Research Article Open Access
Lipid Content Variation in Plantago media Leaves in Response to Light Conditions
Olga Rozentsvet1*, Tamara Golovko2, Elena Bogdanova1, Galina Tabalenkova2, Ekaterina Kokovkina2, Igor Dalke2 and Viktor Nesterov1
1Institute of Ecology of the Volga Basin Russian Academy of Science, Togliatti, Russia
2Institute of Biology of the Komi Republic of the Scientific Center of Russian Academy of Science Ural Branch, Syktyvkar, Russia
- *Corresponding Author:
- Olga Rozentsvet
Institute of Ecology of the
Volga Basin Russian Academy of Science
Komzin’s Str. 10, Tolyatti, 445003, Russia
Tel: +8482-48-99-77
Fax: +7 8482 48 96 09
E-mail: olgarozen55@mail.ru
Received Date: June 16, 2015 Accepted Date: July 13, 2015 Published Date: July 15, 2015
Citation: Rozentsvet O, Golovko T, Bogdanova E, Tabalenkova G, Kokovkina E, et al. (2015) Lipid Content Variation in Plantago media Leaves in Response to Light Conditions, Cuba. J Ecosys Ecograph 5:163. doi:10.4172/2157-7625.1000163
Copyright: © 2015 Rozentsvet O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
The aim of the present work was to study the variation of lipid and fatty acids (FA) composition as well as morphometric characteristics of Plantago media leaves from the different light conditions in northeastern Russia. The content of lipids in the leaves was measured for several years during the blooming period, and the level of lipid peroxidation was estimated. For lipid analysis, the mid leaf part from 12-15 typical plants was cut in small parts and three samples of 1-2 g were chosen from the total biomass. Lipids were extracted three times using three times chloroform/methanol. The quantification of phospholipids (ÃÂ L) was performed by the content of inorganic phosphorus. Glycolipids (GL) and non-polar lipids (NL) were quantified using a densitometer. The leaves of the plants grown under lower light had lower leaf mass/area ratio (LMA) but larger areas of lamina. They accumulated lower levels of lipid peroxide products. Daily content of malondialdehyde changed more significantly during periods with clear and warm weather. It was found that as the duration of sunshine increased, the content of total lipids (TL) increased (r=0.78), but an increase in temperature resulted in a decrease of their content (r=-0.70), especially for plants in high-sunshine habitats. Concentration of GL in leaves of shaded plants increased with increasing precipitation but decreased with increasing temperature and the duration of sunshine. The same effect is exerted by rainfall on PL content. Amount of saturation FA (SFA) increased with increasing temperature and the duration of sunshine. Precipitation contributed to the accumulation of unsaturation FA (USFA). Thus, the content of lipids in the leaves depended on weather and microclimate conditions.
Keywords
Plantago media; Glycolipids; Fatty acids; Non-polar lipids; Phospholipids; Total lipids; plasticity
Introduction
In nature, plants are subjected to simultaneous effects of various ecological factors, which may induce coordinated eco-physiological reactions of the plants [1,2]. With climate changing and ecological problems aggravating, the knowledge of responses of various functional groups of plants will be of particular importance in determining plants’ persistence under climate change [3]. The properties of plants that determine their adaptation are important for understanding geographic distribution of species [4]. Of special interest in this respect are ecologically flexible species widely represented in the natural flora of different botanical and geographical zones.
The genus Plantago is characterised by a large specific, structural, and ecological diversity. Some Plantago species have been found to be genetically differentiated and phenotypically plastic toward illumination [5] mineral nutrition [6] and soil humidity [7]. Plantago media L. is characterised having moderate soil and climatic requirements. The plant can be found in bottomland meadows, thinned forests, fallow lands, pastures, and along roads. Its area covers Europe, Siberia, and Western and Central Asia. In the European northeastern part of Russia, it reaches the Arctic (Vorkuta) [8].
Plasticity of plants is a combination of morphological, biochemical, and physiological characteristics [9]. Estimations of the influence of climatic changes on plants are based mainly on morphogenetic criteria [7]. Biochemical characteristics are used more infrequently, though they determine the genotype plasticity and are the basis of physiological processes, many of which occur with membranes. Basic building blocks of membranes are lipid molecules [10]. There are universal and specific roles of lipids that affect plant adaptation to many ecological factors. For example, significant adaptive changes include the changing in the unsaturated and saturated fatty acids (USFA/SFA) balance [11,12] ratios phospholipids (PL) and glycolipids (GL) [13] individual classes lipids [14]. Lipid peroxidation (LPO), induced by reactive oxygen species (ROS) formation, is one of the plant’s early responses to the effect of disturbing factors, including salinization [15,16]. Changes caused by LPO are viewed as the base for defensive and recovery processes.
