Light Absorption of the Aluminium Effect Pigment Coated Textile: An Investigation Using TiO2 Nanoparticle in the Basecoat and Al2O3 Nanoparticle in the Topcoat
Received: 06-Feb-2019 / Accepted Date: 27-Mar-2019 / Published Date: 05-Apr-2019
Abstract
Effect pigments were used to coat cotton and polyester textiles. After preparation of sol, the textile was coated and cured. After curing of already coated fabric, transmission and reflection characteristics were investigated through UV-Vis spectrophotometry. Subtracting these values from 100%, light absorption% by textile has been found. In this work, light absorption of effect pigment coated textile and their effect will be discussed.
Keywords: Special effect pigments; Aluminium pigment; Textile coating; Surface modification; Functionalized textile; Titanium dioxide; Nanoparticle
Introduction
Natural sunlight consists of 5% UV (300-400 nm), 43% visible (400-700 nm), and 52% infrared (700-2500 nm) [1]. When light falls onto fabric, a small portion of the light beam is absorbed rather than to be reflected or transmitted. If a light wave strikes a material with electrons having the same vibrational frequencies, then those electrons will absorb the energy of the light wave and transform it into vibrational motion and the electrons interact with neighbouring atoms and convert its vibrational energy into thermal energy. Subsequently, the light wave with that given frequency is absorbed by the object and never reverse in the form of light. To give a silvery and shiny lustre to the substrate, aluminium pigments are commonly used in coatings under the slightly alkaline conditions of most waterborne paints, aluminium reacts with water and forms aluminium oxide and aluminium hydroxide. This reaction process leads a big problem because there is a loss of the metallic appearance of the pigment particles. Surface modification of aluminium is necessary to avoid reaction and to be protected from water [2]. The introduction of hydroxyl groups would be expected to lead to a surface with a stronger interaction with water [3].
Different optical properties such as absorption, transmission, and reflection properties of the fabrics are determined primarily by the constructional parameters of textiles [4,5]. But these properties can be influenced when the textile surface is modified by different nanoparticles. Levinson suggested optical properties of a freely suspended film (i.e., reflectance, transmittance, and absorptance) depend on three factors such as refractive indices of the pigment and the binder, the construction and concentration of the pigment nanoparticles and obviously the thickness of coated film [6]. Of course, according to the transmission line theory, the performance of a microwave absorption material mainly depends on the intrinsic electrical conductivity, dielectric constant and magnetic permeability [7]. Traditional coloured paints absorb the majority of the incoming solar radiation. An exception has been found for lightly coloured whitish paints [8]. A cool coating must have two criteria: low visible transmittance and low NIR absorptance to minimize NIR heat gain.
Textile modification with nanoparticle provides desirable effects on applied surface without alteration of the fiber’s basic properties. A lot of experiments were conducted based on surface treatment to achieve different developed properties. Experiments are conducted basically on Ti, Ag, Cu, Zn, and other transition metals. At present, research of TiO2 treated textile goes largely on UV protection, antimicrobial, and selfcleaning properties. Depletion of the ozone layer causes TiO2 modified textile to be particularly important for the textile industry because of its UV protection property. TiO2 provides desirable protection when it refracts and/scatters the most UV rays through a high refractive index by absorbing UV rays [9]. Silver improves the performance of TiO2 photocatalyst as it reduces the electron-hole recombination rate and by extending the range of light absorbed by TiO2 to visible region [10,11]. TiO2 rutile is a strongly scattering and weakly absorbing pigment. Titanium dioxide white scatters strongly in most of the solar spectrum but absorbs strongly in the UV (below 400 nm). There is little absorption in most of the visible and infrared spectra [1].
