nodule initially Tracheal mass 9 months later Suspicious right neck nodes on PET-CT 5 months later | Right lobectomy Interval radical excision of tracheal mass and left lobectomy Interval right radical ND |
No | Adjuvant CTX and RT after 2nd surgery | 2.1 × 1.5 | Right thyroid nodule: benign Tracheal mass: ATC, 3 LN positive Right ND: negative |
Yes Trachea and strap muscles | Stage IVB T4bN1aM0 |
>3 years after second surgery |
| 4 | Noguchi et al. [28] | 51/M | Rapidly growing right thyroid nodule with mild tenderness | Right lobectomy with right levels 2-4 and level 6 ND with intraoperative RT | No | Oral valproic acid daily NeoadjuvantCRT Adjuvant CTX | 3 × 4.1 × 3.5 | Tumour surrounded by fibrous tissue divided by thin ring of PTC; encapsulating fibrous tissue contained remnants of ATC LN: 4 out of 26 positive | Yes Strap muscles | Stage IVB T4bN1M0 |
>2 years |
| 5 | Olthof et al. [31] | 76/F | Enlarging anterior neck mass | TT with left modified radical neck dissection (levels 2-6) | Yes | Adjuvant radioactive iodine therapy I131 Adjuvant RT | 7 × 6 × 4 | Hürthle cell carcinoma dedifferentiated to ATC with differentiation along rhabdomyoblastic cell lines Level 2 LN positive | Yes Regional cervical LN | Stage IVB T4aN1aM0 |
>3 years |
| 6 | Liu et al. [23] | 68/M | Anterior neck mass with compressive symptoms and dyspnoea | TT with removal of enlarged LNs | No | Adjuvant RT | 5 × 3 × 4 | ATC LN negative | Yes Strap muscles | Stage IVB T4bN0M0 |
>10 years |
| 7 | Pichardo-Lowden et al. [26] | 26/F | Rapidly growing anterior neck mass with odynophagia and voice change | TT and left modified radical ND | No | Adjuvant CRT | 5 × 4 | Undifferentiated carcinoma LN negative | Yes Tracheal wall, strap and sternocleidomastoid muscles, encasing left internal jugular vein and adhering to common carotid artery | Stage IVB T4bN0M0 |
>2 years |
| 8 | Akaishi et al. [24] | 48/F | Not known | Now known but completely resected | No | No | <5 | Incidental small focus of ATC | Yes Not known | Stage IVB | >12 years 7 months |
| 9 | Akaishi et al. [24] | 68/F | Not kwown | Not known but debulking done | Not known | Adjuvant RT and CTX | <5 | Incidental small focus of ATC | Yes Not known | Stage IVB | 9 years |
| 10 | Akaishi et al. [24] | 66/F | Not known | Not known but completely resected | No | Adjuvant RT and CTX | >5 | ATC | Yes Not known | Stage IVB | >3 years |
| 11 | Kihara et al. [32] | 82 | Immobile thyroid mass | Subtotal thyroidectomy | Not known | Adjuvant CRT | 4.9 | ATC | Yes Surrounding muscle | Stage IVB T4bN0M0 |
6 years 4 months |
| 12 | Kim et al. [33] | 57/F | Not known | TT | Not known | Adjuvant RT and CTX | 5 | ATC | Yes Not known | Stage IVB | 4 years 4 months |
| 13 | Kim et al. [33] | 55/F | Not known | TT | Not known | Adjuvant RT | 4 | ATC | Yes Not known | Stage IVB | >3 years 5 months |
| 14 | Siironen et al. [34] | 85/F | Not known | Thyroid lobectomy | Not known | Adjuvant RT | Not known | ATC | Yes Recurrent laryngeal nerve | Stage IVB T4bN0M0 |
>6 years 6 months |
| 15 | Siironen et al. [34] | 62/M | Not known | Radical surgery including TT | Not known | Adjuvant CRT | Not known | ATC | Yes Not known | Stage IVB T4bN0M0 |
5 years 4 months |
| 16 | De Crevoisier et al. [29] | 75/M | Not known | Macroscopically complete resection | Not known | Adjuvant CRT | Not known | ATC concomitant with PTC or FTC | Yes Not known | Stage IVB T4bN1M0 |
6 years 6 months |
| 17 | De Crevoisier et al. [29] | 72/F | Not known | Incomplete resection | No | Adjuvant CRT | Not known | ATC concomitant with PTC or FTC | Yes Not known | Stage IVB T4bN0M0 |
>4 years |
| 18 | De Crevoisier et al. [29] | 52/F | Not known | Macroscopically complete resection | Not known | Neoadjuvant CRT | Not known | ATC | Yes Regional cervical LN | Stage IVB T4bN1M0 |
2 years 3 months |
| 19 | De Crevoisier et al. [29] | 75/F | Not known | Macroscopically complete resection | Not known | Adjuvant CRT | Not known | ATC | Yes Regional cervical LN | Stage IVB T4bN1M0 |
2 years 1 month |
| 20 | De Crevoisier et al. [29] | 58/M | Not known | Incomplete resection | No | Adjuvant CRT | Not known | ATC concomitant with PTC or FTC | Yes Regional cervical LN | Stage IVB T4bN1M0 |
2 years 1 month |
| 21 | Ito et al. [35] | 77/F | Not known | TT and ND | Yes | Adjuvant CRT | Not known | ATC | Yes Local spread and regional cervical LN | Stage IVB T4bN1M0 |
>4 years 6 months |
| 22 | Rodriguez et al. [36] | 58/M | Not known | Total thyroidectomy and multiple LN dissections (5) | Not known | Adjuvant CTX | Not known | ATC with associated PTC | Yes Local spread and regional cervical LN | Stage IVB T4bN1M0 |
>5 years 10 months |
Table 1: Cases of locally advanced (T4b) ATC with long-term survival of >2 years.
