Short Communication Open Access
Ligands of Receptor for Advanced Glycation End-Products Produced by Activated Microglia are Critical in Neurodegenerative Diseases
Myeongjoo Son1,2* Seyeon Oh2*, Sojung Lee1,2 and Kyunghee Byun1,2†1Department of Anatomy and Cell Biology, Gachon University, Graduate School of Medicine, Incheon, Republic of Korea
2Functional Cellular Networks Laboratory, Lee Gil Ya Cancer and Diabetes Institute, Gachon University, Incheon, Republic of Korea
- Corresponding Author:
- Kyunghee Byun
Department of Anatomy and Cell Biology
Gachon University Graduate School of Medicine
Yeonsu-Gu, Incheon 21999, Republic of Korea
Tel: + 82-899-6511
E-mail: khbyun1@gachon.ac.kr
Received date: March 17, 2017; Accepted date: April 03, 2017; Published date: April 10, 2017
Citation: Son M, Oh S, Lee S, Byun K (2017) Ligands of Receptor for Advanced Glycation End-Products Produced by Activated Microglia are Critical in Neurodegenerative Diseases. J Alzheimers Dis Parkinsonism 7:318. doi:10.4172/2161-0460.1000318
Copyright: © 2017 Son M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Receptor for advanced glycation end products (RAGE) and its ligands have been reported to be involved in the progressions of neurodegenerative diseases, including Alzheimer’s and Parkinson’s disease. Recently microglia activated by immunological stimuli, cytokines, or oxidative stress were reported to synthesize and secrete RAGE ligands including AGEs, HMGB1, and S100 in neurodegenerative diseases. Furthermore, RAGE/ligand binding has been implicated in neuroinflammation and in the progression of neurodegenerative diseases through a RAGEmediated pathway in neurons. A number of RAGE inhibitors, such as, antagonists, small RAGE inhibitors, anti-RAGE antibody, and soluble RAGE, have been shown to interfere with RAGE/ligand binding and to reduce RAGE ligand accumulation, microglia activation, and neuronal cell death in neurodegenerative diseases. Accordingly, RAGE inhibitors present an attractive therapeutic target in neurodegenerative diseases, and RAGE ligands might be useful diagnostic targets. Some human studies have shown RAGE ligand distributions in brain, serum, and cerebrospinal fluid are promising biomarkers for early disease detection and that these ligands might play important roles during early disease stages. Taken together, RAGE ligands and RAGE inhibitors appear to be good therapeutic and diagnostic candidates for neurodegenerative diseases.
Keywords
Amyloid beta (Aβ); Advanced glycated end products (AGEs); HMGB1; S100; Receptor of AGEs (RAGE); Microglia activation; Alzheimer’s disease (AD); Parkinson’s disease (PD); Diagnosis; Therapeutic effects
Relations between Microglial Activation and Neuronal Cell Death in Neurodegenerative Diseases
Neurodegenerative diseases result from the progressive loss of neuronal cell functions and structures, and Alzheimer’s disease (AD) is a chronic type of neurodegenerative disease [1]. The main causes of AD have yet to be determined, although 1% to 5% of cases harbor a genetic mutation [2]. Several studies have shown chronic inflammation contributes to the pathology of AD [3,4] and which is known to be related to microglia activation [5,6]. Although the cause of AD progression is unclear, AD is characterized by inflammatory responses to amyloid-β (Aβ), microglia activation, and astrocyte recruitment by Aβ deposits [7]. Parkinson’s disease (PD) is occurs by loss of dopaminergic neurons in the substantia nigra (SN) and by many other events and agents, such as, genetic events or toxic drugs or chemicals, such as, 1-methyl-4- phenyl-1,2,3-6 tetrahydropyridine or rotenone [8,9]. Pathologic changes in the PD brain are closely related to microglial activation induced inflammation, which accelerates dopamine (DA)-producing neuron death. Interestingly, positron emission tomography (PET) studies have shown noticeable microglial activation in the SN, putamen and subcortical and cortical areas of the PD brain [10,11].