To improve its efficiency is to shift its absorption from the UV region into the visible-light region which allows more photons to be absorbed [12]. One of the most limitations of TiO2 nanoparticle is high rate of electron-hole recombination. So, one way is doping TiO2 with B, P or F atoms by the sol-gel method [13-18]. By adding various dopants into its lattice, much progress has been found in the area of visible light-active TiO2, including metal [19-21] and nonmetal elements [22-26]. Chen et al. examined the Visible-Light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials and found that all those doped TiO2 nanomaterials show a yellow to light yellow color, suggesting their ability to absorb light in the visible region [12]. Mica flakes coated with titanium dioxide and iron oxide are in most cases similar to mica flakes coated with only titanium dioxide, but are more absorbing, less scattering, darker, and somewhat less reflecting in the NIR [27].
Another possibility to modify substrate surface with TiO2 is to investigate in a combination with Ag to achieve desired antimicrobial, UV protective, and photocatalytic characteristics [28-36]. The hybrid functionalization of cotton and viscose fabrics using silver nanowires (AgNWs) colloid and TiO2 was investigated by Giesz et al. [37]. The intercalation of AgNR arrays into the P3HT/PCBM polymer films improved the optical absorption of the films [38]. Low solar absorptance in visible and IR region has been examined with flakes of metal coated with a single or double layer of Fe2O3 on SiO2 under 200 nm layer thickness [39]. Zinc oxide has been used as a white pigment for its absorbance property to light especially UV light [40]. Levinson et al. showed that zinc oxide had an absorption point at approximately 380 nm which means bellow 380 nm it has absorption property to make it opaque [6]. Iron oxide browns exhibit strong absorption in part of the visible spectrum and low absorption in the NIR. But possibly no experiment was conducted on TiO2, in combination with aluminium pigment yet as aluminium pigment doesn’t show antimicrobial, self-cleaning or photocatalytic property which is the most demandable at present. That could one reason why researchers are not interested to work with aluminium pigment. They have another alternative like the compound of Ag, Cu, Zn, Fe or Ti. In this work, we will show the developed absorption functionalization of aluminium and TiO2 modified aluminium effect pigment coated textile in a row.
Materials And Methods
Materials
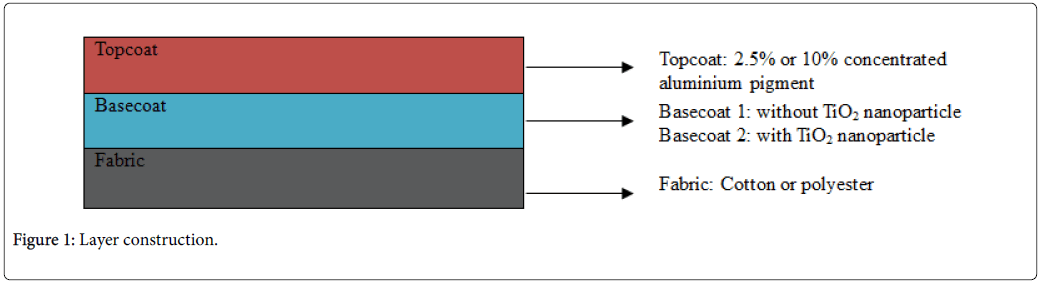
In the experiments, both cotton and polyester textiles were used. Textiles were coated with different pigment concentrations for two different basecoats: basecoat1, without TiO2 nanoparticles and basecoat2 with TiO2 nanoparticles. Eckart white aluminium pigments shinedecor 3500 and shinedecor 5000 were used as metal effect pigments to achieve not only excellent whiteness color but also developed absorbed property of textiles. 30% Helizarin White RTU was added as a source of the TiO2 nanoparticle. Following recipes are followed during metal effect coating preparation process. Two topcoats were prepared by using the bellow recipe (Table 1, Figure 1).