In our earlier work, we studied the daily dynamics of membrane lipids in P. media collected from midland regions of Russia [17]. The aim of the present work was to study the variation of lipid and fatty acids composition, as well as morphometric characteristics, of P. media leaves from the different light conditions in northeastern Russia.
Materials and Methods
Sampling site and plant material
The region of study was in South Timan (62°45’ N, 55°49’ E) in the southern part of the Timan Ridge, which is an important orographical structure of the northeastern section of the European part of Russia [18]. The mean annual air temperature in the region of study is -1.5°C; the mean temperature of the warmest month (July) is +15°C. The frostfree season lasts 76 days, on average. The duration of photoperiod at the end of June until the beginning of July is 19 h.
P. media plants grown in the two closely spaced sites differing light conditions: a sparsely grassed flank of the southeastern exposition (open site 1, S1-light type) and a thick-grassed terrace at the mountain foot (shaded site 2, S2-shadow type). Microclimatic parameters (air temperature and humidity) at the sites were measured with a portable meteorological station (Data Logger LI-1400, USA). The characteristics of weather conditions during the study were provided by the Meteorology Centre of Komi Republic (Table 1). The centre was about 20 km away from the sites of study.
| Parameter | 2007 | 2009 | 2010 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| June | July | June | July | June | July | ||||
| III | I | II | III | I | II | III | I | II | |
| Average air temperature, °C | 17.6 | 24.6 | 21.3 | 10.6 | 11.0 | 16.7 | 16.4 | 16.3 | 16.2 |
| Deviation from the norm | 2.0 | 9.0 | -5.0 | -4.0 | -5.0 | 0 | 2.0 | 1.0 | 0 |
| Precipitation, mm | 84.0 | 1.0 | 18.0 | 18.0 | 50.0 | 17.0 | 4.0 | 7.0 | 2.0 |
| Precipitation, % of the norm | 443.0 | 5.0 | 78.0 | 82.0 | 217.0 | 74.0 | 18.0 | 30.0 | 9.0 |
| Duration of sun shining, h | 90.0 | 141.0 | 97.0 | 100 | 42 | 110 | 105 | 102 | 115 |
Table 1: Meteorological data for the period of study.
Morphometric data of leaves
Plants were collected simultaneously with two sites in the first decade of each year. Leaves from 12-15 typical mature plants were collected from the middle of the rosette plants. To measure the linear sizes (length, width) and leaf area, images of 10 leaves were collected together with a scale rule using a digital camera (DMC-TZ3 Panasonic, Japan). The data were processed in Image Tool for Windows, v.3.00 software. The leaves were further weighted and dried at 80°C. The leaves of S1 and S2 plants were compared by linear size, area, and leaf mass/area ratio (LMA, g m-2).
Biochemical analysis
For lipid analysis, the mid leaf part of 12-15 typical plants was cut in small parts and three samples of 1-2 g were chosen from the total biomass. The samples were treated with hot isopropanol and kept in a cold, dark place prior to analysis.
Lipid peroxidation intensity in the plant leaves was determined by measurement of malonedialdehyde (MDA) concentrations after reaction with thiobarbituric acid [19]. Fluorescence intensity was measured using a spectrophotometer Specol (Germany) at 532 nm.
Lipids were extracted three times using three times chloroform/ methanol (1:2, v/v) by the method of [20]. The combined extracts were purified from non-lipid compounds and concentrated using a rotary vacuum evaporator. The quantification of phospholipids (РL) was performed by the content of inorganic phosphorus [21] with the following calculation of their molar masses. GL were quantified using a densitometer Sorbfil (Russia) with the occasional comparison of the data with those obtained from the galactose measurement. The latter was done using an anthrone reagent [22] using a spectrophotometer Specol (Germany) at 620 nm. Monogalactosyldiacylglycerol (Laroden, Sweden) and galactose (Sigma, USA) were used to create calibration curves. NL were measured spectrophotometrically, and tripalmitate (Sigma, USA) was used as a standard for calibration curve. Total lipids (TL) were calculated as a sum of NL, GL- and PL [23].