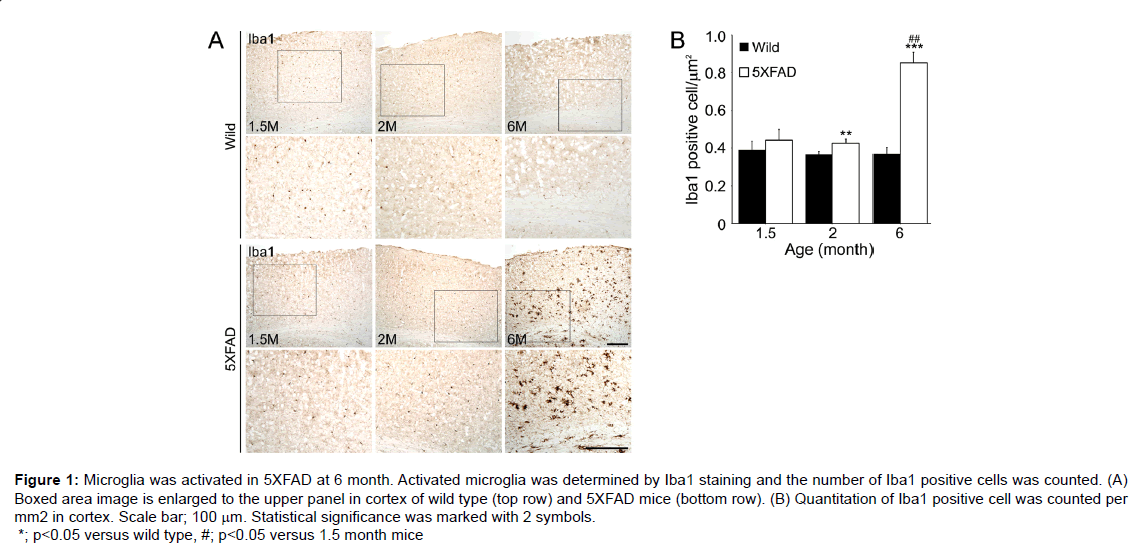
Molecular genetic studies are indispensable for understanding the central role played by Aβ in the pathogenesis of AD. Amyloid precursor protein (APP) mutation mouse models, such as, the PDAPP [12], Tg2576 [13], APP23 [14], APP/presenilin (PS1) models or APP/ PS1/Tau mutation models, such as, the APP/PS1 [15], 3XFAD [16] and 5XFAD [17] models. The 5XFAD model shows an amyloid plaque formation, which exhibits neuron loss in cortical layer 5 and subiculum from 9 months [18] and inn this model; microglia is activated in the cortex (Figure 1), which is a region of neuronal death [18].
Genetic mutation animal models of PD have also been well established, such as, the PINK1, PARKIN, DJ-1, PARK9, LRKK2 and α-Synuclein models [19]. Although genetic models generally show features that appear in the PD, PINK1 [20] and PARKIN [21] genetic models do not exhibit DA related behavior abnormities and the DJ-1 [22], PARK9 [23] and LRRK2 [24,25] models do not exhibit changes in the number of DA-producing neurons in SN.
However, α-Synuclein genetic model exhibits hallmark pathologic features of PD, including progressive loss of the DA-producing neurons in the SN and reduced DA levels in the striatum [26] and formation of Lewy bodies in old animals [19]. In particular, α-synuclein has been reported to be related to microglia activation in the SN and striatum [27,28]. These observations suggest microglial activation is critical for neuronal cell death.
Microglia can be activated by toxins, cytokines, injury, or inflammation [29,30] and their activation has also been reported to be a key contributor [5,6,31]. Cytokines are involved in systemic inflammation and in degenerative disease and can be produced by neurons. In AD, amyloid β (Aβ), chromogranin A (CGA), interferon gamma (IFN-γ), and matrix metalloproteinase-3 (MMP-3) are candidate participants in neuronal apoptosis and microglial activation [32-34] and the cytokines α-synuclein, CGA, IFN-γ, MMP-3, neuromelanin, and tumor necrosis factor alpha (TNF-α) are candidate microglia activators in PD [35-41].