| (a)Basecoat 1 (without TiO2) | ||
|---|---|---|
| Product | Basecoat 1 (%) | |
| Thickener | 3 | |
| softwater | 65.5 | |
| Edulan GS | 30 | |
| Edulan XCL | 1.5 | |
| Helizarin White RTU | - | |
| Pigment | - | |
| (b)Basecoat 2 (with TiO2) | ||
| Product | basecoat 2 | |
| Thickener | 2 | |
| softwater | 36.5 | |
| Edulan GS | 30 | |
| Edulan XCL | 1.5 | |
| Helizarin White RTU | 30 | |
| Pigment | - | |
| (c)Topcoat (2.5% or 10% pigment concentration) | ||
| Product | Topcoat 2.5% | Topcoat 10% |
| Thickener | 2.5 | 0.5 |
| softwater | 56 | 28 |
| Edulan CA | 30 | 30 |
| Edulan XCL | 1.5 | 1.5 |
| Helizarin White RTU | - | - |
| Pigment | 10 (2.5%) | 40 (10%) |
Table 1: Recipe of basecoat 1, basecoat 2 and topcoat
Layer construction
Sixteen samples, eight for cotton and eight for polyester, have been treated with two aluminium effect pigments named Shinedecor 5000 and Shinedecor 5000. The samples have been ordered as bellow (Figure
2 and Table 2).
| Sample no | Pigment name | Basecoat | Topcoat pigment% |
|---|---|---|---|
| 1 | Shinedecor 5000 | basecoat 1 (without TiO2) | Topcoat 2.5% |
| 2 | Topcoat 10% | ||
| 3 | Shinedecor 3500 | basecoat 1 (without TiO2) | Topcoat 2.5% |
| 4 | Topcoat 10% | ||
| 5 | Shinedecor 5000 | basecoat 2 (with TiO2) | Topcoat 2.5% |
| 6 | Topcoat 10% | ||
| 7 | Shinedecor 3500 | basecoat 2 (with TiO2) | Topcoat 2.5% |
| 8 | Topcoat 10% |
Table 2: Details of different pigment-coated textile samples
Methods
Samples are placed on a strand and coated with the previously prepared solutions. The coating was done with a roller following conventional coating procedure. Fabrics were then cured at a 120- degree temperature and after curing for 2 minutes, they are then kept for 24 hours in open air for perfect penetration of the coated particles. It has been done to condense and to fix the chemical compounds on the textile substrate. The strengthened thin film was created by this way which will affect the surface property of employed property.
Results
A UV-VIS Spectrophotometer (model: UV-2600/2700 from Shimadzu), capable of measurement wavelength up to 1400 nm, was used to measure the reflection and transmission
Firstly, the reference cotton and polyester samples were investigated to get reflection% and transmission%. Secondly, aluminium pigment coated cotton and polyester samples (having either basecoat1 or basecoat2) were examined. The sum of the reflection and the transmission% was subtracted from 100% to get absorption% of aluminium effect pigment coated textile.