Fatty acid methyl esters (FAME) were prepared by transmethylation with 5% HCl in methanol. FAMEs were purified by preparative TLC using hexane/diethyl ether/acidic acid (80:20:1, v/v/v). They were analysed using a Cristal 5000.1 gas chromatograph (Perkin-Elmer, Norwalk, Connecticut), fitted with a 105 m × 0.25 mm i.d. capillary column (Restek, USA) under isothermal conditions (column at 180°C; injector and detector at 260°C). The oven temperature was programmed: 170°C for 3 min, heated to 220°C at 4°C/min, held at 220°C for 15 min. FAMEs were identified by comparing retention times with fatty acid standards (Supelco 37, Supelco, USA).
Statistical analysis
The data were processed using the Statistical 6.0 for Windows and Microsoft Excel 2007 software. The data are presented as means ± standard errors (n=10-15 for morphometric data: n=3 for Biochemical date). The effect of environmental factors on the lipid content was estimated by correlation-regression analysis. Statistical significance between groups was assessed by the student t-test (p<0.05). Relationships between morphometric parameters and lipid characteristics were measured using a Spearman’s correlation coefficient.
Results
Habitats of growing plants
The experimental period of 2007 was the warmest and sunniest; in 2009, it was cold, with short periods of sunshine. In 2010, there was a relatively small amount of precipitation interspersed in sunny and warm weather (Table 1). The weather conditions exerted a substantial effect on the microclimate in the plantain habitats. As seen in (Figure 1A), S1 plants (light type) received more light and heat on clear, sunny days during 2007 and 2010, especially in the first half of the day. The intensity of photosynthetic active radiation (PAR) at the plant level was 400-500 μmol/m/s already by the early morning hours; by midday, it reached 1000-1500 μmol/m/s. The maximal light of S2 plants (shadow type) was three times as low as that of S1 plants. The differences in the light regime of the two plantain habitats were also maintained on overcast days with dense cloud cover (2009) (Figure 1B). The relative humidity at the plant level varied widely, dropping considerably at the midday hours, especially on sunny days (2007, 2010) (Figures 1G and 1H). Thus, the habitats of P. media plants differed substantially by microclimate conditions, predominantly by light regime. The environmental conditions constantly changed throughout the day and varied depending on the weather.
Morphometric data
P. media S1 plants formed smaller leaves compared to S2 plants (Table 2). In all years of study, the lamina of S2 plants was approximately twice as long as that of S1 plants. The differences in the lamina width were evident in 2009 and 2010. The specific leaf area (SLA) of S1 plants was 2-4 times lower than that of S2 plants. However, it is worth noting that the year-wise differences in the SLA were more pronounced in S2 plants than in the S1 ones. The largest values of SLA were registered in the dampest and coldest season (2009). Similar data have been observed for P. major plants growing in different light conditions [24]. Plants typically respond to shade by producing leaves with less mass per unit area [25,26]. Leaves with high LMA are usually thick and dense and have low values of specific leaf area (SLA). A plant with such leaves grows more slowly than high-SLA plants [27]. Studies have shown many LMA values are positively correlated with relative growth rate and maximum rate of photosynthesis [28]. Our data showed that the greatest values of the LMA corresponded mostly to cool and wet conditions (2009). Therefore, both, different levels of light during the year, the weather conditions can impact the linear dimensions of P. media leaves, their area, and mass.
| Parameters | 2007 | 2009 | 2010 |
|---|---|---|---|
| Site 1 | |||
| Leaf length, mm | 55±3 | 53±2 | 46±2 |
| Leaf width, mm | 23±2 | 24±2 | 19±1 |
| SLA, mm2 | 830±80 | 880±80 | 650±40 |
| LMA, g/m2 | 87 | 120 | 93 |
| Site 2 | |||
| Leaf length, mm | 91±4* | 115±8* | 100±4* |
| Leaf width, mm | 28±2 | 44±3* | 37±2* |
| SLA, mm2 | 1810±145 | 3580±470* | 2510±150* |
| LMA, g/m2 | 45 | 55 | 52 |
Note: *statistically significant differences between the sites (p ≤ 0.05); means ± SE (n=10-12)
Table 2: The morphometric characteristics of leaves of P. media from the open (S1) and shaded (S2) sites.
