Commentary Open Access
Lithium: A Novel Therapeutic Drug for Traumatic Brain Injury
Seong S Shim*Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta VA Medical Center, USA
- *Corresponding Author:
- Seong S Shim
Department of Psychiatry and Behavioral Sciences
Emory University School of Medicine
Atlanta VA Medical Center
1670 Clairmont Road, Decatur, GA 30033, USA
Tel: 404 321-6111
E-mail: seong.shim@va.gov
Received date: May 02, 2017; Accepted date: May 17, 2017; Published date: May 24, 2017
Citation: Shim SS (2017) Lithium: A Novel Therapeutic Drug for Traumatic Brain Injury. J Alzheimers Dis Parkinsonism 7:327. doi:10.4172/2161-0460.1000327
Copyright: © 2017 Shim SS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Tauopathy and β Amyloid: The Neuropathological Markers of TBI
Traumatic brain injury (TBI) has long been a major public health issue. Approximately 1.7 million individuals currently suffer from TBI [1]. A significant portion of individuals with TBI (up to 24%) suffer “sustained TBI” for many years [2]. A particular concern is that sustained TBI has a tendency to take a chronically deteriorating course as the acute neuropathology of TBI initiates progressive apoptotic cascades leading to chronic neurodegenerative disorders such chronic traumatic encephalopathy (CTE) [3].
A large body of evidence from clinical studies with individuals with TBI and preclinical studies using TBI animal models indicates that hyperphosphorylated tau aggregation (tauopathy) and beta amyloid (Aβ) accumulation are the key neuropathological markers of CTE [3]. Interestingly, there are many similarities between CTE and Alzheimer’s disease (AD) [4] (Table 1). Tauopathy and Aβ deposits are the pathological hallmarks of both disorders. Hyperphosphorylated tau proteins in CTE are chemically similar to that observed in AD. For example, in CTE as well as AD, hyperphosphorylated tau protein contains all six isoforms and the amino acid sites of phosphorylation.
In tau protein are the same between the two disorders [5]. Apolipoprotein E (ApoE) ε4 allele, a strong genetic factor for AD susceptibility and Aβ deposition [6], increases the risk of CTE [7]. Global cerebral atrophy suggesting progressive neuronal loss is also observed in both disorders [8]. Additionally, cognitive impairment is the key symptom of both disorders [9]. In fact, a number of individuals with TBI directly develop AD, suggesting that TBI is an important predisposing factor for AD [10].
Glycogen Synthase Kinase-3 Inhibition: A Novel Therapeutic Target for TBI
Glycogen synthase kinase-3 (GSK-3) is a serine/threonine kinase and is constitutively active, and keeps the large number of its substrates in inactive states [11]. GSK-3 has a strong tendency to block neuroprotection and neuronal survival and promote apoptosis by phosphorylating (thus, inactivating) its substrates which are involved in neuroprotection such as transcription factors and signaling molecules [12]. GSK-3 also exacerbates tauopathy and Aβ accumulation, the key neuropathological markers of both TBI and AD. Conversely, the inhibition of GSK-3β mitigates tauopathy and Aβ deposits and attenuates neurodegeneration in these disorders [13].
| CTE | AD | |
|---|---|---|
| Axonal damage | + | + |
| Synaptic/neuronal loss | + | + |
| Neurite degenration | + | + |
| Microgliasis | + | + |
| Neurofibrially tangles | + | + |
| A√?¬?/APP | + | + |
| Risk of A√?¬?/APP in 3x Transgenic AD Model>normal mice after TBI | ||
| Caspase-3 Induction | + | + |
| APOE4 | Susceptible to CTE | Susceptible to AD |
Table 1: Tauopathy, A√?¬? plaques, cognitive impairment in CTE and AD.