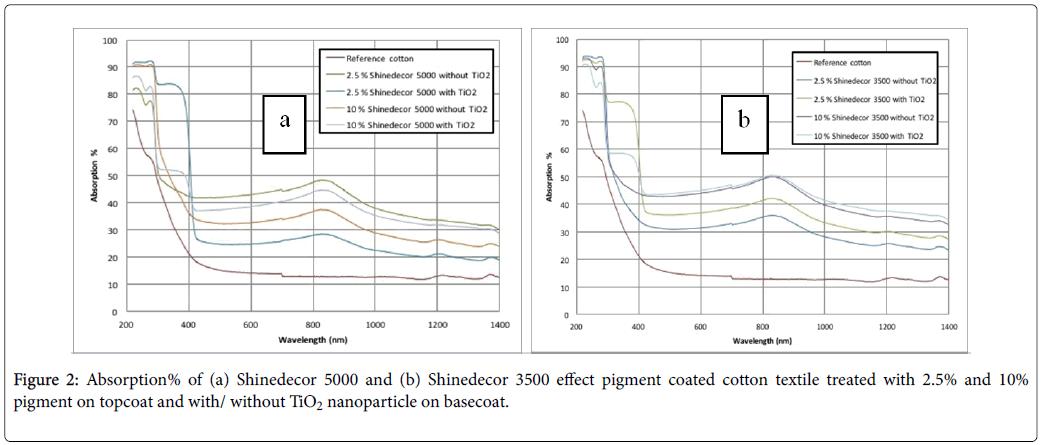
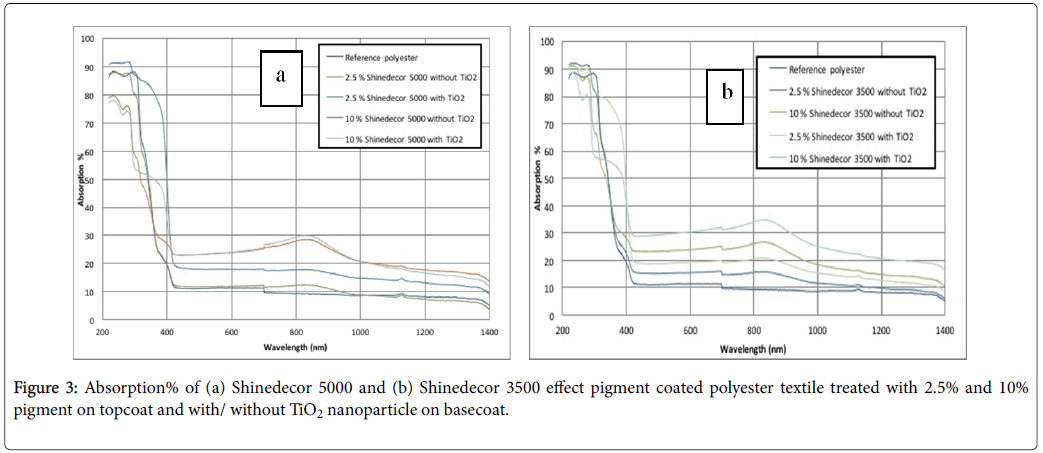
Figure 2a and 1b show absorption% of aluminium effect pigment coated cotton textiles. They contain 2.5% and 10% pigment concentrations, having two different basecoats: basecoat1 (without TiO2 nanoparticles) and basecoat2 (with TiO2 nanoparticles). Figure 2a is for shinedecor 5000 pigment and Figure 2b is for shinedecor 3500 pigment. On the other hand, Figure 3a and 3b show the polyester samples coated with the same metal effect pigments. They include the same sample procedure in composites stated in Figure 2a and 2b.
Discussions
Untreated cotton textile exhibits negative charge due to the presence of hydroxyl (-OH) group at its polymer chain like other cellulosic fibre. On the other hand, polyester textile shows negative charge. It is attributed to the presence of carboxyl (-COOH) end groups. In both cases, these strong negative charges help the fibre to make strong bonding in solution stages, although polyester shows normally hydrophobic and inert nature as very little number of polar groups.
In our experiment, two different aluminium pigments were used. This metal effect pigment consists of metal platelet. This platelet helps textile to achieve both shiny silvery colour and good light scattering property as well. In the UVC region (100 nm-280 nm), the coated textiles absorb about 80-90% of light. The absorption% of untreated reference textile was about 60-70%. The phenomenon was found in both cotton (Figure 2a and 2b) and polyester (Figure 3a and 3b) samples. In the UVB and UVA region, the absorption% was decreased dramatically to 50-20% (Figure 2a and 2b) and 30-10% (Figure 3a and 3b). The coated textiles with the basecoat TiO2 show relatively higher value of absorption% in figures 2 and 3 in the visual and IR region. In the visual and IR spectrum, all of metal effect coated samples exhibit higher absorption% in comparison with uncoated reference cotton and polyester samples.