| Year | Time of day, h | |||||
|---|---|---|---|---|---|---|
| Site 1 | Site 2 | |||||
| 4–6 | 12–14 | 21–23 | 4–6 | 12–14 | 21–23 | |
| 2007 | 11.2±0.5 | 30.2±2.7 | 18.8±1.4 | 10.9±0.6 | 22.1±1.7 | 13.2 ±1.7 |
| 2009 | NA | 3.3±0.3 | 5.8±0.6 | NA | 3.6±0.5 | 5.0±0.5 |
| 2010 | 15.0±1.4 | 20.7±3.3 | 20.3±4.4 | 10.0±2.3 | 15.7±2.7 | 9.3 ±1.5 |
Note: NA–not measured
Table 3: The content of malondialdehyde (MDA, μmol/g dry weight) in the leaves of Plantago media plants growing in open (S1) and shaded (S2) sites.
Lipid peroxidation
The intensity of lipid peroxidation is one of the functional characteristics of plant cells [15]. The content of MDA, an indicator of peroxidation of lipids (Тable 3), in the plantain leaves varied widely from 3.5-30 μmol/g dry weight. In the warm years (2007 and 2010), S1 plants had a higher MDA content in their leaves compared to S2 plants; in the cold, low-sunlight, and rainy year (2009), the level of MDA was equally low in leaves of both S1 and S2 plants. In the warmer season of 2007, an increase in MDA was observed in the afternoon compared to morning hours. In the warm and dry 2010 season, lipid peroxidation was revealed only in the leaves of S2 plants. In the leaves of S1 plants, a high MDA level was maintained until late in the evening. The differences in the content of MDA between S1 and S2 plants were larger in the warm season with sunny days.
Content of lipids
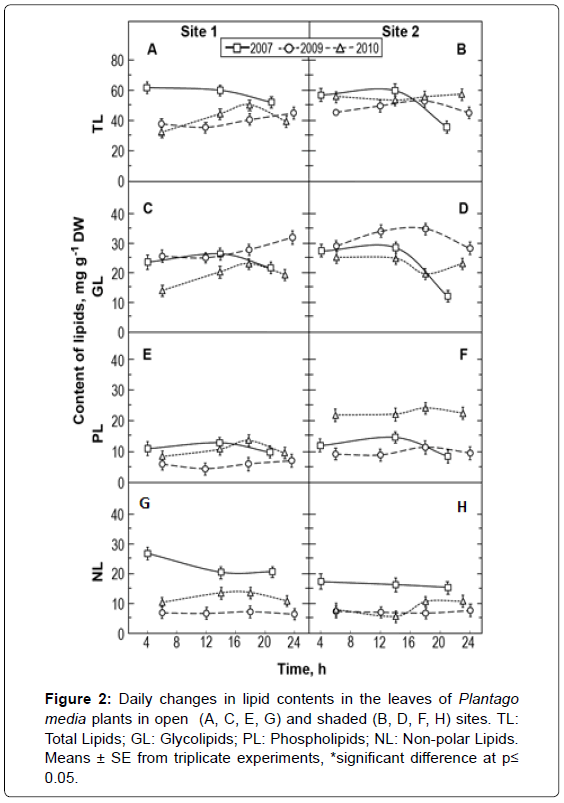
The morphometric parameters’ change of leaves occurred when lipid composition was modified. The content of TL was 1.3−1.5 fold higher in dry and warm years (2007 and 2010) than in cool and rainy ones (2009) (Figures 2A and 2B). The greatest differences in TL between plants at S1 and 2 occurred in 2007 (Figure 2).
The major components of TL (>75%) were polar GL and PL. Leaf concentrations of GL were higher in 2009 in comparison to other years. Also the concentration of GL was higher in shaded S2 plants during the day compared to S1 plants (Figures 2C and 2D). The lowest level of GL was observed when there was the most stable number of hours of sunshine, for instance in 2010.
The PL amounted to about 25% of total lipids. As seen in Figure 2, the year-related climatic conditions affected the PL content. In the cold and rainy 2009 year, PL concentration in leaves was lower than that in the other (drier and warmer) years (Figures 2E and 2F). During all years, the leaf content of PL in S2 plants was higher than that in S1 ones. The differences were larger in the dry and warm season of 2010. However, variations in daily flow of total PL content were less significant in comparison with GL.