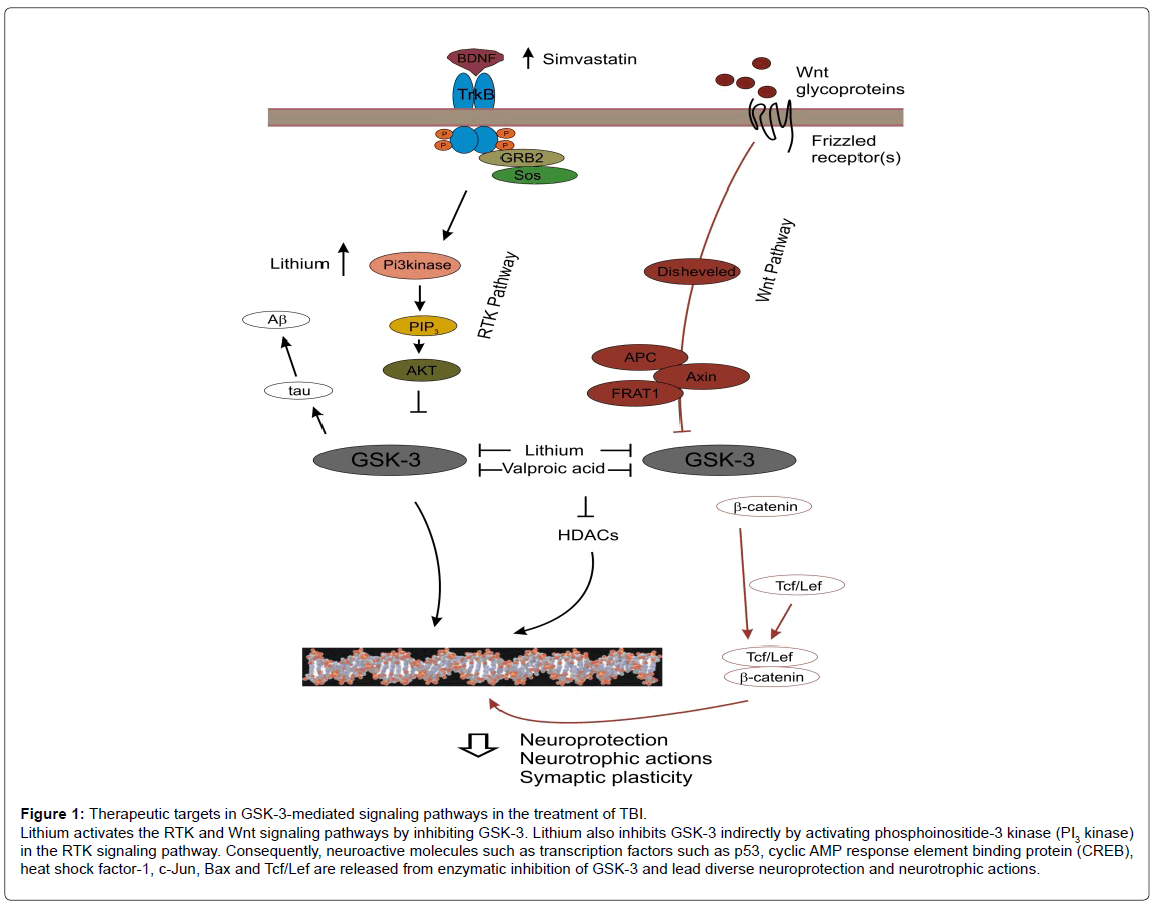
The receptor tyrosine kinase (RTK) and Wnt signaling pathways are the GSK-3 upstream signaling pathways that play important roles in neuroprotective, anti-apoptotic and neurotropic actions [14] (Figure 1). These neuroplastic actions are largely mediated by down-regulating GSK-3 activity. A large number of neuro-active molecules including transcription factors and signaling molecules are constitutively in inactive states as they are inactivated (phosphorylated) by GSK- 3. Activation of these pathways down regulates GSK-3 activity and thereby, releases these molecules from inhibition by the enzyme. These activated molecules lead to diverse neuroprotection and neurotrophic actions. Studies with TBI rodent models have shown that RTK and Wnt signaling pathways are naturally activated shortly after TBI induced, and activation of the pathways is associated with neuroprotective actions against TBI-induced neuropathology [15,16] (Figure 1). Although these pathways may be an innate neuroprotective process against TBI, activation of these pathways is transiently and is not strong enough to produce sustainable therapeutic actions against TBI [15-17].
Figure 1: Therapeutic targets in GSK-3-mediated signaling pathways in the treatment of TBI.
Lithium activates the RTK and Wnt signaling pathways by inhibiting GSK-3. Lithium also inhibits GSK-3 indirectly by activating phosphoinositide-3 kinase (PI3 kinase)
in the RTK signaling pathway. Consequently, neuroactive molecules such as transcription factors such as p53, cyclic AMP response element binding protein (CREB),
heat shock factor-1, c-Jun, Bax and Tcf/Lef are released from enzymatic inhibition of GSK-3 and lead diverse neuroprotection and neurotrophic actions.