Some researchers like Holmberg et al. showed relatively higher value of absorption% Silicon and Germanium Nanowire Fabric in visual spectrum [41]. In UV and IR regions, the results were same. But considering the textile were able property, it is very important to keep the textile cool. It is big challenge for the researchers to combine high absorption property of the coated textile in both UV and IR regions. We think that more works have to be done on this area. At all, the significance of this work is to keep the coated textile in a moderate cool condition especially in visual and IR regions.
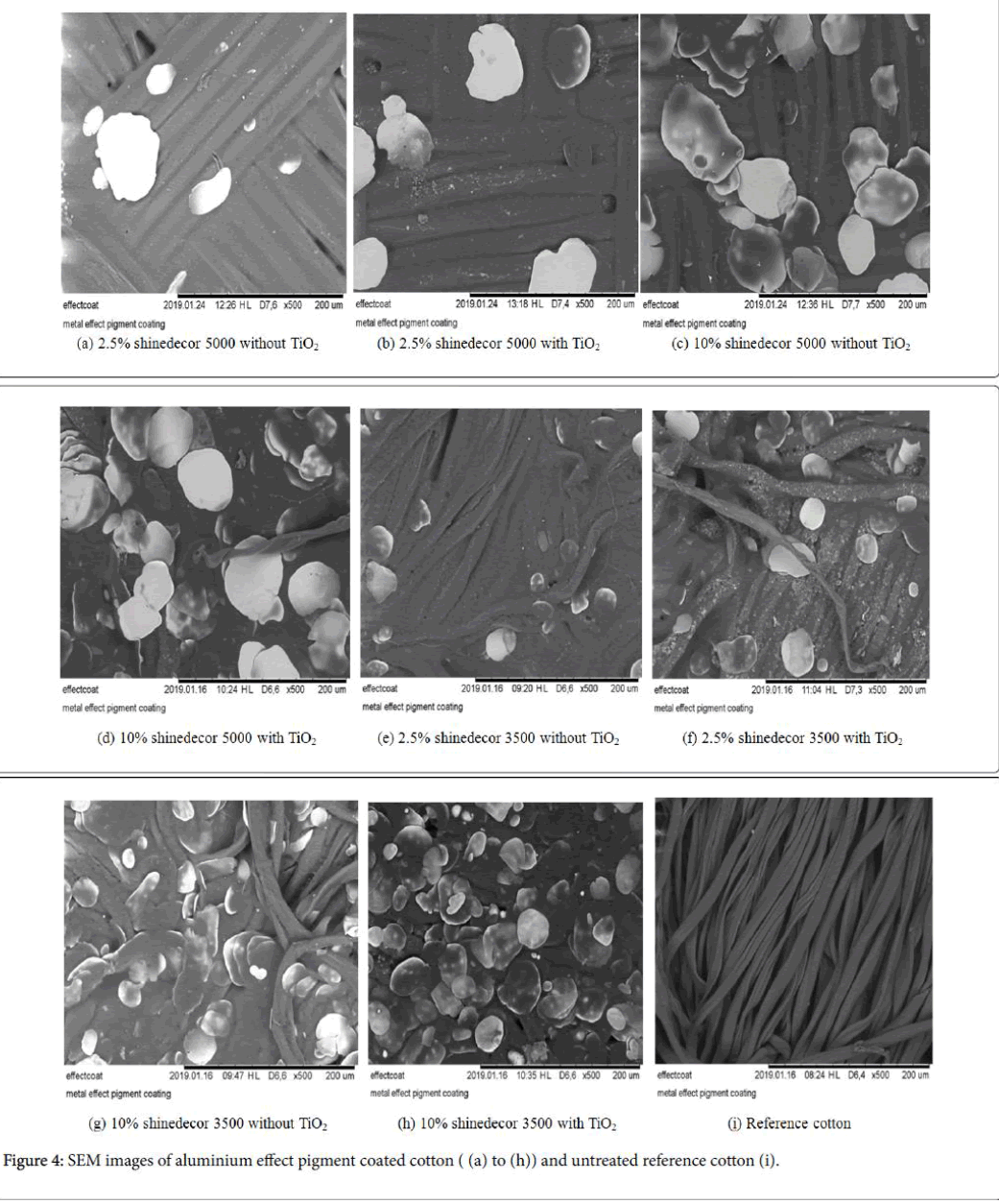
The phenomenon of absorbing more degree of light can be analysed by the SEM image analysis. The aluminium platelets are trapped on textile surface during coating process. They are fixed permanently after the curing process. (Figures 3a-3h) for cotton and (Figure 4a-4h) for polyester show different corn-like platelets on their surfaces. The density of their appearance usually depends on the pigment concentration. If the pigment concentration is higher, the possibility of their appearance on surface is higher. Sometimes, different phenomenon has been found because of uneven distribution of pigment particles. Probably, for this reason (Figures 3b and 3e, and Figure 4a and 4b) present relatively lower amount of pigment particle than the amount to be expected. Aluminium platelets are visualized on textile surfaces by the SEM images. These platelets not only reflect the incident light but also a big amount of incident light is absorbed. In addition, TiO2 nanoparticles also have influence on optical protection of the textile surfaces as it also scatters the incident radiation. The textile surface was treated with TiO2 nanoparticle in combination with aluminium pigment. It showed a higher light absorption% of the coated substrate. Using TiO2 as basecoat increases the absorbency at higher extent (Figures 4 and 5).
Conclusion