Figure 1: Microglia was activated in 5XFAD at 6 month. Activated microglia was determined by Iba1 staining and the number of Iba1 positive cells was counted. (A) Boxed area image is enlarged to the upper panel in cortex of wild type (top row) and 5XFAD mice (bottom row). (B) Quantitation of Iba1 positive cell was counted per mm2 in cortex. Scale bar; 100 μm. Statistical significance was marked with 2 symbols. *; p<0.05 versus wild type, #; p<0.05 versus 1.5 month mice
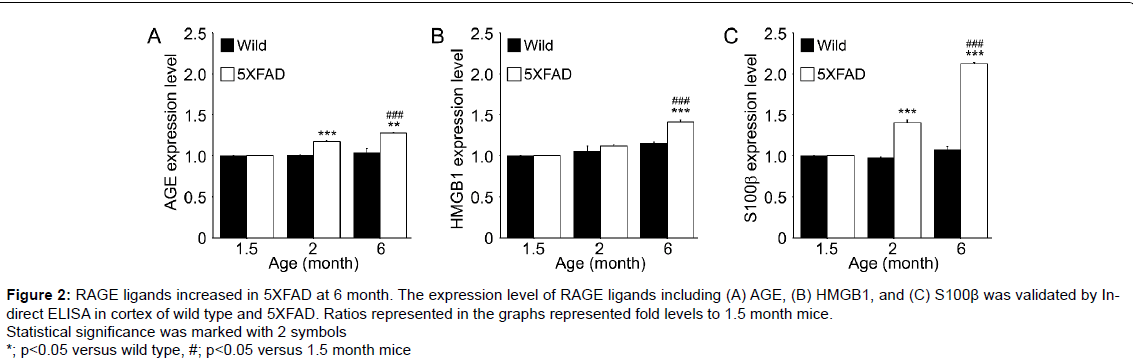
Figure 2:RAGE ligands increased in 5XFAD at 6 month. The expression level of RAGE ligands including (A) AGE, (B) HMGB1, and (C) S100β was validated by Indirect ELISA in cortex of wild type and 5XFAD. Ratios represented in the graphs represented fold levels to 1.5 month mice. Statistical significance was marked with 2 symbols. *; p<0.05 versus wild type, #; p<0.05 versus 1.5 month mice.
Secretion and Synthesis of RAGE Ligands by Activated Microglia in AD and PD
RAGE ligands include advanced glycation end products (AGEs), high mobility group box chromosomal protein 1 (HMGB1), lipopolysaccharide (LPS), macrophage-1 antigen (Mac-1), phosphatidylserine and S100/calgranulin and under pathologic conditions activate microglia [42-46], which then secrete and synthesize RAGE ligands, such as, AGEs, HMGB1 and S100β in AD [29] (Figure 2) and PD [47].
Advanced glycation end products (AGEs)
AGEs are considered to induce the development or support the progression of neurodegenerative diseases and their toxic properties are known to stem from oxidative stress and inflammation [48,49]. AGEs localize in senile plaques and extracellular spaces in AD [50-52] and AGE-albumin and RAGE binding stimulate the activations of diverse signaling cascades through MAPK (mitogen-activated protein kinases) and bcl-2-like protein 4 (Bax) pathways that result in neuron apoptosis [29]. In PD, AGEs act as major structural cross-linkers, and are responsible for the formation of Lewy bodies in human dopaminergic neurons [53]. Furthermore, AGE-albumin and RAGE co-localization promote MAPK and Bax mediated DA-producing neuron apoptosis in the SN of Rotenone-exposed mice [47] in a manner similar to that observed in AD [29].
High mobility group box 1 protein (HMGB1)
Activated microglia secretes HMGB1 during inflammation in neurodegenerative diseases [54] and this secretion is increased by Aβ and promotes neuronal cell death. Furthermore, microglial infiltration and secretion of soluble HMGB1 are significantly elevated in the AD hippocampus and promote neuronal cell death, synaptic destruction and behavioral deficits [55]. The interaction between HMGB1 and RAGE activates the NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathway and leads to neuronal cell death via an autoregulatory loop, which exacerbates neurodegeneration and neuroinflammation in AD [56]. HMGB1 is also released by microglia under inflammatory conditions, and in PD, binds to microglial Mac-1. Furthermore, these activities activate the NF-κB pathway and NADPH (nicotinamide adenine dinucleotide phosphate) oxidase [57].