A number of studies using TBI rodent models strongly suggest that GSK-3 inhibition is a novel therapeutic target for TBI. Among many GSK-3 inhibitors, lithium is known to be a prototype GSK-3 inhibitor, which directly inhibits the enzyme as well as inhibits the enzyme by phosphorylating it [18] (Figure 1). Studies using TBI rodent models have demonstrated that lithium reduces TBI-induced neuropathology, exerts neuroprotective actions, promotes cell survival and reduces tauopathy and Aβ accumulation (Table 2). These effects are correlated with the therapeutic effects of the drug such as improving TBI-induced cognitive impairment, abnormal locomotor coordination, depressive and anxiety-like behaviors (Table 2).
| Animals, drug administration | TBI lesion | Neuron loss | √?¬?-catenin | √?¬†P-tau | A√?¬? | Anxiety/depression | Cognitive function |
|---|---|---|---|---|---|---|---|
| Rat, posttraumatic for 5 days [26] | ↓ | ↓ | ↑ | ↑ | |||
| Mice, posttraumatic for 2 weeks [27] |
↓ | ↓ | ↓ | ||||
| Mice, posttraumatic for 3 weeks [28] | ↓ | ↓ | ↑ | ||||
| Mice, pre-traumatic a single injection [29] | ↑ | ↓ | |||||
| Mice, pre-traumatic for 2 weeks, Post-traumatic for 4 weeks [30] |
↓ | ↓ | ↑ | ||||
| Mice, post-traumatic for 3 weeks, subclinical doses of lithium/valproate [31] | ↓ | ↓ | ↓ |
Table 2: Effects of lithium on the neuropathology and symptoms of TBI in TBI rodent models.
Lithium: Beyond GSK-3 Inhibition
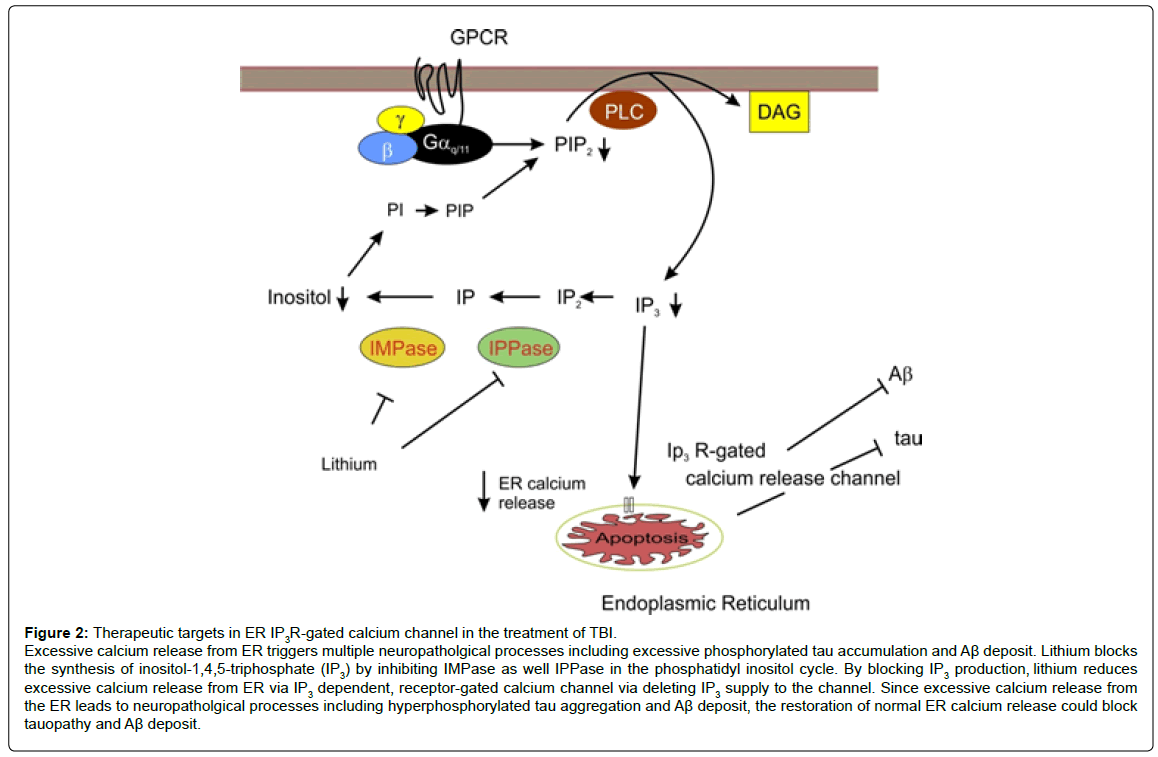
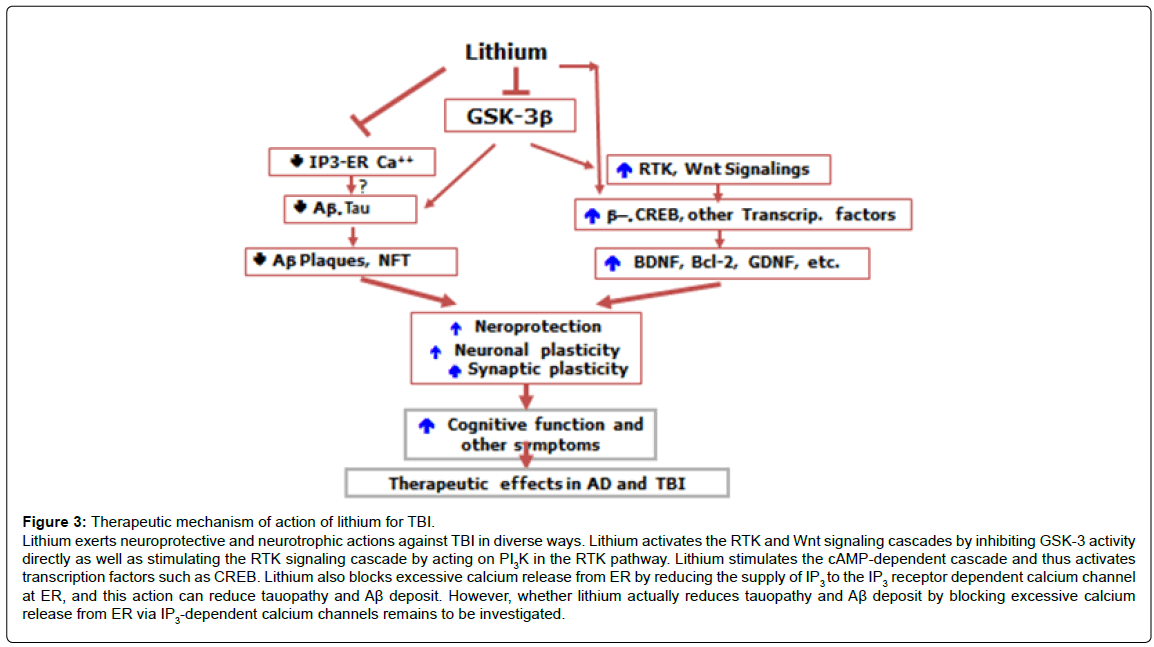