The higher absorption of light has been achieved by using aluminium effect pigment in along with TiO2 or without TiO2. In UV region, specially UVC and UVB region, this metal effect coated film gives fabric UV absorbing property. In UVC spectrum region, about 80-90% of spectrum has been absorbed which is tremendously higher. It also shows that developed absorption is parallel in visual and IR region for all the pigment coated textile and the absorption percentage is low. In NIR region, when a pigment absorbs less, the coating layer remains cool. More research on it can be possible solution to use this coated fabric as good absorber.
Acknowledgments
We would like to thank Thomas Heistermann and Dr. Thomas Grethe, Hochschule Niederrhein, for supplying the chemicals, technical help and ensuring the use of analytical instrument.
References
- Levinson R, Berdahl P, Akbari H (2005a) Solar spectral optical properties of pigments—Part II: survey of common colorants. Sol Energy Mater Sol 89: 351-389.
- Karlsson P, Palmqvist AE, Holmberg K (2006) Surface modification for aluminium pigment inhibition. Adv Colloid Interface Sci 128: 121-134.
- Fujishima A, Zhang X, Tryk DA (2008) TiO2 photocatalysis and related surface phenomena. Surf Sci Rep 63: 515-582.
- Zimniewska M, Batog J, Romanowska EBB (2012) Functionalization of natural fibres textiles by improvement of nanoparticles fixation on their surface. Fiber Bioeng Info 5: 321-339.
- Zimniewska M, Myalski J, Koziol M, Mankowski J, Bogacz E (2012) Natural fiber textile structures suitable for composite materials. J Nat Fibers 9: 229-239.
- Levinson R, Berdahl P, Akbari H (2005b) Solar spectral optical properties of pigments—Part I: model for deriving scattering and absorption coefficients from transmittance and reflectance measurements. Sol Energy Mater Sol 89: 319-349.
- Wang X, Liao X, Zhang W, Shi B (2015) Bio-inspired fabrication of hierarchical Ni-Fe-P coated skin collagen fibers for high-performance microwave absorption. Phys Chem Chem Phys 17: 2113-2120.
- Smith GB, Gentle A, Swift P, Earp A, Mronga N (2003) Coloured paints based on coated flakes of metal as the pigment, for enhanced solar reflectance and cooler interiors: description and theory. Sol Energy Mater Sol 79: 163-177.