The S100 protein family
This low-molecular-weight protein family consists of approximately 25 proteins, which are involved in the regulation of protein phosphorylation, calcium homeostasis, transcription factors, and inflammatory response [58]. S100β is found in degenerative cells of AD and PD [59], and it has been reported S100β overexpression ameliorates AD-like pathology and enhances microgliosis and astrogliosis [60]. S100 proteins have also been reported to cause neuronal cell death via TNF-α and NF-κB in PD [61].
In the 5XFAD model, microglia activation increased at 6 months and significantly increased RAGE ligand levels at 6 months versus 1.5 months, and these ligands can promote neuron cell death at 9 or 12 months (Figure 2) [18]. Interestingly, in PD, secreted α-synuclein can activate microglia directly and LRRK2 has been related with the regulation of microglia activation in PD [62].
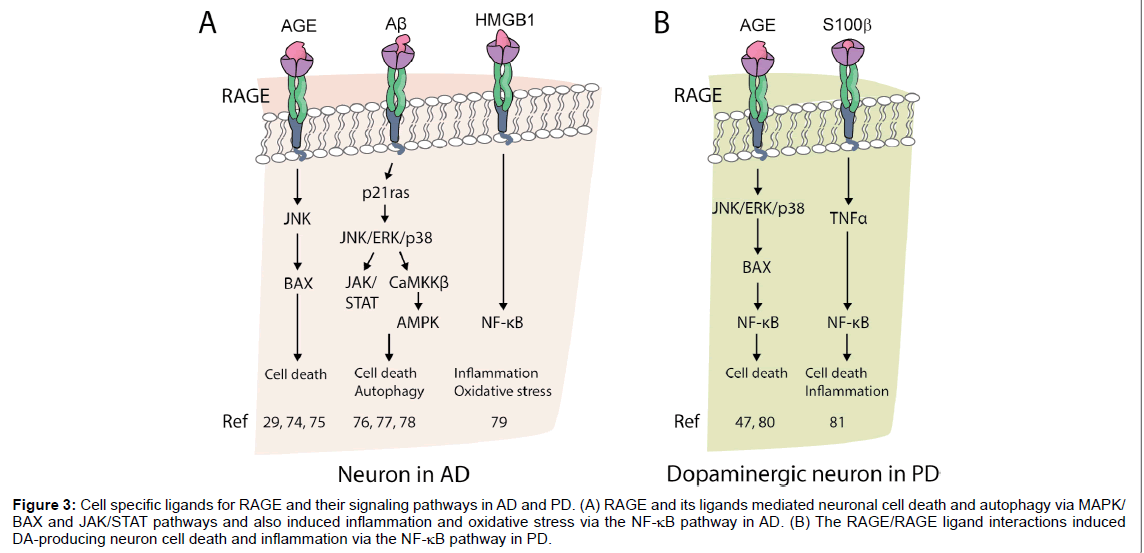
RAGE ligands are secreted and synthesized by activated microglia and accelerate neurodegenerative diseases via RAGE pathway (Figure 3). In particular, JNK and MAPK play important roles in neuronal cell death and seem to be induced by NF-κB in the presence of inflammation.
RAGE Ligands and RAGE as Potential Therapeutic and Diagnostic Targets in AD and PD
RAGE/ligand binding leads to neuronal cell death, and thus, the inhibition of this binding considered an important strategy for treating neurodegenerative diseases [29,47]. Furthermore, anti-RAGE therapy using an antagonist, small molecule RAGE inhibitors, soluble RAGE (sRAGE) or anti-RAGE antibody has been reported to protect neurons [63-66]. In AD, FPS-ZM1 (a high-affinity RAGE-specific inhibitor) was found to specifically bind to the V domain of RAGE, to cross the Blood-Brain-Barrier (BBB), and to inhibit Aβ-induced cellular stress [63], and FPS-ZM1 protected neurons from mitochondrial injury and oxidative stress by reducing RAGE/ligand binding directly or reducing Aβ levels indirectly in brain [67-69]. In addition, a number of RAGE inhibitors are undergoing clinical trials for the treatment of AD. Azeliragon (TTP488), which was developed by vTv Therapeutics (formerly TransTech Pharma), is a candidate for the treatment of mild AD and is currently the subject of a phase 3 clinical trial [70]. TransTech Pharma also developed PF-04494700, which inhibits RAGE/Aβ-42 binding. This agent, which is administered orally, crosses the BBB and helps reduce Aβ accumulation and spatial memory defects [71].