The NL are referred to as storage lipids, and their content is comparable to that of PL (Figures 2G and 2H). In 2007 and 2010, the content of NL in S1 plants was higher than that in S2 plants. Plants of both groups accumulated the largest quantities of NL in their leaves in 2007, when the season was warm and amount of precipitation was sufficient. In the colder and rainy 2009 year, the leaves accumulated less NL.
Fatty acid composition
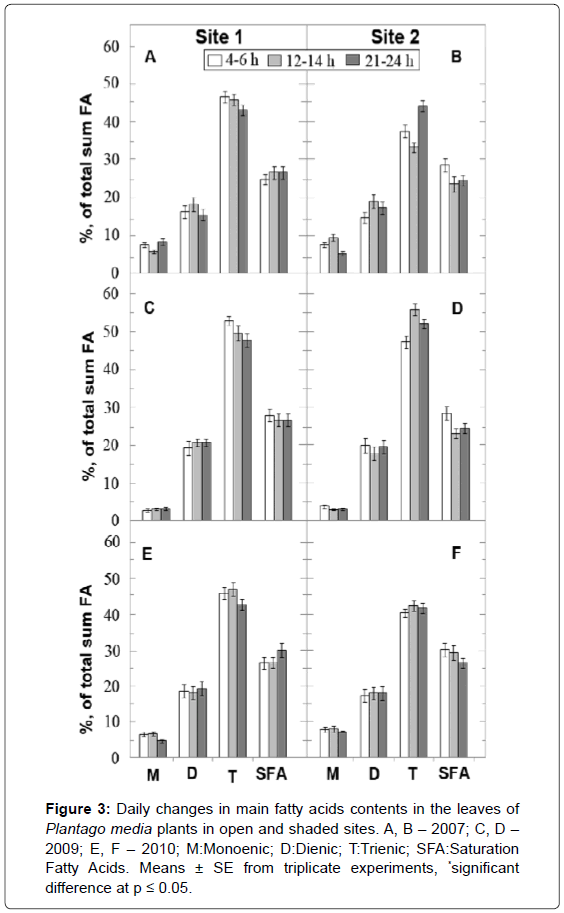
About 20 FA were identified in the leaves of P. media, with C16 and C18 acids comprising more than 90%. The leaves of the plantain plants had a relatively low FA content, containing some hydrocarbon chains shorter than 16 carbons (<5%) and less FA with hydrocarbon chains longer than 20 carbons (<2%). The main component of unsaturated FA (USFA) was linolenic acid (C18:3; 33-58% of total FA) and predominant saturation FA (SFA) was palmitic acid (C16:0; 18-26%). After integrating the data of all three years, the major FA can be depicted in a series based on their content in the following order: C18:3>C16:0>C18:2>C18:1 ≥ С18:0.
The differences in the FA contents between S1 and S2 plants depend on the environmental conditions. So, in 2009 (Figures 3C and 3D) the levels of trienoic FA were higher in S1 and S2 plants compared to those in 2007 (Figures 3A and 3B) and 2010 (by 10−20%) (Figure 3E, F). The lowest content of monoenic FA was registered in 2009 (about 5% of total FA). The highest content of USFA was observed in the leaves of plants in the cold 2009 year.
Discussion
The results of our work show that variation of weather and microclimate conditions affects morphological and biochemical features of leaves of P. media plants growing in different light condition of northeast Russia (South Timan). Plants on the open site formed smaller leaves with a high LMA. Laminas of shaded plants were larger and had a low LMA value. The LMA variability of plantain leaves seems to relate to differences in their anatomy and chemical composition.
Our data also show the effects of environmental on leaf content of MDA, an indicator of lipid peroxidation. In S1 plants, the level of lipid peroxidation was higher than in S2 ones. The differences in the MDA contents between S1 and S2 plants were larger in the warm season with sunny days. This can indicate a stronger effect of reactive oxygen species on the lipid components of cellular membranes in the leaf tissues of plants growing in high amounts of light and warm air temperature. At high light intensities, there is a higher probability of electrons in the electron transport chain to leak onto oxygen molecules, forming free radicals [15]. In the damp and cold season of 2009, when illumination of plants was low, the differences in the MDA contents between S1 and S2 plants almost completely disappeared. A more pronounced daily dynamic of NL and MDA in 2007 and 2010 may indicate a more intensive lipid exchange in warm, dry years. Perhaps, the storage and metabolically important NL, especially triacylglycerides, were used by mesophyll cells to repair the membrane lipids damaged in the process of lipid peroxidation.