Interestingly, SB-216763, a specific GSK-3 inhibitor, showed neither improvement in memory nor overt neuroprotection [17]. In contrast to SB-216763, lithium has neuroprotective actions beyond GSK-3 inhibition. Lithium inhibits GSK-3 activity by activating the RTK signaling pathway by stimulating phosphoinositide-3 kinase (PI3 kinase) in the pathway (Figure 1). Lithium also blocks protein kinase C activity by inhibiting inositol monophosphatase and also does cyclic AMP signaling pathways, leading to activation of transcription factors involved in neuroprotective and neurotrophic actions such as cyclic AMP responsive element (CREB) [19]. Furthermore, lithium has stabilizing properties of the inositol triphosphate receptor (IP3R) calcium channel localized to the membrane of the endoplasmic reticulum (ER), which is the primary storage of calcium as well as the major regulator of calcium concentration within the cell, by depleting IR3 supply to the IP3R [20,21]. Excessive activation of this channel triggers a wide array of neuropathological processes including apoptosis, impairments in synaptic plasticity and memory encoding, inflammatory responses and the formation of tauopathy and Aβ accumulation [22-24] (Figure 2). Our recent study shows that lithium reduces excessive calcium release from ER in 3xTg AD rodent models [25-32]. This finding suggests that lithium can reduce neuropathological processes triggered by excessive calcium release from ER such as tauopathy and Ab deposit. Thus, in addition to the blockade of GSK-3 activity, diverse mechanisms for neuroprotection may be needed to produce robust therapeutic effects against TBI. In this context, lithium is a drug of particular interest for complex pathological conditions such as TBI or AD since the drug targets multiple pathogenic processes simultaneously (Figure 3).