Some evidence indicates sRAGE and anti-RAGE antibody block RAGE/ligand binding in AD. sRAGE is a splice variant of RAGE that binds RAGE ligands more effectively than RAGE, and thus, downregulates the RAGE-mediated pathway. sRAGE and anti-RAGE antibody act by inhibiting Aβ uptake and RAGE/ligand binding [29,63], but sRAGE also reduces cell death of DA-producing neuron through MAPK phosphorylation, Bax expression in PD [47]. RAGE ligands can also be used to support diagnoses of AD or PD. Generally, MRI and cerebrospinal fluid (CSF) biomarkers related to AD, do not provide evidence of neurodegenerative change or amyloid clearance [71,72]. However, it has been reported that some RAGE ligands might be useful diagnostic targets. In the human AD brain, AGEs are distributed in neuron cytoplasm in the hippocampus and para-hippocampal gyrus and in one study, serum and CSF levels of AGEs were suggested as biomarkers for the early detection of AD [72]. Furthermore, AGE accumulation has been observed in incidental Lewy Body diseases (a presymptomatic PD state) as well as in the Lewy bodies of PD patients [73]. These studies suggest AGEs/RAGE binding plays an important role during the early stages of neurodegenerative diseases [73].
Conclusion
The role played by activated microglia in neuronal cell death has been well established in neurodegenerative diseases. Microglia activated by immunological stimuli, oxidative stress or cytokines secrete and synthesize RAGE ligands, such as, AGEs, HMGB1 and S100β, and these ligands trigger deleterious, RAGE mediated, signaling, which results in neuron death in AD and PD. A number of RAGE inhibitors, including antagonists, small molecule RAGE inhibitors, sRAGE and anti-RAGE antibody have been demonstrated to ameliorate the pathologic processes of AD and PD. Moreover, RAGE ligands in serum or CSF can be used diagnostically to detect the presence of AD and PD. Furthermore, evidence at hand indicates RAGE inhibitors and RAGE ligands are good therapeutic and diagnostic candidates in AD and PD [74-81].
Figure 3:Cell specific ligands for RAGE and their signaling pathways in AD and PD. (A) RAGE and its ligands mediated neuronal cell death and autophagy via MAPK/ BAX and JAK/STAT pathways and also induced inflammation and oxidative stress via the NF-κB pathway in AD. (B) The RAGE/RAGE ligand interactions induced DA-producing neuron cell death and inflammation via the NF-κB pathway in PD.
Contribution
Myeongjoo Son and Seyeon Oh have equally contributed.
Acknowledgement
This work was supported by the National Research Foundation of Korea (grant no. 2015R1A2A2A01005212).
References
- Burns A, Iliffe S (2009) Alzheimer's disease. BMJ 338: b158.
- Reitz C, Mayeux R (2014) Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 88: 640-651.
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, et al. (2000) Inflammation and Alzheimer's disease. Neurobiol Aging 21: 383-421.
- Ahn SM, Byun K, Cho K, Kim JY, Yoo JS, et al. (2008) Human microglial cells synthesize albumin in brain. PLoS One 3: e2829.
- Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D (1989) Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol 24: 173-182.
- Luber-Narod J, Rogers J (1988) Immune system associated antigens expressed by cells of the human central nervous system. Neurosci Lett 94: 17-22.
- Sastre M, Klockgether T, Heneka MT (2006) Contribution of inflammatory processes to Alzheimer's disease: molecular mechanisms. Int J Dev Neurosci 24: 167-176.
- Hirsch EC, Höglinger G, Rousselet E, Breidert T, Parain K, et al. (2003) Animal models of Parkinson's disease in rodents induced by toxins: An update. J Neural Transm Suppl 89-100.
- Binienda ZK, Sarkar S, Mohammed-Saeed L, Gough B, Beaudoin MA, et al. (2013) Chronic exposure to rotenone, a dopaminergic toxin, results in peripheral neuropathy associated with dopaminergic damage. Neurosci Lett 541: 233-237.
- Bartels AL, Willemsen AT, Doorduin J, de Vries EF, Dierckx RA, et al. (2010) [11C]-PK11195 PET: quantification of neuroinflammation and a monitor of anti-inflammatory treatment in Parkinson's disease? Parkinsonism Relat Disord 16: 57-59.