The more light and warm temperature P. media plants received, the less GL was found in their leaves. Since GL are the main lipids of the thylakoid membranes, which contain photosystems with lightharvesting pigment-protein complexes, they are probably maintained at a certain ratio to chlorophylls. This observation may indicate a change in the number of chloroplasts and their structure, such as changes in the number and size of grana of chloroplasts that affected the change in the photosynthetic intensity.
Plant leaves contained less PL in the cool year (2009) than in warm and dry years. The greatest changes in the composition of the main PL occurred in 2010. The content of PL and NL were more dependent on light conditions than the content of the GL.
Modification of the FA pool is considered to play a key role in the adaptation of organisms to environmental conditions [11]. Data in the literature indicate that changes in the composition and content of FA have an evident adaptive character; they adjust physiologically vital properties of cellular membranes, primarily fluidity [11,28]. We found that high rate of light (S1) and sunny days (2007, 2010) lead to increased content of monoenoic FA. On the other hand, in a cold year (2009) noted the largest content of trienoic FA. As a result, ratios of the SFA and USFAs varied.
Using a mathematical method, we determined the degree of influence of individual factors on the composition and content of lipids (Table 4). As the duration of sunshine increased, the content of total lipids increased (r=0.78). At the same time, an increase in temperature resulted in a decrease of their content (r=-0.7), especially for plants in high-sunshine habitats. Concentration of GL in leaves of shaded plants increased with increasing precipitation but decreased with increasing temperature and the duration of sunshine. The same effect is exerted by rainfall on PL content. Weather factors had a statistically significant but opposite effect on the contents of FA. Amount of SFA increased with increasing temperature and the duration of sunshine. Precipitation contributed to the accumulation of USFA.
| Lipids | Spearman’s correlation coefficient | |||||
|---|---|---|---|---|---|---|
| Temperature, °С | Precipitation | Duration of sun shining, h | ||||
| Site 1 | Site 2 | Site 1 | Site 2 | Site 1 | Site 2 | |
| TL | -0.70* | -0.05 | -0.78* | 0.34 | 0.78* | 0.34 |
| GL | -0.48* | -0.56* | 0.68* | 0.68* | -0.68* | -0.68* |
| PL | 0.72* | 0.31 | -0.75* | -0.79* | 0.75* | 0.79* |
| NL | 0.94* | 0.75* | -0.54* | -0.20 | 0.54* | 0.20 |
| SFA | 0.72* | 0.75* | -0.59* | -0.44* | 0.59* | 0.44* |
| USFA | -0.83* | -0.79* | 0.57* | 0.61* | -0.57* | -0.61* |
Note: the asterisk indicates a significant difference at p ≤ 0.05
Table 4: Correlation between the content of lipids in the leaves of Plantago media plants and ecologic factors.
Conclusions
Thus, the changes in lipid and FA composition have been studied in P. media leaves in response to light conditions in northeastern Russia (South Timan). The content of lipids in the leaves depended on weather and microclimate conditions. In general, our results suggest that the variability of lipid content, as well as morphological variability of leaves is functional features that define ecological plasticity of P. media. Apparently, due to their ecological plasticity, P. media plants can occupy different ecotopes, and, as a result, they have a wide geographic distribution.
References
- Larcher (2003)Physiological Plant Ecology. In: Ecophysiology and Stress Physiology of Functional Groups(4thedn.) Springer-Verlag, Berlin, New York.
- Zunzunegui M, Barradas M, Alvarez-Cansino AL, Esquivias MP, Novo F (2011)Seasonal physiological рlasticity and recovery capacity after summer stress in Mediterranean scrub communities. PlantEcol 212: 127-142.
- Harrison SP, Prentice IC,Rarboni D,Kofeld KE, Ni J, et.al (2010)Ecophysiological and bioclimatic foundations for a global plant functional classification. J VegetSci21: 300-317.
- Kuiper D, SmidA (1985) Genetic differentiation and phenotypic plasticity in Plantagomajor. The effect of differences in level of irradiance on growth, photosynthesis, respiration, and chlorophyll content.Physiol Plant 65: 520-528.
- Lambers H, Posthumus F,Stulen I, Lanting I, van de Dijk SJ, et.al (1981)Energy metabolism of Plantago major ssp. major as dependent on the supply of mineral nutrients.Physiol Plant 51: 245-252.