- Radetic M (2013) Functionalization of textile materials with TiO2 nanoparticles. J Photochem Photobiol C Photo Chem Rev 16: 62-76.
- Dong H, Wu Z, Lu F, Gao Y, El-Shafei A, et al. (2014) Optics-electrics highways: Plasmonic silver [email protected] 2 core-shell nanocomposites for enhanced dye-sensitized solar cells performance. Nano Energy 10: 181-191.
- Etacheri V, Di Valentin C, Schneider J, Bahnemann D, Pillai SC (2015) Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J Photochem Photobiol C Photo Chem Rev 25: 1-29.
- Chen X, Burda C (2008) The Electronic Origin of the Visible-Light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials. J Am Chem Soc 130: 5018-5019.
- Zhao W, Ma W, Chen C, Zhao J, Shuai Z (2004) Efficient Degradation of Toxic Organic Pollutants with Ni2O3/TiO2-xBxunder Visible Irradiation. J Am Chem Soc 126: 4782-4783.
- Lin L, Lin W, Zhu Y, Zhao B, Xie Y (2005) Phosphor-doped Titania —a Novel Photocatalyst Active in Visible Light. Chemistry Letters, 34:284-285.
- Yu JC, Yu J, Ho W, Jiang Z, Zhang L (2002) Effects of F-Doping on the Photocatalytic Activity and Microstructures of Nanocrystalline TiO2 Powders. Chem Mater 14: 3808-3816.
- Nukumizu K, Nunoshige J, Takata T, Kondo JN, Hara M, et al. (2003) TiNxOyFz as a Stable Photocatalyst for Water Oxidation in Visible Light (<570 nm). Chem Letters 32: 196-197.
- Li D, Haneda H, Labhsetwar NK, Hishita S, Ohashi N (2005) Visible-light-driven photocatalysis on fluorine-doped TiO2 powders by the creation of surface oxygen vacancies. Chem Phys Letters 401: 579-584.
- Li D, Haneda H, Hishita S, Ohashi N (2005b) Visible-Light-Driven N-F-Codoped TiO2 Photocatalysts. 2. Optical Characterization, Photocatalysis, and Potential Application to Air Purification. Chem of Mater 17: 2596-2602.
- Choi W, Termin A, Hoffmann MR (1994) Effects of Metal-Ion Dopants on the Photocatalytic Reactivity of Quantum-Sized TiO2 Particles. Angew Chem Int Ed Engl 33: 1091-1092.
- Umebayashi T, Yamaki T, Itoh H, Asai K (2002) Analysis of electronic structures of 3d transition metal-doped TiO2 based on band calculations. Journal of Physics and Chemistry of Solids 63: 1909-1920.
- Anpo M, Kishiguchi S, Ichihashi Y, Takeuchi M, Yamashita H, et al. (2001) The design and development of second-generation titanium oxide photocatalysts able to operate under visible light irradiation by applying a metal ion-implantation method. Res Chem Intermed 27: 459-467.
- Yang H, Zhu S, Pan N k (2004) Studying the mechanisms of titanium dioxide as ultraviolet-blocking additive for films and fabrics by an improved scheme. J Appl Polym Sci 92: 3201-3210.
- Khan SUM (2002) Efficient Photochemical Water Splitting by a Chemically Modified n-TiO2. Science 297: 2243-2245.
- Burda C, Lou Y, Chen X, Samia ACS, Stout J, et al. (2003) Enhanced Nitrogen Doping in TiO2Nanoparticles. Nano Letters 3: 1049-1051.
- Chen X, Burda C (2004) Photoelectron Spectroscopic Investigation of Nitrogen-Doped Titania Nanoparticles. J Phys Chem B 108: 15446-15449.
- Chen X, Lou YB, Samia ACS, Burda C, Gole JL (2005) Formation of Oxynitride as the Photocatalytic Enhancing Site in Nitrogen-Doped Titania Nanocatalysts: Comparison to a Commercial Nanopowder. Adv Funct Mater 15: 41-49.