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, et al. (2006) In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis 21: 404-412.
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, et al. (1995) Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature 373: 523-527.
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, et al. (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274: 99-103.
- Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, et al. (1997) Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci U S A 94: 13287-13292.
- Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, et al. (2001) Co-expression of multiple transgenes in mouse CNS: A comparison of strategies. Biomol Eng 17: 157-165.
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, et al. (2003) Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39: 409-421.
- Oakley H, Cole SL, Logan S, Maus E, Shao P, et al. (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: Potential factors in amyloid plaque formation. J Neurosci 26: 10129-10140.
- Eimer WA, Vassar R (2013) Neuron loss in the 5XFAD mouse model of Alzheimer's disease correlates with intraneuronal Aβ42 accumulation and Caspase-3 activation. Mol Neurodegener 8: 2.
- Blesa J, Przedborski S (2014) Parkinson's disease: Animal models and dopaminergic cell vulnerability. Front Neuroanat 8: 155.
- Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, et al. (2009) PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell 33: 627-638.
- Kitada T, Pisani A, Karouani M, Haburcak M, Martella G, et al. (2009) Impaired dopamine release and synaptic plasticity in the striatum of parkin-/- mice. J Neurochem 110: 613-621.
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, et al. (2005) Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A 102: 5215-5220.
- Schultheis PJ, Fleming SM, Clippinger AK, Lewis J, Tsunemi T, et al. (2013) Atp13a2-deficient mice exhibit neuronal ceroid lipofuscinosis, limited a-synuclein accumulation and age-dependent sensorimotor deficits. Hum Mol Genet 22: 2067-2082.
- Tong Y, Pisani A, Martella G, Karouani M, Yamaguchi H, et al. (2009) R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci U S A 106: 14622-14627.
- Herzig MC, Kolly C, Persohn E, Theil D, Schweizer T, et al. (2011) LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum Mol Genet 20: 4209-4223.
- Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, et al. (2012) Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338: 949-953.
- Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB (2005) Microglial inflammation in the parkinsonian substantia nigra: Relationship to alpha-synuclein deposition. J Neuroinflammation 2: 14.
- Watson MB, Richter F, Lee SK, Gabby L, Wu J, et al. (2012) Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein. Exp Neurol 237: 318-334.
- Byun K, Bayarsaikhan E, Kim D, Kim CY, Mook-Jung I, et al. (2012) Induction of neuronal death by microglial AGE-albumin: Implications for Alzheimer's disease. PLoS One 7: e37917.
- Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, et al. (1999) Neuroglial activation repertoire in the injured brain: Graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev 30: 77-105.
- Colton CA, Gilbert DL (1987) Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett 223: 284-288.
- Cai Z, Hussain MD, Yan LJ (2014) Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer's disease. Int J Neurosci 124: 307-321.
- Wu Z, Sun L, Hashioka S, Yu S, Schwab C, et al. (2013) Differential pathways for interleukin-1β production activated by chromogranin A and amyloid β in microglia. Neurobiol Aging 34: 2715-2725.
- Badie B, Schartner J, Vorpahl J, Preston K (2000) Interferon-gamma induces apoptosis and augments the expression of Fas and Fas ligand by microglia in vitro. Exp Neurol 162: 290-296.
- Kim YS, Joh TH (2006) Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson's disease. Exp Mol Med 38: 333-347.
- Taupenot L, Ciesielski-Treska J, Ulrich G, Chasserot-Golaz S, Aunis D, et al. (1996) Chromogranin A triggers a phenotypic transformation and the generation of nitric oxide in brain microglial cells. J Neurosci 72: 377-389.
- Twig G, Graf SA, Messerli MA, Smith PJ, Yoo SH, et al. (2005) Synergistic amplification of beta-amyloid- and interferon-gamma-induced microglial neurotoxic response by the senile plaque component chromogranin A. Am J Physiol Cell Physiol 288: C169-175.
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, et al. (2008) Synuclein activates microglia in a model of Parkinson's disease. Neurobiol Aging 29: 1690-1701.
- Upender MB, Naegele JR (1999) Activation of microglia during developmentally regulated cell death in the cerebral cortex. Dev Neurosci 21: 491-505.
- Kim YS, Choi DH, Block ML, Lorenzl S, Yang L, et al. (2007) A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. FASEB j 21: 179-187.