- Mudrik V,Kosobrukhov A, Knyazeva I, Pigulevskaya T(2003) Changes in the photosynthetic characteristics of Plantago major plants caused by soil drought stress. Plant Grow Regul40: 1-6.
- Flora Arctica USSR, Fasc. VIII, Pars 2 (1983) In: Tolmatchev AI, Jurtzev BA (eds.)Orobanchaceae-Plantaginaceae,Nauka, The Leninopoli (in Russian).
- Cornelissen JHC,Lavorel S, Garnier SE et.al (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide Austral. J Bot 51: 335-380.
- Harwood JL (1998) Environment effect on plant lipid biochemistry. In:Harwood JL (ed.)Plant Lipid Biosynthesis: Fundamentals and Agricultural Applications. Cambridge University Press, Cambridge
- Los DA, Murata N (2004) Membrane fluidity and its roles in the perception of environmental signals.Biochim. Biophys. ActaBiomemb1666: 142-157.
- Wu J, Seliskar DM, Gallagher JL (2005) The response of plasma membrane lipid composition in callus of the halophyte Spartina patens (Poaceae) to salinity stress.Amer J Bot 92: 852-858.
- Mansour MMF, Salama KHA, Al-Mutawa MM,AbouHadidAF (2002) Effect of NaCl and polyamines on plasma membrane lipids of wheat roots.Biol Plant 45: 235-239.
- Sui N, Li M, Li K,Song J, Wang BS (2010) Increase in unsaturated fatty acids in membrane lipids of Suaeda salsa L. enhances protection of photosystem II under high salinity.Photosynthetica48: 623-629.
- Frankel EN (1998) Photooxidation of Unsaturated fats. In: Lipid oxidation. Bell and Bain Ltd., Glasgow, pp.13-55.
- Labudda M (2013) Lipid peroxidation as a biochemical marker for oxidative stress during drought. An effective tool for plantbreeding.E-wydawnictwo, Poland.
- Grebenkina TM,Nesterov VN,Rozentsvet OA,Bogdanova ES (2012) The dynamics of lipid and pigment composition in Plantago media (Plantaginaceae) during daylight. Vegetation Resource4: 565-578.
- Biological diversity of nature protected areas (NPA) of the Komi Republic(2006) In: Degteva SV(ed.) Protected Natural Complexes of Timan, Part 1. KomiSci Ctr. The Syktyvkar (in Russian).
- Lukatkin AS,Bashmakov DI,KipaykinaNV (2003) A protective role of thidiazuron treatment for cucumber seedlings affected by heavy metals and chill. Russ. J Plant Physiol 50: 346-348.
- Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can JBiochemPhysiol37: 911-917.
- Vaskovsky VE,LatyshevNA (1975) Modified jungnickels reagent for detecting phospholipids and other phosphorus compounds on thin-layer chromatograms. J Chromat A 115: 246-249.
- KatesM, Elsevier, Amsterdam (1975) Techniques of lipidology. In: Work TS, Work E (eds.) Laboratory techniques in biochemistry and molecular biology, pp.269-610.
- Rozentsvet OA, Nesterov VN,BogdanovaES (2014) Membrane-forming lipids of wild halophytes growing under the conditions of Prieltonie of South Russia.Phytochemistry105: 37-42
- LyubimenkoVN(1963) On the question of the physiological characteristics of light and shadow leaves. The selected works, Kiev No. 1194-202.
- DijkstraP,Lambers H, Cambridge ML, Konings H, Pons TL (1989)Cause and effect of differences in specific leaf area. In Causes and consequences of variation in growth rate and productivity of higher plants. Dordrecht, SPB Academic Publishing, pp. 125-140.
- Onoda Y,Schieving F,AntenТPR (2008) Effect of light and nutrient availability on leaf mechanical properties ofPlantago major: a conceptual approach. Ann Bot 101: 727-736.
- Poorter H,RemkesC (1990) Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate.Oecologia83: 553-559.
- Golovko TK, Dalke IV,Zakhozhiy IG, Dymova OV,TabalenkovaGN (2011) Functional plasticity of photosynthetic apparatus and its resistance to photoinhibition in Plantago media.Russ J Plant Physiol58: 549-559.
- Wada K, Murata N(2009) Lipids in thylakoid membranes and photosynthetic cells. In: Wada H,Murata N (eds.)Lipids in photosynthesis: essential and regulatory functions. Springer Science and Business Media, pp.1-9.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 15605
- [From(publication date):
September-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11017
- PDF downloads : 4588