There have been 3 cases with comparable survival in the literature with survival of more than 9, 10 and 12 years [23,24]. All cases were characterized by having just an incidental focus of ATC within an otherwise well-differentiated thyroid carcinoma with limited extrathyroidal spread. Our case however, was different as our patient presented with a gross ATC tumour with tracheal invasion and we were fortunate that the disease was still surgically resectable.
A widely cited staging system by Shin et al. [30] for papillary thyroid cancer is based on the depth of tracheal invasion. Stage I disease abuts the external perichondrium of the trachea but without cartilaginous erosion. Stage II disease invades into the cartilage or causes cartilage destruction. Stage III disease extends into the lamina propria of the tracheal mucosa. Stage IV disease is full-thickness invasion through the tracheal mucosa. There is no similar staging system for ATC but based on the above, the degree of tracheal invasion for our case would be classified as Stage 3. Based on the histological examination, the tumour was about 2 mm from the closest tracheal resection margin with tumour seen beneath the epithelium of the trachea. The involved tracheal segment was completely resected and primary anastomosis was performed.
Our case represents an anecdotal case wherein it is possible to achieve cure with clear surgical margins. Although there is general reluctance to attempt surgical resection in anaplastic carcinoma due to uniformly poor prognosis, our case concurs with the American Thyroid Guidelines on ATC [14] in which patients with ATC and extra-thyroidal invasion should have en bloc resection if grossly negative margins can be achieved. In our opinion, being able to achieve clear negative margins is the single most important prognostic factor for patients with ATC.
Conclusion
The prognosis of ATC remains poor as it is characterized by aggressive and extensive disease at presentation, the inability in most patients to perform radical enough surgery in order to achieve clear margins, high morbidity of complete extirpation and limited response to radiotherapy or chemotherapy. However, if complete surgical resection is possible, patients should be treated aggressively with a combination of surgery and adjuvant radiotherapy.
References
- Pasieka JL (2003) Anaplastic thyroid cancer. CurrOpinOncol 15: 78-83.
- Ain KB (1998) Anaplastic thyroid carcinoma: behavior, biology, and therapeutic approaches. Thyroid 8: 715-726.
- Giuffrida D, Gharib H (2000) Anaplastic thyroid carcinoma: current diagnosis and treatment. Ann Oncol 11: 1083-1089.
- O'Neill JP, O'Neill B, Condron C, Walsh M, Bouchier-Hayes D (2005) Anaplastic (undifferentiated) thyroid cancer: improved insight and therapeutic strategy into a highly aggressive disease. J LaryngolOtol 119: 585-591.
- Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A (2005) Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer 103: 1330-1335.
- Goutsouliak V, Hay JH (2005) Anaplastic thyroid cancer in British Columbia 1985-1999: a population-based study. ClinOncol (R CollRadiol) 17: 75-78.
- Besic N, Auersperg M, Us-Krasovec M, Golouh R, Frkovic-Grazio S, et al. (2001) Effect of primary treatment on survival in anaplastic thyroid carcinoma. Eur J SurgOncol 27: 260-264.
- McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, et al. (2001) Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery 130: 1028-1034.
- Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A (2001) Prognostic factors and therapeutic strategy for anaplastic carcinoma of the thyroid. World J Surg 25: 617-622.
- Passler C, Scheuba C, Prager G, Kaserer K, Flores JA, et al. (1999) Anaplastic (undifferentiated) thyroid carcinoma (ATC). A retrospective analysis. Langenbecks Arch Surg 384: 284-293.
- Nilsson O, Lindeberg J, Zedenius J, Ekman E, Tennvall J, et al. (1998) Anaplastic giant cell carcinoma of the thyroid gland: treatment and survival over a 25-year period. World J Surg 22: 725-730.
- JunorEJ, Paul J, Reed NS (1992) Anaplastic thyroid carcinoma: 91 patients treated by surgery and radiotherapy. Eur J SurgOncol 18: 83-88.
- Demeter JG, De Jong SA, Lawrence AM, Paloyan E (1991) Anaplastic thyroid carcinoma: risk factors and outcome. Surgery 110: 956-961.
- Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, et al. (2012) American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 22: 1104-1139.
- VenkateshYS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, et al. (1990) Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer 66: 321-330.
- Are C, Shaha AR (2006) Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann SurgOncol 13: 453-464.