- Wißling P (2006) Metallic effect pigments: fundamentals and applications. Vincentz Network GmbH & Co KG.
- Mihailovic D, Å aponjic Z, Vodnik V, Potkonjak B, Jovancic P, et al. (2010) Multifunctional PES fabrics modified with colloidal Ag and TiO2 nanoparticles. Polym Adv Technol 22: 2244-2249.
- Cheng Q, Li C, Pavlinek V, Saha P, Wang H (2006) Surface-modified antibacterial TiO2/Ag+ nanoparticles: Preparation and properties. Appl Surf Sci 252: 4154-4160.
- Li J, Liu X, Qiao Y, Zhu H, Ding C (2014) Antimicrobial activity and cytocompatibility of Ag plasma-modified hierarchical TiO2 film on titanium surface. Colloids Surf B Biointerfaces 113: 134-145.
- Yang Y, Wen J, Wei J, Xiong R, Shi J, et al. (2013) Polypyrrole-decorated Ag-TiO2 nanofibers exhibiting enhanced photocatalytic activity under visible-light illumination. ACS Appl Mater Interfaces 5: 6201-6207.
- Méndez-Medrano MG, Kowalska E, Lehoux A, Herissan A, Ohtani B, et al. (2016) Surface modification of TiO2 with Ag nanoparticles and CuO nanoclusters for application in photocatalysis. J Phys Chem C 120: 5143-5154.
- Wang Q, Yang X, Liu D, Zhao J (2012) Fabrication, characterization and photocatalytic properties of Ag nanoparticles modified TiO2 NTs. J Alloy Compd 527: 106-111.
- Xin B, Jing L, Ren Z, Wang B, Fu H (2005) Effects of simultaneously doped and deposited Ag on the photocatalytic activity and surface states of TiO2. J Phys Chem B 109: 2805-2809.
- Liu C, Yang D, Jiao Y, Tian Y, Wang Y, et al. (2013) Biomimetic synthesis of TiO2-SiO2-Ag nanocomposites with enhanced visible-light photocatalytic activity. ACS Appl Mater Interfaces 5: 3824-3832.
- Daghrir R, Drogui P, Robert D (2013) Modified TiO2 for environmental photocatalytic applications: a review. Ind Eng Chem Res 52:3581-3599.
- Giesz P, Mackiewicz E, Grobelny J, Celichowski G, Cieslak M (2017) Multifunctional hybrid functionalization of cellulose fabrics with AgNWs and TiO2. Carbohydr Polym 177: 397-405.
- Park JB, Bae TS, Sohn JI, Cha S, Lee J, et al. (2015) Fabrication of Ag Nanorods-Embedded P3HT/PCBM Films for the Enhancement of Light Absorption. ECS Solid State Lett 4: 5-9.
- Smith GB, Gentle A, Swift P, Earp A, Mronga N (2003) Coloured paints based on coated flakes of metal as the pigment, for enhanced solar reflectance and cooler interiors: description and theory. Sol Energy Mater Sol 79: 163-177.
- Saito M (1993) Antibacterial, Deodorizing, and UV Absorbing Materials Obtained with Zinc Oxide (ZnO) Coated Fabrics. Journal of Coated Fabrics 23:150-164.
- Holmberg VC, Bogart TD, Chockla AM, Hessel CM, Korgel BA (2012) Optical properties of silicon and germanium nanowire fabric. J Phys Chem C 116 (42): 22486-22491.
Citation: Rashid MM, Mahltig M (2019) Light Absorption of the Aluminium Effect Pigment Coated Textile: An Investigation Using TiO2 Nanoparticle in the Basecoat and Al2O3 Nanoparticle in the Topcoat. J Mater Sci Nanomater 3: 110.
Copyright: © 2019 Rashida MM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.