Figure 2: Therapeutic targets in ER IP3R-gated calcium channel in the treatment of TBI.
Excessive calcium release from ER triggers multiple neuropatholgical processes including excessive phosphorylated tau accumulation and Aβ deposit. Lithium blocks
the synthesis of inositol-1,4,5-triphosphate (IP3) by inhibiting IMPase as well IPPase in the phosphatidyl inositol cycle. By blocking IP3 production, lithium reduces
excessive calcium release from ER via IP3 dependent, receptor-gated calcium channel via deleting IP3 supply to the channel. Since excessive calcium release from
the ER leads to neuropatholgical processes including hyperphosphorylated tau aggregation and Aβ deposit, the restoration of normal ER calcium release could block
tauopathy and Aβ deposit.
Figure 3: Therapeutic mechanism of action of lithium for TBI. Lithium exerts neuroprotective and neurotrophic actions against TBI in diverse ways. Lithium activates the RTK and Wnt signaling cascades by inhibiting GSK-3 activity directly as well as stimulating the RTK signaling cascade by acting on PI3K in the RTK pathway. Lithium stimulates the cAMP-dependent cascade and thus activates transcription factors such as CREB. Lithium also blocks excessive calcium release from ER by reducing the supply of IP3 to the IP3 receptor dependent calcium channel at ER, and this action can reduce tauopathy and A√?¬? deposit. However, whether lithium actually reduces tauopathy and A√?¬? deposit by blocking excessive calcium release from ER via IP3-dependent calcium channels remains to be investigated.
The molecular mechanism underlying tauopathy and Aβ accumulation is poorly understood. A number of studies have shown that tau protein is excessively hyperphosphorylated, and Aβ accumulates shortly after TBI produced. These neuropatholgical events contribute to the conversion of acute TBI to chronic neurodegeneration [4]. The molecular mechanism that triggers tauopathy and Aβ accumulation following TBI is unknown. Understanding of that mechanism may lead to developing a novel therapeutic strategy in treating TBI and AD at the very early stages of the disorders.
References
- Centers for Disease Control and Prevention (2011) Injury prevention and control: Traumatic injury. How many people have TBI? Atlanta, GA.
- Ruff RM (2011) Mild traumatic brain injury and neural recovery: Rethinking the debate. Neurorehabilitation 28: 167-180.
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, et al. (2009) Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68: 709-735.
- Blennow K, Hardy J, Zetterberg H (2012) The neuropathology and neurobiology of traumatic brain injury. Neuron 76: 886-899.
- Schmidt ML, Zhukareva V, Newell KL, Lee VM, Trojanowski JQ (2001) Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer's disease. Acta Neuropathol 101: 518-524.
- Xu F, Vitek MP, Colton CA, Previti ML, Davis J, et al. (2012) Human apolipoprotein E2 promotes parenchymal amyloid deposition and neuronal loss in vasculotropic mutant amyloid-√?¬? protein Tg-SwDI mice. J Alzheimers Dis 31: 359-369.
- Jordan BD, Relkin NR, Ravdin LD, Jacobs AR, Bennett A, et al. (1997) Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA 278: 136-140.
- Shively S, Scher AI, Perl DP, Diaz-Arrastia R (2012) Dementia resulting from traumatic brain injury: What is the pathology? Arch. Neurol 69: 1245-1251.
- Spikman JM, Timmerman ME, Milders MV, Veenstra WS, van der Naalt J (2012) Social cognition impairments in relation to general cognitive deficits, injury severity and prefrontal lesions in traumatic brain injury patients. J Neurotrauma 29: 101-111.
- Sivanandam TM, Thakur MK (2012) Traumatic brain injury: A risk factor for Alzheimer√ʬ?¬?s disease. Neurosci Biobehav Rev 36: 1376-1381.
- Cole AR (2013) Glycogen synthase kinase 3 substrates in mood disorders and schizophrenia. FEBS J 280: 5213-5227.
- Hur EM, Zhou FQ (2010) GSK3 signaling in neural development. Nat Rev Neurosci 11: 539-551.
- Engel T, Go√?¬Īi-Oliver P, G√?¬≥mez de Barreda E, Lucas JJ, Hern√?¬°ndez F, et al. (2008) Lithium, a potential protective drug in Alzheimer's disease. Neurodegener Dis 5: 247-249.