- Tan RK, Finley RK 3rd, Driscoll D, Bakamjian V, Hicks WL Jr, et al. (1995) Anaplastic carcinoma of the thyroid: a 24-year experience. Head Neck 17: 41-47.
- Haigh PI, Ituarte PH, Wu HS, Treseler PA, Posner MD, et al. (2001) Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer 91: 2335-2342.
- Kobayashi T, Asakawa H, Umeshita K, Takeda T, Maruyama H, et al. (1996) Treatment of 37 patients with anaplastic carcinoma of the thyroid. Head Neck 18: 36-41.
- Orita Y, Sugitani I, Amemiya T, Fujimoto Y (2011) Prospective application of our novel prognostic index in the treatment of anaplastic thyroid carcinoma. Surgery 150: 1212-1219.
- Patel KN, Shaha AR (2006) Poorly differentiated and anaplastic thyroid cancer. Cancer Control 13: 119-128.
- Kurukahvecioglu O, Ege B, Poyraz A, Tezel E, Taneri F (2007) Anaplastic thyroid carcinoma with long term survival after combined treatment: case report. EndocrRegul 41: 41-44.
- Liu AH, Juan LY, Yang AH, Chen HS, Lin HD (2006) Anaplastic thyroid cancer with uncommon long-term survival. J Chin Med Assoc 69: 489-491.
- Akaishi J, Sugino K, Kitagawa W, Nagahama M, Kameyama K, et al. (2011) Prognostic factors and treatment outcomes of 100 cases of anaplastic thyroid carcinoma. Thyroid 21: 1183-1189.
- Kanaseki T, Harabuchi Y, Wakashima J, Asakura K, Kataura A, et al. (1999) A case of anaplastic thyroid carcinoma surviving disease free for over 2 years. AurisNasus Larynx 26: 217-220.
- Pichardo-Lowden A, Durvesh S, Douglas S, Todd W, Bruno M, et al. (2009) Anaplastic thyroid carcinoma in a young woman: a rare case of survival. Thyroid 19: 775-779.
- Shinohara S, Kikuchi M, Naito Y, Fujiwara K, Hori S, et al. (2009) Successful treatment of locally advanced anaplastic thyroid carcinoma by chemotherapy and hyperfractionated radiotherapy. AurisNasus Larynx 36: 729-732.
- Noguchi H, Yamashita H, Murakami T, Hirai K, Noguchi Y, et al. (2009) Successful treatment of anaplastic thyroid carcinoma with a combination of oral valproic acid, chemotherapy, radiation and surgery. Endocr J 56: 245-249.
- De Crevoisier R, Baudin E, Bachelot A, Leboulleux S, Travagli JP, et al. (2004) Combined treatment of anaplastic thyroid carcinoma with surgery, chemotherapy, and hyperfractionated accelerated external radiotherapy. Int J RadiatOncolBiolPhys 60: 1137-1143.
- Shin DH, Mark EJ, Suen HC, Grillo HC (1993) Pathologic staging of papillary carcinoma of the thyroid with airway invasion based on the anatomic manner of extension to the trachea: a clinicopathologic study based on 22 patients who underwent thyroidectomy and airway resection. Hum Pathol 24: 866-870.
- Olthof M, Persoon AC, Plukker JT, van der Wal JE, Links TP (2008) Anaplastic thyroid carcinoma with rhabdomyoblastic differentiation: a case report with a good clinical outcome. EndocrPathol 19: 62-65.
- Kihara M, Miyauchi A, Yamauchi A, Yokomise H (2004) Prognostic factors of anaplastic thyroid carcinoma. Surg Today 34: 394-398.
- Kim TY, Kim KW, Jung TS, Kim JM, Kim SW, et al. (2007) Prognostic factors for Korean patients with anaplastic thyroid carcinoma. Head Neck 29: 765-772.
- Siironen P, Hagstrom J, Maenpaa HO, Louhimo J, Heikkila A, et al. (2010) Anaplastic and poorly differentiated thyroid carcinoma: therapeutic strategies and treatment outcome of 52 consecutive patients. Oncology 79: 400-408.
- Ito K,Hanamura T, Murayama K, Okada T, Watanabe T, et al. (2012) Multimodality therapeutic outcomes in anaplastic thyroid carcinoma: improved survival in subgroups of patients with localized primary tumors. Head Neck 34: 230-237.
- Rodriguez JM, Pinero A, Ortiz S, Moreno A, Sola J, et al. (2000) Clinical and histological differences in anaplastic thyroid carcinoma. Eur J Surg 166: 34-38.
Citation: Fu WZE, Lim MY, Chuah KL, Chuah KL, Khoo LCM (2015) Locally Advanced Anaplastic Thyroid Carcinoma with Long-Term Survival of More Than 7 Years after Combined Surgery Including Tracheal Resection and Radiotherapy: Case Report. Otolaryngology 5:197. DOI: 10.4172/2161-119X.1000197
Copyright: © 2015 Fu WZE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16334
- [From(publication date): 7-2015 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 11775
- PDF downloads: 4559