- Mount MP, Lira A, Grimes D, Smith PD, Faucher S, et al. (2007) Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J Neurosci 27: 3328-3337.
- Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, et al. (1999) RAGE mediates a novel proinflammatory axis: A central cell surface receptor for S100/calgranulin polypeptides. Cell 97: 889-901.
- Schmidt AM, Yan SD, Yan SF, Stern DM (2001) The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 108: 949-955.
- Chavakis T, Bierhaus A, Nawroth PP (2004) RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect 6: 1219-1225.
- He M, Kubo H, Morimoto K, Fujino N, Suzuki T, et al. (2011) Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep 12: 358-364.
- Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, et al. (2011) HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci 31: 1081-1092
- Bayarsaikhan E, Bayarsaikhan D, Lee J, Son M, Oh S, et al. (2016) Microglial AGE-albumin is critical for neuronal death in Parkinson's disease: A possible implication for theranostics. Int J Nanomedicine 10 Spec Iss: 281-292.
- Li J, Liu D, Sun L, Lu Y, Zhang Z (2012) Advanced glycation end products and neurodegenerative diseases: mechanisms and perspective. J Neurol Sci 317: 1-5.
- Grillo MA, Colombatto S (2008) Advanced glycation end-products (AGEs): involvement in aging and in neurodegenerative diseases. Amino Acids 35: 29-36.
- El Khoury J, Luster AD (2008) Mechanisms of microglia accumulation in Alzheimer's disease: Therapeutic implications. Trends Pharmacol Sci 29: 626-632.
- Shuvaev VV, Laffont I, Serot JM, Fujii J, Taniguchi N, et al. (2001) Increased protein glycation in cerebrospinal fluid of Alzheimer's disease. Neurobiol Aging 22: 397-402.
- Münch G, Cunningham AM, Riederer P, Braak E (1998) Advanced glycation endproducts are associated with Hirano bodies in Alzheimer's disease. Brain Res 796: 307-310.
- Castellani R, Smith MA, Richey PL, Perry G (1996) Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res 737: 195-200.
- Andersson U, Tracey KJ (2011) HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 29: 139-162.
- Jang A, Liew H, Kim YM, Choi H, Kim S, et al. (2013) p35 deficiency accelerates HMGB-1-mediated neuronal death in the early stages of an Alzheimer's disease mouse model. Curr Alzheimer Res 10: 829-843.
- Wei X, Yang D, Shi T, Wang J, Deng Y, et al. (2016) Metabotropic Glutamate Receptor 7 (mGluR7) as a Target for the Modulating Pain-Evoked Activities of Neurons in the Hippocampal CA3 Region of Rats. CNS Neurol Disord Drug Targets.
- Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, et al. (2011) HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci 31: 1081-1092.
- Wolf R, Howard OM, Dong HF, Voscopoulos C, Boeshans K, et al. (2008) Chemotactic activity of S100A7 (Psoriasin) is mediated by the receptor for advanced glycation end products and potentiates inflammation with highly homologous but functionally distinct S100A15. J Immunol 181: 1499-1506.
- Watanabe Y, Kato H, Araki T (2008) Protective action of neuronal nitric oxide synthase inhibitor in the MPTP mouse model of Parkinson's disease. Metab Brain Dis 23: 51-69.
- Mori T, Koyama N, Arendash GW, Horikoshi-Sakuraba Y, Tan J, et al. (2010) Overexpression of human S100B exacerbates cerebral amyloidosis and gliosis in the Tg2576 mouse model of Alzheimer's disease. Glia 58: 300-314.
- Abdelsalam RM, Safar MM (2015) Neuroprotective effects of vildagliptin in rat rotenone Parkinson's disease model: role of RAGE-NFkappaB and Nrf2-antioxidant signaling pathways. J Neurochem 133: 700-707.
- Schapansky J, Nardozzi JD, LaVoie MJ (2015) The complex relationships between microglia, alpha-synuclein, and LRRK2 in Parkinson's disease. Neuroscience 302: 74-88.
- Deane R, Singh I, Sagare AP, Bell RD, Ross NT, et al. (2012) A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest 122: 1377-1392.