- Gould TD, Manji HK (2005) Glycogen synthase kinase-3: A putative molecular target for lithium mimetic drugs. Neuropsychopharmacology 30: 1223-1237.
- Noshita N, Lew√?¬©n A, Sugawara T, Chan PH (2002) Akt phosphorylation and neuronal survival after traumatic brain injury in mice. Neurobiol Dis 9: 294-304.
- Shapira M, Licht A, Milman A, Pick CG, Shohami E, et al. (2007) Role of glycogen synthase kinase-3beta in early depressive behavior induced by mild traumatic brain injury. Mol Cell Neurosci 34: 571-577.
- Dash PK, Johnson D, Clark J, Orsi SA, Zhang M, et al. (2011) Involvement of the glycogen synthase kinase-3 signaling pathway in TBI pathology and neurocognitive outcome. PLoS ONE 6: e24648.
- Shim SS, Stutzmann GE (2016) Inhibition of glycogen synthase kinase-3: An emerging target in the treatment of traumatic brain injury. J Neurotrauma 33: 2065-2076.
- Atack JR, Cook SM, Watt AP, Ragan CI (1992) Measurement of lithium-induced changes in mouse inositol(1)phosphate levels in vivo. J Neurochem 59: 1946-1954.
- Bosche B, Sch√?¬§fer M, Graf R, H√?¬§rtel FV, Sch√?¬§fer U, et al. (2013) Lithium prevents early cytosolic calcium increase and secondary injurious calcium overload in glycolytically inhibited endothelial cells. Biochem Biophys Res Commun 434: 268-272.
- Schlecker C, Boehmerle W, Jeromin A, DeGray B, Varshney A, et al. (2006) Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium. J Clin Invest 116: 1668-1674.
- Lichtenthaler SF (2012) Alpha-secretase cleavage of the amyloid precursor protein: Proteolysis regulated by signaling pathways and protein trafficking. Curr Alzaheimer Res 9: 165-177.
- Leeds PR, Yu F, Wang Z, Chiu CT, Zhang Y, et al. (2014) A new avenue for lithium: Intervention in traumatic brain injury. ACS Chem Neurosci 5: 422-433.
- Wang ZF, Fessler EB, Chuang DM (2011) Beneficial effects of mood stabilizers lithium, valproate and lamotrigine in experimental stroke models. Acta Pharmacol Sinica 32: 1433-1445.
- Shim SS, Kapecci, Briggs NC, Stutzmann GE (2016) Lithium treatment suppresses IP3-gated calcium signaling and synaptic plasticity in the hippocampus of 3xTg-AD mice. Society for Neuroscience, 2017 annual meeting, San Diego CA.
- Dash PK, Johnson D, Clark J, Orsi SA, Zhang M, et al. (2011) Involvement of the glycogen synthase kinase-3 signaling pathway in TBI pathology and neurocognitive outcome. PLoS ONE 6: e24648.
- Yu F, Wang Z, Tchantchou F, Chiu CT, Zhang Y, et al. (2012) Lithium ameliorates neurodegeneration, suppresses neuroinflammation and improves behavioral performance in a mouse model of traumatic brain injury. J Neurotrauma 29: 362-374.
- Yu F, Zhang Y, Chuang DM (2012) Lithium reduces BACE1 overexpression, √?¬? amyloid accumulation, and spatial learning deficits in mice with traumatic brain injury. J Neurotrauma 29: 2342-2351.
- Shapira M, Licht A, Milman A, Pick CG, Shohami E, et al. (2007) Role of glycogen synthase kinase-3beta in early depressive behavior induced by mild traumatic brain injury. Mol Cell Neurosci 34: 571-577.
- Zhu ZF, Wang QG, Han BJ, William CP (2010) Neuroprotective effect and cognitive outcome of chronic lithium on traumatic brain injury in mice. Brain Res Bull 83: 272-277.
- Yu F, Wang Z, Tanaka M, Chiu TC, Leeds P, et al. (2013) Posttraumatic co-treatment with lithium and valproate: Reduction of lesion volume, attenuation of blood brain barrier disruption and improvement in motor coordination in mice with traumatic brain injury. J Neurosurg 119: 766-773.
- Chalecka-Franaszek E, ChuangDM (1999) Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate induced inhibition of Akt-1 activity in neurons. Proc Natl Acad Sci U S A 96: 8745-8750.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 4195
- [From(publication date):
June-2017 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 3266
- PDF downloads : 929