- Han YT, Choi GI, Son D, Kim NJ, Yun H, et al. (2012) Ligand-based design, synthesis, and biological evaluation of 2-aminopyrimidines, a novel series of receptor for advanced glycation end products (RAGE) inhibitors. J Med Chem 55: 9120-9135.
- Han YT, Kim K, Choi GI, An H, Son D, et al. (2014) Pyrazole-5-carboxamides, novel inhibitors of receptor for advanced glycation end products (RAGE). Eur J Med Chem 79: 128-142.
- Han YT, Kim K, Son D, An H, Kim H, et al. (2015) Fine tuning of 4,6-bisphenyl-2-(3-alkoxyanilino)pyrimidine focusing on the activity-sensitive aminoalkoxy moiety for a therapeutically useful inhibitor of receptor for advanced glycation end products (RAGE). Bioorg Med Chem 23: 579-587.
- Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, et al. (2009) RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci U S A 106: 20021-20026.
- Yan SD, Chen X, Fu J, Chen M, Zhu H, et al. (1996) RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature 382: 685-691.
- Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, et al. (2004) RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. EMBO J 23: 4096-4105.
- Therapeutics v (2014) Evaluation of the Efficacy and Safety of Azeliragon (TTP488) in Patients With Mild Alzheimer's Disease (STEADFAST).
- Galasko D, Bell J, Mancuso JY, Kupiec JW, Sabbagh MN, et al. (2014) Clinical trial of an inhibitor of RAGE- Aβ interactions in Alzheimer disease. Neurology 82: 1536-1542.
- Takeuchi M, Sato T, Takino J, Kobayashi Y, Furuno S, et al. (2007) Diagnostic utility of serum or cerebrospinal fluid levels of toxic advanced glycation end-products (TAGE) in early detection of Alzheimer's disease. Med Hypotheses 69: 1358-1366.
- Munch G, Luth HJ, Wong A, Arendt T, Hirsch E, et al. (2000) Crosslinking of alpha-synuclein by advanced glycation endproducts--an early pathophysiological step in Lewy body formation? J Chem Neuroanat 20: 253-257.
- Wang CY, Xie JW, Xu Y, Wang T, Cai JH, et al. (2013) Trientine reduces BACE1 activity and mitigates amyloidosis via the AGE/RAGE/NF-κB pathway in a transgenic mouse model of Alzheimer's disease. Antioxid Redox Signal 19: 2024-2039.
- Wang X, Yu S, Hu J, Wang P, Wang CY, et al. (2014). Streptozotocin-induced diabetes increases amyloid plaque deposition in AD transgenic mice through modulating AGEs/RAGE/NF-?B pathway. Int J Neurosci 124: 601-608.
- Lv C, Wang L, Liu X, Cong X, Yan S, et al. (2014). Geniposide attenuates oligomeric Aß(1-42)-induced inflammatory response by targeting RAGE-dependent signaling in BV2 cells. Curr Alzheimer Res 11: 430-440.
- Origlia N, Bonadonna C, Rosellini A, Leznik E, Arancio O, et al. (2010) Microglial receptor for advanced glycation end product-dependent signal pathway drives ß-amyloid-induced synaptic depression and long-term depression impairment in entorhinal cortex. J Neurosci 30: 11414-11425.
- Son SM, Jung ES, Shin HJ, Byun J, Mook-Jung I (2012) Aß-induced formation of autophagosomes is mediated by RAGE-CaMKKβ-AMPK signaling. Neurobiol Aging 33: 1006.
- Bortolotto V, Grilli M (2016) Every cloud has a silver lining: Proneurogenic effects of Aß oligomers and HMGB-1 via activation of the RAGE-NF-?B axis. CNS Neurol Disord Drug Targets 15: 1-14.
- Teismann P, Sathe K, Bierhaus A, Leng L, Martin HL, et al. (2012) Receptor for advanced glycation endproducts (RAGE) deficiency protects against MPTP toxicity. Neurobiol Aging 33: 2478-2490.
- Sathe K, Maetzler W, Lang JD, Mounsey RB, Fleckenstein C, et al. (2012) S100B is increased in Parkinson's disease and ablation protects against MPTP-induced toxicity through the RAGE and TNF- α pathway. Brain 135: 3336-3347.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 4020
- [From(publication date):
April-2017 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 3095
- PDF downloads : 925